Abstract
NS1 is an elusive dengue protein, involved in viral replication, assembly, pathogenesis, and immune evasion. Its levels in blood plasm are positively related to disease severity like thrombocytopenia, hemorrhage, and vascular leakage. Despite its pathogenic roles, NS1 is being used in various vaccine formulations due to its sequence conservancy, ability to produce protective antibodies and low risk for inducing antibody-dependent enhancement. In this study, we have used bioinformatics tools and reported literature to develop an NS1 variant (dNS1). Molecular docking studies were performed to evaluate the receptor-binding ability of the NS1 and dNS1 with TLR4. NS1 and dNS1 (153 to 312 amino acid region) genes were cloned, expressed and protein was purified followed by refolding. Docking studies showed the binding of NS1 and dNS1 with the TLR4 receptor which suggests that N and C-terminal sequences of NS1 are not critical for receptor binding. Antibodies against NS1 and dNS1 were raised in rabbits and binding affinity of anti-dNS1 anti-NS1 sera was evaluated against both NS1 and dNS1. Similar results were observed through western blotting which highlight that N and C-terminal deletion of NS1 does not compromise the immunogenic potential of dNS1 hence, supports its use in future vaccine formulations as a substitute for NS1.
Similar content being viewed by others
Introduction
Dengue virus (DENV) is the most common mosquito-borne flavivirus. It has approximately 11 kb long ssRNA genome, four serotypes (DENV1–4), and has wide range of clinical implications: from mild fever to life-threatening conditions, the latter includes thrombocytopenia and vascular leakage with or without the development of hemorrhage1,2. The clinical symptoms associated with severe dengue are attributed to the pathological response of B and T immune cells i.e., antibody-dependent enhancement (ADE) and antigenic-sin respectively3. According to WHO, 390 million cases of DENV infection are reported annually and 9.6 million of them require hospitalization1, and countries from tropical and sub-tropical areas are facing seasonal dengue epidemics since the last decade. To overcome people's sufferings and global economic loss, there is a dire need for an effective DENV medication. Although scientists have developed various vaccines and therapeutic formulations against dengue, but none of them have received global acceptance. Dengvaxia, the only commercial vaccine against dengue, is reported to be effective only in primary infected dengue patients with 9 to 16 years of age. A newly developed vaccine, Q-DENGA, has recently been approved in Brazil to vaccinate healthy individuals but the follow-up studies are still required to measure the efficiency of this vaccine. Meanwhile, continuous efforts are being made for the identification of better vaccine candidates.
DENV has ten proteins; three of them are structural proteins i.e., envelope, capsid, and membrane protein, whereas seven are non-structural proteins i.e., NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). To develop an effective vaccine formulation, different research groups are targeting a different set of proteins, based on their role in viral replication and pathogenesis4. NS1 is widely used in DENV diagnosis and for the development of drug and vaccine against dengue5. It is approximately 45 kDa long, multi-functional glycoprotein that has an indispensable role in virus replication, pathogenesis, and immune evasion4. It expresses as a monomer and attains dimeric configuration due to the attachment of high mannose oligosaccharide in the ER lumen. From Golgi apparatus, the protein has three destinations; it may involve in virus replication or is transferred to cell surface where the protein could reside in membrane association in a dimeric form, moreover, three NS1 dimers upon homo-oligomerization can attain a hexameric (310 kDa) conformation and thus secreted outside the cell4,6. There are two conserved glycosylation sites in NS1 i.e., N-130 and N-207. Studies have revealed that complex glycans attached at N‐130 are involved in the secretion and stability of NS1 hexameric structure, whereas high mannose glycans tethered at N‐207 are associated with secretion and stability of NS1 in dimeric form7. Using mannose glycans, NS1 binds with lectin and evades immune complementation as well as prohibits DENV neutralization via lectin pathway8.
Among the non-structural proteins, only NS1 is secreted outside the cell. It is secreted into the blood plasma before the production of antibodies against the virus, which makes it an ideal marker for early diagnosis of dengue9,10. Higher levels of NS1 in a patient’s sera are directly related to disease severity, it destabilizes cell junctions by interacting with thrombin protein and stimulates glycocalyx degradation, followed by tissue-specific endothelial hyper-permeability and vascular leakage11,12. NS1 is reported to activate macrophages, platelets, and mononuclear peripheral cells via binding to Toll-like receptors 4 (TLR4)13. As a result of immune cell activation, a cascade of pro-inflammatory cytokines is released that destabilize the cellular tight junctions14. Furthermore, the interaction of NS1 with the host protein (fibronectin), inhibits membrane attack complex formation and C9 polymerization during the complement activation pathway, hence evading the immune response and favoring viral replication15.
