Abstract
The negative impact of refinery wastewater is of great concern to the aquatic, terrestrial, and aerial environment. In this study, N-hexadecylchitosan (NHDC) was successfully synthesized to deal with low mechanical strength, poor adsorption capacity, and limited selectivity of native chitosan. The NHDC was characterized by fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and X-Ray diffraction analysis (XRD) to study its composition, morphology, and structural attributes. The adsorption of hydrocarbon pollutants from refinery wastewater was studied in batch mode experiments. The results indicated that the removal of COD attained by chitosan and NHDC was 21 and 63%, respectively. COD removal was found to be maximal, i.e., 96% using 0.08 g of NHDC at 60 min in a solution of pH 6.5 maintained at 60 °C. Furthermore, kinetic data revealed that the adsorption system followed pseudo-second order kinetics, whereas equilibrium studies supported both monolayer and multilayer adsorption mechanisms. The designed adsorption platform was able to capture hydrocarbon pollutants under very mild optimized conditions. Furthermore, NHDC demonstrated long term stability when subjected to five successive cycles, which contributed to the sustainability of water treatment systems. On the basis of the outcome of this work, it is advocated that new biobased NHDC can be used as an efficient adsorbent for the remediation of organic contaminants laden wastewater streams generated from oil refineries.
Similar content being viewed by others
Introduction
The oil petroleum industries consume a massive amount of water and generate an oily wastewater stream which is heavily loaded with organic contaminants, thereby posing a serious threat to the environment1. According to the oil and gas statistics report 2018, the estimated daily water consumption for energy-related purposes during the petrochemical and refining processes was 3.95 × 107 m3/day (about 15% of the global water supply)2. During various processing steps, large amounts of water supplies are needed for the crude oil extraction process and as a consequence huge volumes of effluents are generated3. The amount of effluent produced by petroleum refineries during oil processing is about 0.4–1.6 times the total of crude oil products processed. Therefore, according to the current report, more than 250 million barrels of wastewater are generated per day in gas and oil fields, and more than 40% of it is released into the environment globally4. These statistics clearly show the current state of this grave problem related to the drainage of effluent from the petrochemical industry around the world that has caused negative impact on humans, aquatic life, crop production and our environment4.
It is important to mention that the wastewater produced by the petroleum industry contains a variety of hazardous materials, including xylene, toluene, phenol, benzene, ethylbenzene, polycyclic aromatic hydrocarbons (PAHs), among others5. Refinery processes use a large amount of water, which makes them the main source of organic pollutants in wastewater6. As several species cannot survive near the effluent production source, the land around the oil refinery has a comparatively low abundance and diversity of fauna. The refinery’'s effluent included several toxic inorganic and organic compounds, such as phenols, which are extremely toxic to humans. Benzene is the most hazardous of all petrochemical pollutants that is known to cause liver cancer, fibrosis, necrosis, edoema, haemorrhage, and bone marrow damage7. Phenolic compounds harm the liver and lungs and cause infections in the vascular system in animals, even in low concentrations, these compounds can be extremely harmful. Moreover, xylene can cause memory loss, insomnia, and fatigue in humans8. Refinery wastewater discharged into water bodies is toxic to aquatic life because it comprises oil and grease, while the oil layer formed on the surface of the water reduces light penetration and thus reduces the activity of food synthesis and oxygen formation9,10,11,12.
