Abstract
The main objective of this study was to evaluate the association of the insomnia-anxiety comorbidity with incident type 2 diabetes (T2D) in a large prospective cohort. We selected adults without diabetes at baseline from the French NutriNet-Santé cohort who had completed the trait anxiety subscale of the Spielberger State-Trait Anxiety Inventory (STAI-T, 2013–2016) and a sleep questionnaire (2014); insomnia was defined according to established criteria. Using multivariable Cox models, we compared T2D risk across 4 groups: no insomnia or anxiety (reference), insomnia alone, anxiety alone (STAI-T ≥ 40), and comorbid anxiety and insomnia. Among 35,014 participants (mean baseline age: 52.4 ± 14.0 years; 76% women), 378 (1.1%) developed T2D over a mean follow-up of 5.9 ± 2.1 years. Overall, 28.5% of the sample had anxiety alone, 7.5%—insomnia alone, and 12.5%—both disorders. In the fully-adjusted model, a higher T2D risk was associated with anxiety-insomnia comorbidity (HR = 1.40; 95% CI 1.01, 1.94), but not with each disorder separately, compared to the group without insomnia or anxiety. The findings supported a positive association between anxiety-insomnia comorbidity and incident T2D among general-population adults. Future research using clinical diagnoses of mental disorders could confirm the findings and guide diabetes prevention programs.
Similar content being viewed by others
Introduction
Diabetes mellitus represents a major public health challenge owing to its prevalence, chronicity and deleterious impact on various organs and tissues1,2. Type 2 diabetes (T2D), which accounts for 90% of all diabetes cases, has multifactorial etiology, including genetic, psychosocial, behavioral, and psychiatric causes1. It is therefore critical to advance knowledge about modifiable factors in order to better target primary prevention of the disease.
A recent umbrella review provided strong support for the increased prevalence of T2D among individuals with psychiatric conditions, particularly anxiety and insomnia3. Moreover, comorbidity, defined as the simultaneous presence of ≥ 2 conditions in the same individual, of these two mental disorders is also frequent4. Anxiety is underscored by anticipation of a future threat and presents symptoms such as agitation, tiredness, difficulties concentrating, irritability, and muscle tension5. Insomnia—the most prevalent sleep disorder—is defined by a difficulty falling asleep, maintaining sleep or early morning waking, with such symptoms occurring ≥ 3 nights/week over the past ≥ 3 months5. According to the most recent estimates by the French national public health agency, the general population prevalence of anxiety and insomnia was 25% and 13%, respectively6,7.
A systematic review of observational studies reported that elevated symptoms of anxiety were present in 40% of individuals with diabetes8. Further, one meta-analysis of 14 longitudinal studies with a follow-up ranging from 2 to 14 years reported a 47% increased risk of incident T2D associated with baseline anxiety9. It included studies with heterogeneous methodologies featuring different types of anxiety disorders (e.g., generalized anxiety disorder, panic disorder, phobic disorders, post-traumatic stress disorder)9.
On the other hand, numerous prospective studies have investigated the association between various sleep disorders and T2D; the findings have been relatively consistent and summarized in several meta-analyses10,11,12,13. Prior research has included different symptoms or types of sleep disorders, such as acute insomnia, difficulty falling asleep, non-restorative sleep, and short total sleep time without considering the duration or frequency of experiencing the symptoms. Some studies have focused on insomnia (without considering its impact on daily life) in different general and specific (i.e., pre-diabetic) populations and have found positive associations with incident T2D14,15,16.
To our knowledge, the anxiety-insomnia comorbidity has not been investigated as a predictor of incident T2D in an epidemiological context. However, anxiety and insomnia form a vicious circle and share many biological mechanisms17. They also share several pathways affecting the central nervous system and metabolism, and may represent mechanisms for the development of T2D17. Thus, our objective was to evaluate the association of anxiety, insomnia and their comorbidity with incident T2D among adults. We hypothesized that individuals with anxiety-insomnia comorbidity would have a greater risk of developing T2D than would individuals with anxiety or insomnia alone or those without either disorder.
Materials and methods
Study overview
This prospective analysis is part of the 4-year multidisciplinary MEMORIES project focused on mental-physical comorbidity. Specifically, the associations between a number of individual and concurrent mental health conditions (e.g., anxiety, insomnia, depressive symptoms, eating disorders) and metabolic disorders (notably T2D and obesity) are investigated among general-population adults in order to advance knowledge about risk factors and primary prevention options.