Although NS1 contributes to inflammation, vascular leakage, and hemorrhage, recovery of mice from lethal DENV challenge has been reported when anti-NS1 antibodies were administrated16,17. Moreover, the pathogenic role of NS1 is strain-dependent and is dispensable in highly virulent strain18. Anti-NS1 antibodies play a dual role, either protective or pathogenic, in the host. On one hand, cross-reactivity of anti-NS1 with epithelial cells causes impaired biological functions and tissue damage with symptoms like hemorrhage, coagulopathy, plasma leakage, and vascular permeability19. On the other hand, vaccine formulations based on NS1 protein, are reported to generate protective antibodies which activate Fc-mediated immune functions i.e., antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cell lysis, and phagocytosis20,21. Anti-NS1 antibodies also inhibit the interaction between NS1 and cell membrane and prevent NS1-mediated vascular leakage16. Furthermore, NS1 is the most conserved protein not only among DENV serotypes but also in other flaviviruses (i.e., Zika, West Nile, etc.), and flavivirus cross-reactive NS1 antibodies offer protection against dengue, Zika, and West Nile virus in murine models22. Overall, high secretion levels, high sequence conservancy, high immunogenicity, and low risk of inducing antibody-dependent enhancement (ADE) endorse the NS1-based vaccine formulations23,24. Variants of NS1 could be developed to classify the protective and pathogenic NS1 epitopes, which allows the prevention of pathological effects of NS1 in future vaccine formulations.
In this study, by using bioinformatics tools, we have selected regions from DENV NS1 protein containing conserved epitopes to achieve balanced immunity against four serotypes. Conserved epitopes that are reported to produce cross-reactive antibodies against cellular machinery are excluded and finally the resultant N- and C-terminal deleted variant of NS1 (dNS1) is experimentally validated for its immunogenic potential, to replace NS1 in future vaccine formulations.
Methodology
Ethical statement
The study was approved by the ethical committee of faculty of life sciences, university of the Punjab. All methods were carried out in accordance with relevant guidelines and regulations. The study complied with ARRIVE guidelines. To take the control bleed, commercial preparation of lignocaine-prilocaine cream (EMLA cream, Astra Pharmaceuticals) was applied to desensitize the skin followed by the venipuncture of the rabbit marginal ear vein. For deep anaesthesia, 35 mg of ketamine HCl and 7 mg of xylazine was injected per kg bodyweight of rabbit, followed by extraction of maximum bleed through cardiac puncture25. Various computational tools and softwares (i.e., T-cell and B-cell epitope prediction tools for T and B-cell prediction, Phyre2 server for protein tertiary structure prediction, Chimera 1.16 for protein visualization, PATCHDOCK for molecular docking, and Clustal Omega for multiple sequence alignment) were used to design deleted NS1 (dNS1) from full-length non-structural protein 1 (NS1) of the dengue virus.
dNS1 synthesis
For the synthesis of dNS1, the first step was to analyze the sequences of full-length (351 amino acid residues) NS1 protein from each DENV serotype. This was done by downloading the NS1 sequences from DENV polyproteins with their accession number available at NCBI i.e., ACF49259.1:776-112726, NP_056776.2:776-112727,28, YP_001621843.1:774-112529,30, and NP_073286.1:775-112630,31 for DENV serotypes 1, 2, 3 and 4 respectively, and aligning them through multiple sequence alignment (MSA) using clustal omega. dNS1 was synthesized by targeting the regions, containing relatively conserved residues followed by the identification of B-cell, CTL (cytotoxic T-lymphocyte), and HTL (helper T-lymphocyte) epitopes from the conserved regions, which were predicted through BCPred, NetCTL 1.2 server, and IEDB server respectively. Further processing of T-cell epitopes was also predicted through proteasomal cleavage/TAP transport/MHC class I combined predictor, available on IEDB server. The regions of NS1 containing maximum antigenic sites with relatively conserved amino acid residues among all serotypes was found to be in the middle of the NS1 protein, therefore N and C-terminal regions of NS1 were deleted and the resultant protein was named dNS1.
Tertiary structure prediction
The designed sequence of NS1 and dNS1 were submitted to Phyre2 server for the prediction of protein tertiary structure. The results from the server were visualized using Chimera 1.16 and the tertiary structure of both proteins was compared to determine the structural differences between the two proteins.
Docking of NS1 and dNS1 with TLR4 receptor
The receptor binding ability of a protein is a prerequisite for its functioning as a vaccine candidate. Therefore, docking of NS1 and dNS1 against the TLR4 receptor (PDB ID: 2Z62) was performed32,33 to analyze their relative receptor binding ability. TLR4 receptor belongs to the pattern recognition receptor family and is primarily involved in the activation of inflammatory cytokine in response to various ligands. These ligands can be bacterial lipopolysaccharide, polysaccharides, or viral proteins as well34. DENV NS1 is reported to have an affinity with the TLR4 receptor35. Therefore, we performed docking of both NS1 and dNS1 against TLR4, to observe whether the deletion of N and C terminal residues from NS1 affect its interaction with the TLR4 receptor. The PDB structure of the TLR4 receptor was retrieved through the PDB databank and water crystals were removed using UCSF chimera software. PATCHDOCK server was used for molecular docking of TLR4 with both NS1 and dNS136.