Study population
NutriNet-Santé is an ongoing web-based prospective cohort launched in 2009 in France. Its main aims are: (1) to elucidate the relationship between nutrition, health status, chronic morbidity, and mortality, and (2) to examine the determinants of dietary habits and nutritional status. The cohort’s design and methods have been described elsewhere18. Eligible participants are men and women aged ≥ 18 years, with desktop or mobile Internet access and able to follow an online protocol in French. They are recruited from the general population primarily via recurrent multimedia campaigns.
Once enrolled, participants complete online (https://etude-nutrinet-sante.fr/) a set of 5 questionnaires related to their sociodemographic and anthropometric characteristics, lifestyle choices, physical activity and sedentariness, physical and mental health status, and dietary intake.
Data collection
Type 2 diabetes mellitus assessment
Health status is self-reported at enrollment and is reassessed every 6 months over the follow-up, using a specifically developed health status questionnaire. At any time during the follow-up, participants can also report a major or minor health event, a new prescribed treatment, a medical exam or a hospitalization via a dedicated and secure online platform. T2D cases were defined using ICD-10 code E11 and ATC classification codes for T2D medication. Moreover, in NutriNet-Santé, all self-reported diagnosis and medication use data are linked to the national health insurance database (SNIIRAM), providing information about reimbursement of prescribed medication and hospitalizations. For this study, we considered all incident cases occurring between inclusion and April 2022. Next, T2D mortality cases were identified using a linkage to CépiDC, the French national mortality registry. Details about case ascertainment are provided in Supplementary Table S1. T2D incidence was the main outcome in this study.
Trait anxiety assessment
Trait anxiety was one of the two main exposures in this analysis. It was evaluated by self-reports on the trait anxiety subscale of the State-Trait Anxiety Inventory Form Y (STAI-T). The STAI is one of the most widely used tools for assessing anxiety, particularly as it distinguishes it from depression19. The French version of STAI has been validated in adults from the general population19. STAI-T consists of 20 items (e.g. "I worry too much about things that are not worth it"), scored on a 4-point Likert scale ranging from "Almost never" to "Almost always". The total score range is 20–80 points; the higher the score, the higher the anxiety proneness. Participants were considered as having high trait anxiety if their STAI-T score was ≥ 4020. Anxiety as measured by the STAI-T is highly correlated with generalized anxiety disorder21. In NutriNet-Santé, this questionnaire was administered between November 2013 and December 2016 to participants enrolled in the cohort for ≥ 2.5 years, which represented a total of 119,451 enrollees solicited. Each participant was allowed to complete it once, on a voluntary basis. The STAI-T completion date was considered as baseline for this analysis.
Insomnia assessment
Chronic insomnia was also one of the main exposures in this analysis. A questionnaire assessing sleep habits and characteristics was administered between February and July 2014 to all participants of NutriNet-Santé at that time. To define chronic insomnia, we followed the International Classification of Sleep Disorders—Third Edition (ICSD-3)22 and the Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition (DSM-5)5. For chronic insomnia the criteria include experiencing sleep problems (difficulties falling asleep, frequent nighttime waking), ≥ 3 nights/week over the past ≥ 3 months, and experiencing negative repercussions of such problems in daily life.
Covariate data collection
Socio-demographic data including sex, age, education (< high school, high school diploma, college/undergraduate degree, graduate degree), employment category (manual/blue collar/farmer, office work/administrative staff, professional/executive staff, retired, without professional activity), children < 18 years in the household (yes/no), and lifestyle data including smoking status (never, former or current smoker) and alcohol use (g ethanol/day) are provided by a validated self-reported questionnaire23. Physical activity (low, moderate, high) and sedentariness (minutes spent sitting/day) were assessed with the International Physical Activity Questionnaire using established scoring guidelines24. Anthropometric data (height and weight) were self-reported using a validated questionnaire25. Data on hypertension, dyslipidemia, gestational diabetes and family history of diabetes were obtained from the health status questionnaire. As all of the above-mentioned questionnaires are administered on an annual basis, we used data provided within a 2.5-year window around baseline.
Depressive symptomatology was also modelled as a covariate; it was assessed with the Center for Epidemiologic Studies-Depression Scale (CES-D)26,27, which is administered every 2 years during the follow-up. This is a 20-item questionnaire where participants report their experience of symptoms associated with depression over the past week; the items are rated on 4-point scale ranging from 1 = “Rarely or never” to 4 = “Most or all the time”. Higher scores indicate higher depressive symptomatology. The internal consistency assessed by Cronbach’s alpha coefficient was high (> 0.80) at each CES-D assessment. Participants were considered as having depressive symptoms if their CES-D score was ≥ 17 (for men) or ≥ 23 (for women)26, corresponding to the validated cut-offs for the French population.
Finally, mean energy intake (kcal/day) and proportion of energy from carbohydrates were assessed using a set of three 24-h dietary records which participants were asked to complete every 6 months. We selected CES-D and dietary data within the 2.5-year window around baseline.
Statistical analyses
For the following covariates with < 5% missing values, we used multiple imputation techniques: education (4.0% missing values), employment (4.5%), alcohol consumption (3.2%), sedentariness (4.6%), smoking (3.2%), physical activity (4.5%) and depressive symptoms (3.0%). There were no covariates with > 5% missing values. Body mass index (BMI, kg/m2) was calculated using the self-reported weight and height data. The BMI values were then divided into four categories: underweight (< 18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obesity (≥ 30.0). For the analyses, morbidity and comorbidity groups were created as follows: neither anxiety nor insomnia, anxiety alone, insomnia alone, and comorbid anxiety and insomnia. Descriptive characteristics according to these morbidity groups are presented in Table 1, showing the number (%) and p-values of the chi-squared tests for categorical variables, and the mean values (± SD) and p-values obtained by ANOVA for continuous variables.
We tested the interaction between anxiety and insomnia as regards T2D risk. Next, using the method applied by Deschênes et al.28, the risk estimates of T2D in the different groups were compared to the “no anxiety or insomnia” group in the main model. In a secondary model, the risk estimates were compared to the “anxiety only” group, as anxiety is a more prevalent disorder than chronic insomnia in the general population6,7. These associations were investigated using Cox proportional hazards models, providing hazard ratios (HR) and 95% confidence intervals (CI). Given that the STAI-T completion date (baseline) varied across participants, age was used as a time scale. Participants contributed person-time from their age at baseline (i.e. age at which they completed STAI-T) to their age at T2D onset, age at the time of death (obtained from the CépiDC registry), age at the time of the last follow-up, or age as of April 29, 2022, whichever occurred first. The proportional hazards assumption of the Cox model was tested and confirmed with the rescaled Schoenfeld-type residuals method. The partially-adjusted models (Model 1) were controlled for age (time scale), sex, and obesity (BMI ≥ 30 kg/m2). The fully-adjusted models (Model 2) were adjusted for age (time scale), sex, education, employment, children aged < 18 years in the household, obesity (BMI ≥ 30 kg/m2), physical activity, sedentariness, alcohol consumption, smoking status, hypertension, dyslipidemia, family history of T2D, and depressive symptoms.
In a sensitivity analysis, we added total energy intake and proportion of the total energy from carbohydrates to model 2. In a second sensitivity analysis, we fit our main model restricting the sample to participants with ≥ 2 years of follow-up.
As differences in anxiety and insomnia prevalence according to sex have been highlighted in the literature29,30, we performed tests for interaction (significance level p < 0.10) by sex. The main tests were two-sided and p < 0.05 was considered as evidence for statistical significance. R version 4.1.2 (R Foundation, Vienna, Austria) was used for the analyses.
Ethical standards
The NutriNet-Santé cohort study is conducted according to the Declaration of Helsinki guidelines. It was approved by the Institutional Review Board of the French Institute for Health and Medical Research (INSERM # 00000388FWA00005831) and by the National Commission on Informatics and Liberty (CNIL # 908450 and # 909216). NutriNet-Santé is registered at: https://clinicaltrials.gov/ct2/show/NCT03335644. Electronic informed consent was obtained from all volunteers prior to inclusion in the cohort.
Results
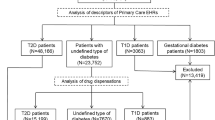
A total of 40,809 participants in NutriNet-Santé completed STAI-T and were thus eligible for this analysis. Of these, 1583 had incomplete anxiety data and were excluded from the sample. Next, those who did not have sleep/insomnia data were ineligible for the study (n = 2753). Participants with type 1 diabetes (incident or prevalent), gestational diabetes or prevalent T2D at the time of completion of STAI-T were also excluded (n = 1321), as were those lost to follow-up (n = 138). Thus, the final sample was composed of 35,014 participants (76.2% women, mean age = 52.4 ± 14.0 years) (Fig. 1).