Cloning and expression of NS1 and dNS1
After initial screening of dNS1 through in-silico approaches, specific primers were designed to amplify dNS1 (containing NS1 sequence from 153 to 312 amino acids). A full-length NS1 (sequence ID: ADM63678.1:776-1128 polyprotein) provided by School of Biological Sciences (SBS, PU, LHR), was used for dNS1 synthesis. Primers for both genes were designed such that NdeI and HindIII sites would be present at the 5′ and 3′ end of the genes respectively, followed by their ligation into pTZ57R/T and transformation in E. coli DH5α cells. After confirming the gene sequences through Sanger sequencing, the gene was excised and ligated in expression vector (pET 28a) followed by their expression analysis in BL codon plus cells. For expression analysis, pET28a, NS1-pET28a, and dNS1-pET28a were transformed in the expression host separately and IPTG (10 µM) was used for induction when cell density was reached near 0.6 OD600nm. Protein expression was analyzed through 15% SDS-PADE, and inclusion bodies were solubilized in Tris–HCl buffer containing 8 M urea and proceeded for refolding. All chemicals used in this study were of analytical grade and were purchased from Sigma Aldrich, however, low temperature chemicals including enzymes, DTT, and IPTG were purchased from Thermo Fisher Scientific.
Refolding of dNS1 and NS1
Only refolded protein in soluble form can ensure the presence of epitopes on the surface to interact with immune machinery and elicit an efficient immune response. Both proteins exhibit different refolding methods. For dNS1, our lab-optimized protocol for refolding cysteine-containing protein was followed37. However, NS1 was not refolded through this method and got precipitated. So for NS1 refolding, the protein was diluted up to 0.4 mg/ml followed by gradual removal of urea through dialysis. Both NS1 and dNS1 proteins were concentrated and analyzed on 12% and 15% SDS PAGE respectively. For SDS PAGE analysis of refolded proteins, samples were prepared using two different methods; reductive samples contain SDS and DTT in their loading dye and were boiled for 5 min, however, non-reductive samples did not contain SDS and DTT in their loading dye and were not heated.
Antibody raising against NS1 and dNS1 in rabbits
For polyclonal antisera production in rabbits, institutional animal experimentation guidelines approved by SBS IRB committee (SBS/431/19) and EU Directive 2010/63/EU of animal experiments were followed. Rabbits (6 months old; 1–1.5 kg in weight) were acclimatized in SBS husbandry and control bleed was taken from the each rabbit, before the experiment, to nullify the occurrence of any false-positive results during the experiment. Polyclonal anti-sera were raised against NS1 and dNS1 by following the guidelines on antibody production by the Canadian council on animal care38. Briefly, 200 µg of each antigen i.e., NS1 and dNS1 was separately mixed with Freund’s adjuvant, to prepare the zero dose. The dose was subcutaneously injected at the dorsal side of the rabbit (one rabbit for each antigen) on multiple sites to avoid lesion formation on one site. Two booster doses (100 µg of respective antigen with incomplete Freund’s adjuvant) were injected in rabbits after four and seven weeks of the zero dose, respectively. Final bleed was taken through the cardiac puncture technique after 10 days of the second booster. Serum was isolated by centrifuging the blood at 5000 rpm for 15 min for further analysis.
ELISA and western blotting for NS1 and dNS1
To compare the antibody-raising potential of NS1 and dNS1 in rabbits, ELISA and western blotting were performed. For ELISA, NS1 and dNS1 (200 ng each) were coated on ELISA plates and incubated at 4 °C for overnight. The layout of the ELISA plate is shown in Table A (Supplementary data) and serum dilution (1:500) was added accordingly followed by the addition of. 1:10,000 dilution of HRP conjugated goat anti-rabbit IgG (Invitrogen). Serum isolated from the control bleed was used as a control for this study. Finally, TMB substrate (thermoscientific) was added to each well to develop blue color followed by the addition of 2 M H2SO4 used to stop the reaction. The differential absorbance at 450/630 nm was measured by an ELISA reader. The ELISA experiment was performed in triplicate, and a two-way t-test was conducted to analyze the reliability of the data. The average of all replicates was calculated, followed by the determination of the standard deviation among replicates to incorporate error bars on the plotted data. Statistical analysis was conducted using Microsoft Excel software.
For western blotting, NS1 and dNS1 were run on 12% and 15% SDS PAGE gel respectively. Gels were transferred on nitrocellulose membrane, blocked with 5% skim milk, and incubated the transferred proteins with their respective as well as cross-reactive serum dilution (1:1250) followed by the addition of HRP-conjugated goat anti-rabbit IgG (Invitrogen) as secondary. Finally, the blots were developed using a TMB substrate.
Results
Prediction of conserved epitopes in NS1 protein among all dengue serotypes
To design the dNS1 (deleted NS1) peptide which could have the potential to replace NS1 in vaccine formulations, the region in DENV NS1 protein that carries maximum conserved epitopes, among all four serotypes was targeted (Fig. A, Supplementary data). The immunogenic potential of NS1 epitopes in terms of eliciting B cell, CTL, and HTL responses were predicted using bioinformatic tools. Epitopes that can elicit each aforementioned response are enlisted separately in Tables 1, 2, and 3 respectively. The position of starting amino acid residue of each selected epitope in respective serotypes is also mentioned. Moreover, the conserved amino acid residues in all DENV serotypes are highlighted with bold letters, and their percentage homology is also enlisted.