The mean follow-up time in this study was 5.9 ± 2.1 years. By the end of the follow-up, there were 378 (1.1%) incident T2D cases. Interaction tests by sex were not statistically significant (p > 0.2) thus baseline descriptive characteristics are presented in the full sample, according to morbidity group (Table 1).
The final sample was predominantly female, relatively well educated, non-smoking and with a normal-range BMI. The anxiety-insomnia group (n = 4386; 12.5% of the full sample) had the highest percentage of women, participants with depressive symptoms, history of hypertension, low physical activity and obesity. In that group, 1.5% developed T2D, which represented a greater proportion than in the other study groups (Table 1). The interaction tests between insomnia and anxiety regarding risk of T2D were not statistically significant (p > 0.3).
Table 2 presents Cox model results of the associations between the 4 morbidity groups and incident T2D, with the “no anxiety or insomnia” group as the reference. In Model 1 (adjusted for age, sex, and obesity), anxiety, unlike insomnia, emerged as a significant predictor of T2D (HR: 1.37; 1.07–1.76). In the fully-adjusted model (Model 2), high trait anxiety alone and chronic insomnia alone were not associated with incident T2D. However, participants with both high trait anxiety and chronic insomnia had an increased risk of incident T2D (HR: 1.40; 95% CI 1.01, 1.95) compared with participants without insomnia or anxiety.
To better understand the relationship between the anxiety-insomnia comorbidity and T2D and to test our secondary hypothesis, we compared participants having both disorders to those having only high trait anxiety. Table 3 presents these Cox model results. In Model 1, controlled for age, sex and obesity, participants with no anxiety or insomnia were significantly less likely to develop T2D during follow-up compared to participants with only high trait anxiety (HR = 0.73; 0.57–0.93). In Model 2, that associated was attenuated. Likewise, Model 2 revealed that participants with anxiety-insomnia comorbidity were not significantly more likely to develop T2D compared to participants with high trait anxiety alone.
Supplementary Table S2 presents the results of the two sets of sensitivity analysis. In the first set (Model 3), we added to Model 2 covariates regarding total mean energy intake without alcohol (kcal/day) and proportion (%) of the total energy from carbohydrates. The addition of these data resulted in the reduction of the sample to 22,819 participants of whom n = 231 were incident T2D cases. The results were consistent with those obtained in the main analysis (HR = 1.61; 1.06–2.45).
In the second set of sensitivity analyses (Model 4), we fit the same models as those in the main analysis, while restricting our sample to participants with ≥ 2 years of follow-up (N = 32,376; n = 226 incident T2D cases). The results (Model 4) likewise remained consistent with those obtained in the main analysis.
Discussion
As reduction of T2D rates is a public health priority, it is important to advance knowledge about modifiable risk factors for the disease in order to inform prevention efforts. We focused on mental health-related determinants and more specifically on the impact of the comorbidity between two prevalent mental disorders. To our knowledge, this prospective study is the first to provide estimates of the association between anxiety-insomnia comorbidity and incident T2D. It included 35,014 men and women, of whom 378 developed T2D during a mean follow-up of nearly 6 years. We employed the validated STAI-T tool for the assessment of trait anxiety and based our insomnia definition on DSM-5 and ICSD-3 criteria. We compared T2D risk across 4 morbidity groups: no anxiety or insomnia, anxiety alone, chronic insomnia alone, and anxiety-insomnia comorbidity. In the main analysis, high trait anxiety alone and chronic insomnia alone were not significantly associated with T2D. However, participants with anxiety-insomnia comorbidity were significantly more likely to develop T2D compared to those without anxiety or insomnia. Our hypothesis was therefore partially supported.
Our main findings regarding anxiety alone are in contrast with a recent meta-analysis which concluded that anxiety is a significant risk factor for T2D9. It should be noted that the 14 observational studies included in that meta-analysis addressed different types of anxiety (e.g., trait anxiety, generalized anxiety disorder, post-traumatic stress disorder, social phobia) rendering the methodology heterogeneous. However, null findings regarding anxiety as a predictor of T2D (measured with STAI-T on a continuous scale) were reported by Abraham et al. in the prospective Multi-Ethnic Study of Atherosclerosis31 included in the meta-analysis. In that meta-analysis, adjustment for depression did not have an impact on the estimated association between anxiety and T2D9. Likewise, our findings were independent of depressive symptoms. Depression has in fact been the most studied mental health determinant of T2D32, alone or together with other mental health conditions. For example, symptoms of depression, anxiety, and insomnia, were found to increase the risk of pre-diabetes and T2D in Swedish middle-aged men but not women33. Next, one large prospective study reported that the anxiety-depression comorbidity was a risk factor for incident T2D28. These authors revealed that participants with an anxiety-depression comorbidity had a higher risk of T2D than did participants with anxiety alone or depression alone28. Whereas anxiety and depression are related disorders, their distinctive features are numerous and important5.
Next, no association between chronic insomnia and incident T2D was found in our study. An umbrella review of two meta-analyses10,11 concluded that a positive association exists between baseline sleep disorders (i.e., difficulty maintaining sleep and difficulty initiating sleep) and T2D onset12. None of the studies included in the meta-analyses adjusted for anxiety10,11. Regarding insomnia itself, prospective studies that have investigated its association with incident T2D concluded to a positive association14,15,16. However, prior research has focused on populations with prediabetes, or has investigated insomnia without considering its impact on daily life14,15. Moreover, Green et al. observed that the association between insomnia and T2D was attenuated when psychiatric distress was viewed as a cofounding variable14.
As hypothesized, NutriNet-Santé participants with anxiety-insomnia comorbidity were more likely to develop T2D compared to their counterparts without anxiety or insomnia. This finding was reinforced by the sensitivity analyses. Given the frequent comorbidity of these two disorders4, some authors suggest that they might in fact represent different symptoms of the same pathology34. However, in our study, there was a significantly increased T2D risk when the reference group was ‘no anxiety or insomnia’ and a non-significant risk when the reference group was ‘anxiety alone’. When comparing the ‘anxiety-insomnia comorbidity’ group with the ‘no anxiety or insomnia’ group, there is a clearer difference in health status than when the comparison is made with the ‘anxiety alone’ group. In addition, our study is not based on clinical diagnoses of anxiety; it is also likely that individuals with the most severe symptoms were not included in the analysis35.
It has been estimated that anywhere from a quarter to about half of individuals with anxiety also have insomnia symptoms36. In addition, 36% of those with insomnia symptoms also have anxiety symptoms37. Uhde and Cortese suggest 3 theoretical models to explain this comorbidity34. First, anxiety and insomnia could represent a single mental health disorder; second, they could be separate conditions, one of which precedes and influences the development of the other; finally, they could be separate conditions caused by an independent factor34. Ohayon and Roth described this comorbidity according to the onset of the two disorders. Insomnia appeared before anxiety in 18% of cases, at the same time in 39%, and after anxiety in 44% of cases38, arguing for a complex, possibly bidirectional relationship between the two disorders17,34. At present, each disorder is regarded as having sufficiently distinct characteristics and is described individually in the DSM-55.
Anxiety and insomnia have several common pathways impacting the central nervous system and metabolism and may represent mechanisms in the pathogenesis of T2D17. There is strong evidence for the role of the hypothalamus–pituitary–adrenal (HPA) axis in sleep regulation39. Also, stress activates the sympathetic nervous system and the HPA axis, resulting in the release of glucocorticoids, such as cortisol, leading to an increase in blood glucose concentrations40. In addition, excessive activation of the HPA axis induces sleep fragmentation, a stress-prone state, further increasing cortisol levels17. On the other hand, insomnia symptoms and anxiety disorders are also associated with markers of systemic inflammation41, such as increased C-reactive protein or interleukin-6, which are themselves associated with T2D42. In addition, anxiety and insomnia are associated with behavioral and lifestyle risk factors that have themselves been linked to T2D. For example, both disorders are strongly associated with weight gain and obesity43,44, and with physical inactivity and sedentariness45. Finally, cognitive mechanisms have also been explored, as anxiety is associated with decreased concentration, lack of motivation and impaired decision making, all of which favor certain unfavorable behaviors, such as reduced adherence to medical regimens12.