The binding of dNS1 with MHC I and MHC II alleles was also predicted by selecting netmhciipan_el 4.1 as prediction method and using reference HLA allele set (Supplementary data; Fig. B,C).
dNS1 synthesis
NS1 is a 349 amino acid long protein. Based on the conserved immunogenic epitopes, 153 to 312 amino acid residues were selected for dNS1 synthesis. Although the epitope starting from 325 is also conserved and predicted to be a potential B-cell epitope (Table 1), it was not included in our designed peptide as antibodies produced against this region are cross-reactive to human blood clotting antigens such as fibrinogen, thrombocytes, platelets, and endothelial cells19, and binding of these antibodies to any of their target is reported to produce hemorrhage in a mouse model39. Similarly, epitope starting from position 105 is also conserved and predicted to be potential B-cell epitopes, but excluded from the dNS1 sequence because it shares sequence similarity with human LYRIC protein, and binding of antibodies to this protein induces endothelial cell damage40. So after thorough consideration, 153–312 amino acid residues from full-length NS1 was selected for dNS1 synthesis. Moreover, the proteasomal cleavage and TAP transport predictor suggested that the selected epitopes have the intrinsic potential to be a T-cell epitopes.
In this study, we used DENV III-NS1 (sequence ID: ADM63678.1) as a template to synthesize dNS1 as it shares 86% sequence similarity (from 153 to 312 amino acid residues) of NS1 of other serotypes. The amino acid sequence of NS1 is shown in Fig. 1, and dNS1 sequence is highlighted with yellow color. The underlined and bold highlighted C (C) in the NS1 sequence is representing the cysteine residues (Fig. 1). NS1 contains twelve cysteine residues whereas dNS1 contains only six. The deletion of six cysteine residues in dNS1 impair the stability of NS1 dimer due to loss of disulfide bridges41.
Tertiary structure analysis of NS1 and dNS1
To make a better comparison between NS1 and dNS1, the tertiary structures of both proteins were predicted through the Phyre2 server and compared (Fig. 2). As the 3D structure of NS1 is already available (PDB ID: 4o6b), the confidence value of the dNS1 predicted structure provided by the server is high. In Fig. 2, α-helices are represented with red color, β-sheets are shown in cyan blue color, whereas coils are colored with white. NS1 contains 6 α-helices, 13 β-sheets, and coils in its structure. dNS1, on the other hand, is a smaller molecule with 2 α-helix, 9 β-sheets, and coils in its structure. The results suggest that dNS1 comes with the deletion of 3 α-helix and 4 β-sheets from the N-terminal whereas, only one α-helix from the C-terminal of NS1 is deleted. Moreover, cysteine residues in both structures are colored magenta. In the light of the 3-dimensional structure of NS1, favorable disulfide bridges are predicted to be form between C23/C34, C74/C162, C198/C242, C299/C350, C331/C346, and C310/C332, however in the case of dNS1, only two disulfide bridges are predicted to be form between C27/C71, and C139/C161. The pattern of predicted disulfide bridges pattern is in line with the findings of Wallis and his group42. The reduction of disulfide bridges in NS1-β ladder ___domain is associated with destabilization of NS1 dimeric structure41,43. Therefore, chances of dNS1 to form dimers are extremely low. Moreover, N-130 which is an important site for N-glycosylation and is responsible for the stability of the hexameric NS1 is missing in dNS1, which reflects the inability of dNS1 to form secretory hexameric structure. Although glycosylation at N-130 is not possible in prokaryotic expression system where post-translational modification do not occur, it is insightful if yeast or mammalian expression is to be used. The soluble tetrameric/hexameric conformation of NS1 is majorly associated with vascular leakage and severe dengue44,45, inability of dNS1 to form the oligomeric structure supports its safer profile in vaccine formulation.
Docking studies of NS1 and dNS1 with TLR4
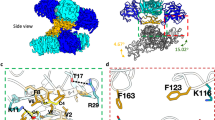
The in-silico designed dNS1 was further validated for its vaccine potential by performing its docking with TLR 4 receptor. As NS1 is reported to activate inflammatory cytokine response in response to its binding with TLR4 receptor, we docked dNS1 with TLR4 receptor to see whether the C and N-terminal deletion have any effect on its receptor binding efficiency. Although NS1 never exists in the monomeric form and dNS1 is unable to form oligomeric structures (as described in section “Tertiary structure analysis of NS1 and dNS1”), therefore to compare the differences in their docking patterns both NS1 and dNS1 were separately docked, in their monomeric form, with TLR4 receptor using the PATCHDOCK server (Fig. 3). Images of the docked complexes at 90° rotations were captured and compared. TLR4 is shown in yellow colored solid surface whereas NS1 and dNS1 proteins are represented with dark-colored licorice sticks (Fig. 3). Both NS1 and dNS1 tend to bind with the TLR4 receptor which suggests that N and C-terminal sequences of NS1 does not have a crucial role in receptor binding. However, different binding patterns of NS1 and dNS1 with TLR4 are observed (Fig. 3). Further studies to evaluate whether the activation of TLR4 receptor in response to dNS1 (unable to form oligomeric structures) induces pathological response is required.