This study has some limitations. As NutriNet-Santé is focused on nutrition and health, it is possible that it attracts individuals interested in these topics. This may lead to an underestimation of studied exposure-outcome associations. Our sample included a relatively small number and percentage of participants with chronic insomnia alone, reducing the available statistical power. NutriNet-Santé participants are predominantly women (> 70%) of high socioeconomic status23, limiting generalizability of the findings. Next, as elsewhere around the world, T2D is generally underdiagnosed in France: it has been estimated that about 20% of cases remain undetected46. In our study, incident T2D cases were assessed and validated through multiple sources. Regarding our exposure variables, one-time subjective measures of anxiety and insomnia were used, although for the former we used a validated tool (STAI) and for the latter we followed the ICSD-3 and DSM-5 criteria. Also, even though the STAI-T is one of the most widely used tools in epidemiological research concerning anxiety, it cannot replace a complete clinical diagnosis. Finally, even though the analyses were adjusted for a number of potential cofounders, omitted variables such as other sleep variables (e.g., total sleep time, obstructive sleep apnea, etc.), other mental health conditions, family history of anxiety or insomnia disorders or ethno-racial status might have impacted the observed associations. Future studies could address questions of interaction, confounding, and/or mediation by such variables.
Nonetheless, this study presents several strengths. To our knowledge, it is the first study investigating the association between anxiety-insomnia comorbidity and incident T2D on a large scale. It uses a very large and diverse sample of French adults recruited from the general population. As our exposure variable was composed of 4 morbidity groups and the models allowed us to calculate separate T2D risk estimates. Next, the analysis was adjusted for well-established T2D risk factors, such as smoking, alcohol use, sedentariness and obesity1, among other covariates. Strengths also include the longitudinal design, with almost 6 years of follow-up, thus reverse causality is an unlikely explanation for the obtained results.
Conclusion
In this large population-based sample, participants with anxiety-insomnia comorbidity were more likely to develop T2D than were participants with no anxiety or insomnia. Future prospective population-based studies are needed to confirm the findings. Given the high prevalence of anxiety and insomnia in the general population, the results highlight the need for prevention programs to address these mental health conditions before they lead to metabolic complications. Moreover, future analyses in specific subgroups (e.g., individuals with poor dietary quality, individuals with concurrent chronic physical and/or mental health conditions) could advance knowledge and help inform targeted T2D prevention efforts.
Data availability
Data described in the manuscript can be made available upon request pending application and approval. Researchers from public institutions can submit a collaboration request including information on the institution and a brief description of the project to [email protected]. All requests are reviewed by the steering committee of the NutriNet-Santé study. A financial contribution may be requested. If the collaboration is accepted, a data access agreement will be necessary and appropriate authorizations from the competent administrative authorities may be needed. In accordance with existing regulations, no personal data will be accessible.
Abbreviations
- BMI:
-
Body mass index
- CES-D:
-
Center for epidemiologic studies-depression scale
- CI:
-
Confidence interval
- DSM-5:
-
Diagnostic and statistical manual of mental disorders—fifth edition
- HPA:
-
Hypothalamus–pituitary–adrenal (axis)
- HR:
-
Hazard ratio
- ICSD-3:
-
International classification of sleep disorders—third edition
- STAI:
-
State-trait anxiety inventory
- STAI-T:
-
Trait anxiety subscale of the state-trait anxiety inventory
- T2D:
-
Type 2 diabetes mellitus
References
World Health Organization. Global report on diabetes. https://apps.who.int/iris/handle/10665/341248 (2016).
International Diabetes Federation. Key global findings 2021. https://diabetesatlas.org/ (2022).
Lindekilde, N. et al. Prevalence of type 2 diabetes in psychiatric disorders: An umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetologia 65, 440–456 (2022).
Aernout, E. et al. International study of the prevalence and factors associated with insomnia in the general population. Sleep Medicine 82, 186–192 (2021).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). (American Psychiatric Association, 2013).
Santé Publique France. CoviPrev : une enquête pour suivre l’évolution des comportements et de la santé mentale pendant l’épidémie de COVID-19. https://www.santepubliquefrance.fr/etudes-et-enquetes/coviprev-une-enquete-pour-suivre-l-evolution-des-comportements-et-de-la-sante-mentale-pendant-l-epidemie-de-covid-19 (2023).
Léger, D., Zeghnoun, A., Faraut, B. & Richard, J.-B. temps de sommeil, la dette de sommeil, la restriction de sommeil et l’insomnie chronique des 18–75 ans: résultats du Baromètre de Santé Publique France 2017. Bull. Epidémiol. Hebd 8–9, 149–160 (2019).
Grigsby, A. B., Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. Prevalence of anxiety in adults with diabetes A systematic review. J. Psychosom. Res. 1053–1060 (2002).
Smith, K. J., Deschênes, S. S. & Schmitz, N. Investigating the longitudinal association between diabetes and anxiety: A systematic review and meta-analysis. Diabet Med. 35, 677–693 (2018).
Anothaisintawee, T., Reutrakul, S., Van Cauter, E. & Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 30, 11–24 (2016).
Cappuccio, F. P., D’Elia, L., Strazzullo, P. & Miller, M. A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 33, 414–420 (2010).
Lindekilde, N. et al. Psychiatric disorders as risk factors for type 2 diabetes: An umbrella review of systematic reviews with and without meta-analyses. Diabetes Res. Clin. Pract. 176, 108855 (2021).
Johnson, K. A. et al. The association of insomnia disorder characterised by objective short sleep duration with hypertension, diabetes and body mass index: A systematic review and meta-analysis. Sleep Med. Rev. 59, 101456 (2021).
Green, M. J., Espie, C. A., Popham, F., Robertson, T. & Benzeval, M. Insomnia symptoms as a cause of type 2 diabetes Incidence: a 20 year cohort study. BMC Psychiatry 17, 94 (2017).
LeBlanc, E. S., Smith, N. X., Nichols, G. A., Allison, M. J. & Clarke, G. N. Insomnia is associated with an increased risk of type 2 diabetes in the clinical setting. BMJ Open Diab. Res. Care 6, e000604 (2018).
Lin, C.-L., Chien, W.-C., Chung, C.-H. & Wu, F.-L. Risk of type 2 diabetes in patients with insomnia: A population-based historical cohort study. Diabetes Metab. Res. Rev. 34, e2930 (2018).
Hirotsu, C., Tufik, S. & Andersen, M. L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 8, 143–152 (2015).
Hercberg, S. et al. The Nutrinet-Santé Study: a web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health 10, 242 (2010).
Spielberger, C., Gorsuch, R., Lushene, R., Vagg, P. R. & Jacobs, G. Manual for the State-Trait Anxiety Inevntory. (Consulting Psychologists Press, 1983).
Kose, J. et al. A Comparison of sugar intake between individuals with high and low trait anxiety: Results from the NutriNet-Santé study. Nutrients 13, 1526 (2021).
Chambers, J. A., Power, K. G. & Durham, R. C. The relationship between trait vulnerability and anxiety and depressive diagnoses at long-term follow-up of generalized anxiety disorder. J. Anxiety Disord. 18, 587–607 (2004).
American Academy of Sleep Medicine. International Classification of Sleep Disorders (ICSD-3). (American Academy of Sleep Medicine, 2014).
Andreeva, V. A. et al. Comparison of the sociodemographic characteristics of the large NutriNet-Santé e-cohort with French Census data: The issue of volunteer bias revisited. J. Epidemiol. Community Health 69, 893–898 (2015).
Craig, C. L. et al. International Physical Activity Questionnaire: 12-country reliability and validity: Med. Sci. Sports Exerc. 35, 1381–1395 (2003).
Touvier, M. et al. Comparison between web-based and paper versions of a self-administered anthropometric questionnaire. Eur. J. Epidemiol. 25, 287–296 (2010).
Fuhrer, R. & Rouillon, F. La version française de l’échelle CES-D (Center for Epidemiologic Studies-Depression Scale). Description et traduction de l’échelle d’autoévaluation. Psychiatry Psychobiol. 4, 163–166 (1989).
Radloff, L. S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977).
Deschênes, S. S., Burns, R. J. & Schmitz, N. Comorbid depressive and anxiety symptoms and the risk of type 2 diabetes: Findings from the Lifelines Cohort Study. J. Affect. Disord. 238, 24–31 (2018).
Jalnapurkar, I., Allen, M. & Pigott, T. Sex differences in anxiety disorders: A review. J. Psychiatr. Depress. Anxiety 4, 1–9 (2018).
Zhang, B. & Wing, Y.-K. Sex differences in insomnia: A meta-analysis. Sleep 29, 85–93 (2006).
Abraham, S. et al. Trait anger but not anxiety predicts incident type 2 diabetes: The multi-ethnic study of atherosclerosis (MESA). Psychoneuroendocrinology 60, 105–113 (2015).
Kremers, S. H. M. et al. The role of mental disorders in precision medicine for diabetes: A narrative review. Diabetologia 65, 1895–1906 (2022).