Docking of NS1 and dNS1 with TLR4 receptor using PATCHDOCK server: Left view of docked complexes, NS1.TLR4 (left), dNS1.TLR4 (right) (A); Front view of docked complexes, NS1.TLR4 (left), dNS1.TLR4 (right) (B); Right view of docked complexes, NS1.TLR4 (left), dNS1.TLR4 (right) (C); Back view of docked complexes, NS1.TLR4 (left), dNS1.TLR4 (right) (D).
Cloning and expression of NS1 and dNS1
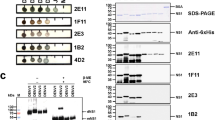
After the in-silico confirmation of the immunogenic potential of dNS1 and its intact receptor binding ability, NS1 and dNS1 were amplified, by using NS1 from DENV-III as a template. 1% agarose gel confirmed the successful amplification of both genes. A band near 500 bp of DNA ladder was found in the case of dNS1 whereas a band for NS1 was found near 1 kb as shown in Fig. 4A. After the validation of gene sequences, expression of both genes was observed in BL codon plus cells through 15% SDS PAGE as shown in Fig. 4B. Cells with pET-28a vector only, without any gene was taken as control for expression analysis. dNS1 gene expression was observed at approx. 20 kDa, mainly in the induced sample, a band of similar size was also observed in the un-induced sample as well, however the band intensity suggested relatively low leaky expression. Similarly, NS1 gene expression was observed near 45 kDa in both the induced sample and the un-induced sample with similar band intensity. Leaky expression in both cases is attributed to weak regulation of the lac operator46.
Cloning and expression of NS1 and dNS1, 1% agarose gel results (A); 500 bp fragment observed after dNS1 gene amplification (left, A), 1 kb fragment observed after NS1 gene amplification (right, A), 15% SDS PAGE results (B); L protein ladder, pET-28a, NS1, dNS1. (U un-induce fraction, I induced fraction). Original gels are provided in Supplementary data Fig. D.
Refolding of NS1 and dNS1
After the successful expression of both genes, the localization of both proteins was observed using 15% SDS PAGE (Fig. 5A). The results suggested that both NS1 and dNS1 express in the form of inclusion bodies, as bands of approx. 45 kDa and 20 kDa were observed either in the pellet fraction or the whole-cell lysate fraction and supernatant fraction did not contain any protein band at the corresponding position. In-vitro refolding of both dNS1 and NS1 proteins are shown in Fig. 5B,C respectively. Fast moving, intact dNS1 protein band in the non-reductive sample, with intensity similar to the reductive sample affirms the proper refolding of dNS1 (Fig. 5B). Similarly, the presence of the NS1 protein band under non-reducing conditions confirms the refolding of NS1 (Fig. 5C). Absence of NS1 protein bands corresponding to dimeric and hexameric conformations under non-reducing conditions is attributed to the lack of post-translational modification (glycosylation) of protein in prokaryotic expression system.
15% SDS PAGE analysis for NS1 and dNS1 localization; WCL whole cell lysate, S proteins in the supernatant fraction, P proteins in the pellet fraction (A), 15% SDS PAGE analysis for dNS1 after refolding; R reductive, NR non-reductive (B), 12% SDS PAGE analysis for NS1 after refolding; R reductive. Reductive samples were heated under reducing condition whereas, non-reductive samples were neither heated nor treated with reducing agent (C). Original gels are provided in Supplementary data Fig. E.
ELISA
After confirming the refolding of NS1 and dNS1 proteins, their immunogenic potential was compared by raising antibodies against them in rabbits. Comparison between the antibody raising potential between the NS1 and dNS1 was evaluated through ELISA as shown in Fig. 6. The administration of only Freund’s adjuvant in rabbits without the antigen do not give significant readings in ELISA, as previously reported by our lab group47. Therefore, the data for this control group is not included in this study. In this experiment, on one hand, the antibody-raising potential of individual proteins by treating them with their respective anti-sera is evaluated. While on the other hand, we have evaluated the cross-reactive affinity of anti-NS1 sera and anti-dNS1 sera with dNS1 and NS1 respectively. As equal amount of antigen is coated on the ELISA plate and same dilution of anti-sera is used for both antigens during the assay, the results showed that both proteins have maximum affinity with their corresponding anti-sera, whereas antibodies raising the potential of dNS1 is greater than NS1. Moreover, the cross-reactive affinity assay revealed that the binding efficiency of anti-NS1 sera with dNS1 is 73% whereas, the binding efficiency of anti-NS1 sera with dNS1 is 80%. The results reflect that N and C-terminal deletion from NS1, does not have a significant effect on antibody production in rabbits.
Antibody raising potential of NS1 and dNS1 through ELISA: Control sera contains sera from rabbits before injecting the zero dose to nullify false positive results; control sera when applied to NS1 coated ELISA well (green bar) and to dNS1 coated ELISA well (blue bar), anti-NS1 sera (raised in rabbits) when applied to NS1 coated ELISA well (green bar) and to dNS1 coated ELISA well (blue bar), anti-dNS1 sera (raised in rabbits) when applied to NS1 coated ELISA well (green bar) and to dNS1 coated ELISA well (blue bar). Among replicates p values > 0.05 were observed, which reflects the reproducibility of data, whereas among different groups (i.e., control, NS1, and dNS1) p values < 0.05 were observed which infers that findings of each group are statistically different from each other.