Eriksson, A.-K. et al. Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet. Med. 25, 834–842 (2008).
Uhde, T. W., Cortese, B. M. & Vedeniapin, A. Anxiety and sleep problems: Emerging concepts and theoretical treatment implications. Curr. Psychiatry Rep. 11, 269–276 (2009).
Haapea, M. et al. Non-participation in a field survey with respect to psychiatric disorders. Scand. J. Public Health 36, 728–736 (2008).
Soehner, A. M. & Harvey, A. G. Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: Results from a nationally representative sample. Sleep 35, 1367–1375 (2012).
Breslau, N., Roth, T., Rosenthal, L. & Andreski, P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol. Psychiatry 39, 411–418 (1996).
Ohayon, M. M. & Roth, T. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 37, 9–15 (2003).
Steiger, A. Neurochemical regulation of sleep. J. Psychiatr. Res. 41, 537–552 (2007).
De Oliveira, C. et al. High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations. Lipids Health Dis. 10, 11 (2011).
Irwin, M. R., Olmstead, R. & Carroll, J. E. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52 (2016).
Brunner, E. J. et al. Inflammation, insulin resistance, and diabetes—Mendelian randomization using CRP haplotypes points upstream. PLOS Med. 5, e155 (2008).
Gariepy, G., Nitka, D. & Schmitz, N. The association between obesity and anxiety disorders in the population: A systematic review and meta-analysis. Int. J. Obes. (Lond) 34, 407–419 (2010).
Cappuccio, F. P. et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 31, 619–626 (2008).
Booth, J. N. et al. Reduced physical activity in adults at risk for type 2 diabetes who curtail their sleep. Obesity 20, 278–284 (2012).
Fagot-Campagna, A., Romon, I., Fosse, S. & Roudier, C. Prévalence et incidence du diabète, et mortalité liée au diabète en France - Synthèse épidémiologique. www.invs.sante.fr (2010).
Acknowledgements
The authors thank Cédric Agaësse (dietary data manager), Alexandre De-Sa and Rebecca Lutchia (dietitians); Selim Aloui (IT manager), Thi Hong Van Duong, Régis Gatibelza, Jagatjit Mohinder and Aladi Timera (computer scientists); Julien Allegre, Nathalie Arnault, Laurent Bourhis, Nicolas Dechamp (biostatisticians) and Fabien Szabo de Edelenyi, Ph.D. (biostatistics team manager); Maria Gomes (NutriNet-Santé participant support); Paola Yvroud, M.D. (health event validator) and Marine Ricau, Ph.D. (operations coordinator) for their technical contribution to the NutriNet-Santé study. The authors sincerely thank all the volunteers of the NutriNet- Santé cohort.
Funding
The MEMORIES project is funded by the French National Research Agency (ANR) (grant #ANR-21-CE36-0003; Dr. Andreeva-PI). The NutriNet-Santé study is supported by the French Ministry of Solidarity and Health, the National Agency for Public Health (Santé Publique France), the National Institute for Health and Medical Research (INSERM), the National Research Institute for Agriculture, Food and Environment (INRAE), the National Conservatory of Arts and Crafts (CNAM), and Sorbonne Paris Nord University. Pauline Duquenne is funded by a doctoral fellowship from the French National Research Agency (ANR) (grant #ANR-21-CE36-0003). The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
VAA, CS, SC, MCB and LKF developed the MEMORIES project on which this study is based; EKG, SH, PG and MT designed and implemented the NutriNet-Santé cohort study; PG and VAA implemented the STAI questionnaire and coordinated anxiety data collection; VAA, PG and DL implemented the sleep questionnaire and coordinated insomnia data collection; VAA conceptualized the study, designed the analytic strategy, and provided theoretical and empirical guidance; PD performed the literature review, statistical analyses and led the writing; LKF provided methodological guidance; all authors assisted with interpretation of the data, critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and its submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duquenne, P., Samieri, C., Chambaron, S. et al. Chronic insomnia, high trait anxiety and their comorbidity as risk factors for incident type 2 diabetes mellitus. Sci Rep 14, 11927 (2024). https://doi.org/10.1038/s41598-024-62675-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62675-y
Keywords
This article is cited by
-
7RSSDI position statement: Mind–body medicine in diabetes by the Mind–Body Medicine Task Force of the RSSDI
International Journal of Diabetes in Developing Countries (2025)