Western blotting
To further validate the ELISA results, western blotting of NS1 and dNS1 was performed against anti-NS1 and anti-dNS1 sera raised in rabbits (Fig. 7). Similar to ELISA experiments, two different blots were produced. Firstly, both NS1 and dNS1 were detected on the nitrocellulose membrane after treating them with anti-dNS1 sera (Fig. 7A). Secondly, both NS1 and dNS1 were detected on the nitrocellulose membrane through their treatment with anti-NS1 sera (Fig. 7B). The results correlate with ELISA findings and showed the significant antibody-raising potential of NS1 and dNS1. Moreover, the cross-reactive affinity of both proteins was also confirmed through blots. dNS1 band is appeared around 20 kDa when treated with either anti-NS1 sera or anti-dNS1 sera, and the band of NS1 appeared at around 45 kDa when treated with either anti-NS1 or anti-dNS1 did not show any significant difference. Overall, these findings support that N and C-terminal deletion of NS1 does not compromise the antibody-raising potential in rabbits.
Antibody raising potential of NS1 and dNS1 through western blotting; dNS1 lane; dNS1 treated with anti-dNS1 serum, NS1 lane; NS1 treated with anti-dNS1 serum (A), NS1 lane; NS1 treated with anti-NS1 serum, dNS1 lane; NS1 treated with anti-NS1 serum (B). Original blots are provided in Supplementary data Fig. F.
Discussion
NS1 is an immune-reactive protein that interacts with both humoral and cellular immune machinery. Its immune-stimulating ability without inducing ADE response makes it a choice of target in various vaccine formulations. However, there are several concerns associated with the use of NS1 in vaccine formulations including the cross-reactivity of anti-NS1 with host proteins leading to the onset of severe dengue (DHF and DSS). In this study, we have focused to overcome these pathological responses by deleting the less conserved, non-immunogenic, and cross-reactive sequences from the NS1 protein. The resultant protein (dNS1) is synthesized by deleting 152 residues from the N-terminal, and 37 residues from the C-terminal, of the NS1 protein. The comparison between NS1 and dNS1 is made to determine the potential of dNS1 to replace NS1 in dengue vaccine formulations. Our results suggest that dNS1 is a relatively conserved, immunogenic molecule and with the removal of N and C-terminal pathological sequences from full-length NS1, it can replace NS1 in future vaccine formulations.
Sequence selection, for dNS1 synthesis, was done by taking the reported literature and results of in-silico experiments into consideration. Sequence conservancy among dengue serotypes ensures balanced immunity. Although consensus sequence was not obtained for each serotype, NS1 is the most conserved protein among DENV virus, and its sequence similarity within a serotype is > 95%. Therefore only one sequence from each serotype is used for conservational analysis, in this study. Although the risk of ADE is low in the case of NS1, for the activation of a balanced immune response against all serotypes, homology among selected sequences is still required. Therefore, we have targeted the region containing relatively conserved epitopes (Tables 1, 2, 3). After getting relatively conserved regions (Fig. A; Supplementary data), sequences that are reported to be non-immunogenic, or responsible for pathogenic effects are omitted. 3D structural studies of NS1 have revealed its three distinct domains; β-roll (1–29), wing ___domain (30–180), and β-___domain (181–352)48. The β-roll ___domain is hydrophobic and assists the protein to interact with membranous structures49. Removal of this sequence from NS1 would compromise its ability to interact with endothelial cells and severe symptoms like vascular leakage could be prevented, without any significant effect on its immunogenic potential. Similarly, epitopes that lead to cross-reactive antibodies are reported to present at the N and C-terminal of NS1 protein39,40, therefore these sequences were deleted to synthesize dNS1 (Fig. 1). In contrast to NS1, where 12 cysteine residues form 6 disulfide bridges, dNS1 contain 6 cysteine residues and only four of them have the ability to form 2 disulfide bridges (Fig. 1; cysteines residues are denoted with C). Out of six, four disulfide bridges in NS1 form between C1/C2, C3/C4, C5/C6, and C7/C12 as revealed by the comparison of the peptide masses before and after reduction and by post-source decay analysis, whereas the remaining two disulfide linkages C8/C10 and C9/C11 were determined by tandem mass spectrometry42. In dNS1 C1, C2, C3, C4, C11, and C12 are deleted, and only C5/C6 and C8/C10 can form the two disulfide bridges (Fig. 3). The reduction in disulfide bridges allosterically destabilize the dimer formation in NS141, therefore the ability of dNS1 to form dimers is significantly compromised. As a result, the formation of other oligomeric structures i.e., tetrameric and hexameric structures is also effected. The soluble tetrameric/hexameric conformation of NS1 is majorly associated with vascular leakage and severe dengue44, therefore, the inability of dNS1 to form the oligomeric structure supports its safer profile in vaccine formulation.
The receptor binding ability of synthesized dNS1 and NS1 are compared using molecular docking. Both NS1 and dNS1 proteins have successfully docked against the TLR4 receptor, however, their binding pattern is different (Fig. 3). Although NS1 binding to TLR4 receptor is subjective to its hexameric conformation, monomeric NS1 is predicted to bind at the surface of the TLR4 receptor and a small region from the protein is interacting with the receptor. However, dNS1 covers the groove of the receptor and the whole molecule is predicted to interact with the receptor. As NS1 is reported to induce cytokine response leading to vascular leakage through its binding with TLR450, further in-vivo studies are required to make this claim true for dNS1.
After the in-silico studies, dNS1 and NS1 were cloned and expressed using bacterial cells (Fig. 4). Both proteins were refolded and antibodies against these refolded proteins were raised in rabbits. ELISA and western blotting were performed to evaluate the antibody-raising potential of both proteins (Figs. 6, 7). Results from ELISA experiments revealed that antibody titer for dNS1 is three times greater than NS1. The NS1-based vaccine is reported to show weak antibodies in cell culture experiments51. Low antibody titers in the case of NS1 might be due to its large size (40 kDa), which could have masked some of its epitopes as compared to the small-sized dNS1 (20 kDa). Moreover, the detection efficiency of anti-dNS1 serum to detect both NS1 and dNS1 is better than that of anti-NS1 serum.
Conclusion
Although many factors such as in-lab validation of the pathological effects of dNS1 in response to its binding with TLR4 and its cross-reactivity with host machinery are still needed to be addressed. The results from this study revealed that that N and C-terminal deletions from the full-length NS1 protein do not attenuate immunogenic potential of dNS1. This supports the utilization of dNS1 in future vaccine formulations.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
Kyle, J. L. & Harris, E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 62, 71–92 (2008).
World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control (World Health Organization, 1997).
Nasar, S., Rashid, N. & Iftikhar, S. Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: A review. J. Med. Virol. 92, 941–955 (2020).
Beatty, P. R. et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 7, 304ra141 (2015).
Flamand, M. et al. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 73, 6104–6110 (1999).
Somnuke, P., Hauhart, R. E., Atkinson, J. P., Diamond, M. S. & Avirutnan, P. N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement. Virology 413, 253–264 (2011).
Thiemmeca, S. et al. Secreted NS1 protects dengue virus from mannose-binding lectin-mediated neutralization. J. Immunol. 197, 4053–4065 (2016).
Kassim, F. M., Izati, M. N., TgRogayah, T., Apandi, Y. M. & Saat, Z. Use of dengue NS1 antigen for early diagnosis of dengue virus infection. Southeast Asian J. Trop. Med. Public Health 42, 562–569 (2011).
Datta, S. & Wattal, C. Dengue NS1 antigen detection: A useful tool in early diagnosis of dengue virus infection. Indian J. Med. Microbiol. 28, 107 (2010).
Puerta-Guardo, H., Glasner, D. R. & Harris, E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 12, e1005738 (2016).
Puerta-Guardo, H. et al. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 26, 1598-1613.e8 (2019).
Modhiran, N. et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 7, 304ra142 (2015).
Puerta-Guardo, H. et al. The cytokine response of U937-derived macrophages infected through antibody-dependent enhancement of dengue virus disrupts cell apical-junction complexes and increases vascular permeability. J. Virol. 87, 7486–7501 (2013).
Conde, J. N. et al. Inhibition of the membrane attack complex by dengue virus NS1 through interaction with vitronectin and terminal complement proteins. J. Virol. 90, 9570–9581 (2016).
Chu, Y.-T. et al. Antibodies against nonstructural protein 1 protect mice from dengue virus-induced mast cell activation. Lab. Investig. 97, 602–614 (2017).
Henchal, E., Henchal, L. & Schlesinger, J. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 69, 2101–2107 (1988).
Lee, P. X. et al. Relative contribution of nonstructural protein 1 in dengue pathogenesis. J. Exp. Med. 217, e20191548 (2020).
Falconar, A. The dengue virus nonstructural-1 protein (NS1) generatesantibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to humanendothelial cells: Potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 142, 897–916 (1997).
Chung, K. M., Thompson, B. S., Fremont, D. H. & Diamond, M. S. Antibody recognition of cell surface-associated NS1 triggers Fc-γ receptor-mediated phagocytosis and clearance of West Nile virus-infected cells. J. Virol. 81, 9551–9555 (2007).
Biering, S. B. et al. Structural basis for antibody inhibition of flavivirus NS1-triggered endothelial dysfunction. Science 371, 194–200 (2021).
Modhiran, N. et al. A broadly protective antibody that targets the flavivirus NS1 protein. Science 371, 190–194 (2021).
Alcalá, A. C. et al. The dengue virus non-structural protein 1 (NS1) is secreted efficiently from infected mosquito cells. Virology 488, 278–287 (2016).
Alcon, S. et al. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40, 376–381 (2002).
Suckow, M. A., Schroeder, V. & Douglas, F. A. The Laboratory Rabbit (CRC Press, 2010).
Qi, H. et al. Rapid production of virus protein microarray using protein microarray fabrication through gene synthesis (PAGES). Mol. Cell. Proteom. 16, 288–299 (2017).
Kinney, R. M. et al. Construction of infectious cDNA clones for dengue 2 virus: Strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 230, 300–308 (1997).
Sabetian, S., Nezafat, N., Dorosti, H., Zarei, M. & Ghasemi, Y. Exploring dengue proteome to design an effective epitope-based vaccine against dengue virus. J. Biomol. Struct. Dyn. 37, 2546–2563 (2019).
Peyrefitte, C. N. et al. Genetic characterization of newly reintroduced dengue virus type 3 in Martinique (French West Indies). J. Clin. Microbiol. 41, 5195–5198 (2003).
Hoque, H. et al. Implementation of in silico methods to predict common epitopes for vaccine development against Chikungunya and Mayaro viruses. Heliyon 7, e06396 (2021).
Whitehead, S. S. et al. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 77, 1653–1657 (2003).
Abdi, S. A. H., Ali, A., Sayed, S. F., Ali, A. & Alam, P. Multi-epitope-based vaccine candidate for monkeypox: An in silico approach. Vaccines 10, 1564 (2022).
Enayatkhani, M. et al. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: An in silico study. J. Biomol. Struct. Dyn. 39, 2857–2872 (2021).
Vaure, C. & Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014(5), 316 (2014).
Modhiran, N. et al. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol. Cell Biol. 95, 491–495 (2017).
Schneidman-Duhovny, D., Inbar, Y., Nussinov, R. & Wolfson, H. J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 33, W363–W367 (2005).
Ahsan, F., Arif, A., Mahmood, N., Rashid, N. & Akhtar, M. Characterization and bioassay of post-translationally modified interferon α-2b expressed in Escherichia coli. J. Biotechnol. 184, 11–16 (2014).
Canadian Council on Animal Care. Guidelines on Antibody Production (Canadian Council on Animal Care, 2002).
Chuang, Y.-C., Lin, J., Lin, Y.-S., Wang, S. & Yeh, T.-M. Dengue virus nonstructural protein 1-induced antibodies cross-react with human plasminogen and enhance its activation. J. Immunol. 196, 1218–1226 (2016).
Lin, Y.-S. et al. Molecular mimicry between virus and host and its implications for dengue disease pathogenesis. Exp. Biol. Med. 236, 515–523 (2011).
Roy, P., Roy, S. & Sengupta, N. Disulfide reduction allosterically destabilizes the β-ladder subdomain assembly within the NS1 dimer of ZIKV. Biophys. J. 119, 1525–1537 (2020).
Wallis, T. P., Huang, C.-Y., Nimkar, S. B., Young, P. R. & Gorman, J. J. Determination of the disulfide bond arrangement of dengue virus NS1 protein. J. Biol. Chem. 279, 20729–20741 (2004).
Pryor, M. J. & Wright, P. J. The effects of site-directed mutagenesis on the dimerization and secretion of the NS1 protein specified by dengue virus. Virology 194, 769–780 (1993).
Shu, B. et al. CryoEM structures of the multimeric secreted NS1, a major factor for dengue hemorrhagic fever. Nat. Commun. 13, 6756 (2022).
Quirino-Teixeira, A. C. et al. Inflammatory signaling in dengue-infected platelets requires translation and secretion of nonstructural protein 1. Blood Adv. 4, 2018–2031 (2020).
Nielsen, B. L., Willis, V. C. & Lin, C. Y. Western blot analysis to illustrate relative control levels of the lac and ara promoters in Escherichia coli. Biochem. Mol. Biol. Educ. 35, 133–137 (2007).
Hassan, N. Studies on Genetically Engineered Precursors of Human Insulin and their Derivatives (University of the Punjab, 2019).
Akey, D. L., Brown, W. C., Jose, J., Kuhn, R. J. & Smith, J. L. Structure-guided insights on the role of NS1 in flavivirus infection. Bioessays 37, 489–494 (2015).
Scaturro, P., Cortese, M., Chatel-Chaix, L., Fischl, W. & Bartenschlager, R. Dengue virus non-structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog. 11, e1005277 (2015).
Chao, C.-H. et al. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 15, e1007625 (2019).
Wu, S.-F. et al. Evaluation of protective efficacy and immune mechanisms of using a non-structural protein NS1 in DNA vaccine against dengue 2 virus in mice. Vaccine 21, 3919–3929 (2003).
Funding
The study was funded by High Education Commission, Pakistan under NRPU project #7136.
Author information
Authors and Affiliations
Contributions
Sitara Nasar has conducted the laboratory experiments and written the manuscript. Saima Iftikhar has designed the research, supervised and reviewed the manuscript critically. Rida Saleem was also involved in carrying lab experiments, Muhammad Shahid Nadeem helped in bioinformatics studies and Muhammad Ali was involved in raising antibodies in rabbits.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nasar, S., Iftikhar, S., Saleem, R. et al. The N and C-terminal deleted variant of the dengue virus NS1 protein is a potential candidate for dengue vaccine development. Sci Rep 14, 18883 (2024). https://doi.org/10.1038/s41598-024-65593-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65593-1