In order to find a way around, various traditional procedures such as direct hydration reaction, photoinduced reaction, electrochemical treatment, biological denitrification treatment, chemical precipitation, adsorption, ion exchange, biosorption, solid phase adsorption, electrochemical precipitation, filtration, complexation, flocculation or coagulation, membrane separation, photocatalytic reduction, phytoremediation, chemical reduction/oxidation, chemical precipitation, reduction, aerobic granule in continuous flow, and membrane inlet mass spectrometry method and adsorption13,14,15,16,17 have been proposed to deal with wastewater contamination due to oil refineries. In addition, some new treatment approaches have been proposed such as membrane technology18 and microwave-assisted catalytic wet air oxidation in recent past19. However, these techniques have their own limitations, such as high cost, generation of secondary pollutants and poor removal efficiency. Particularly, adsorption appears to be quite appealing because of its efficiency, lower costs, and lack of secondary pollution20,21,22,23. Adsorption is the method by which ions, atoms, or molecules of liquids, gases, or total suspended solids are deposited on a surface area. Activated carbons, activated alumina, zeolites, or less expensive materials (limestone, saw dust, peat, etc.), sugarcane bagasse, and biopolymers are applied as adsorbents in the adsorption process24. Carbonaceous materials are among the most common bio-adsorbents and have attracted the greatest attention among scientific community in the current literature25. Although, activated carbon (AC) possesses many desirable features, particularly, its high affinity for pollutants, its production from non-renewable raw materials, such as coal carbon comes at a high cost and limits its wide spread applicability26.
Due to the environmental sustainability perspective, biopolymers have recently been suggested as an alternative to toxic synthetic and chemical materials for wastewater treatment due to their regenerative characteristics such as sustainability, good biocompatibility, non-toxicity, and financial impact27,28,29,30. Among bio-based materials, chitosan has gained worldwide attention, which is a copolymer comprised of β-(1,4)-glycosidic linkages formed by N-acetyl-D-glucosamine and D-glucosamine (Fig. 1). Chitosan is commercially produced by the deacetylation of chitin, the second most abundant organic compound on earth, having an estimated annual biosynthesis rate of 10 billion tons31. Chitosan has several attractive characteristics, such as hydrophilicity, biocompatibility, biodegradability, nontoxicity, and the presence of highly reactive amino (-NH2) and hydroxyl (-OH) groups in its structure, making it a promising adsorbent for the elimination of contaminants from wastewater32. The fundamental benefit of chitosan over other polysaccharides (such as cellulose or starch) is its chemical structure, which can be altered in specialized ways to produce polymers for particular uses. Several studies have examined different chitosan based material in wastewater treatment33. However, native chitosan suffers from several drawbacks such as low mechanical strength, low solubility, poor adsorption capacity, and limited selectivity of native chitosan.
This study aims to improve the affinity of chitosan, a readily available and inexpensive biopolymer, for hydrocarbon contaminants found in refinery wastewater by functionalizing it by the addition of a long alkyl chain. In order to improve the performance characteristics of native chitosan, in this case, N-alkylated chitosan-based material was synthesized, henceforth referred to as N-hexadecylchitosan (NHDC), using conventional and microwave-assisted methods. It was then assessed as to whether it could be used as a new platform for the treatment of refinery wastewater (removal of oily pollutants and COD), as the reduction of these contaminants is required to meet the World Health Organization (WHO) requirements. To the best of our knowledge, no literature has been published on the synthesis of N-Hexadecylchitosan utilizing chitosan and 1-bromo-hexadecane as precursors or its subsequent use in the removal of hydrocarbon contaminants from refinery wastewater.
Materials and methods
Chemicals and reagents
All the required chemicals were purchased from commercial chemical suppliers. They include 85% deacetylated chitosan (Primex Norway), cetyl bromide (wako), sodium hydroxide (Sigma Aldrich), ethyl acetate (Dae-Jung), hydrochloric acid (Analar), Glacial acetic acid (Sigma Aldrich), silver sulfate (Sigma Aldrich), ferrous ammonium sulfate (Sigma Aldrich) potassium dichromate (Riedel–de haen). All of these chemicals were not further purified and used as received from the company. Synthetic wastewater was prepared by dissolving appropriate amounts of benzene, toluene, and naphthalene (Sigma–Aldrich) in distilled water. The refinery wastewater sample was taken from the effluent discharge of a nearby petroleum refinery (Attock Oil Refinery, Pakistan). The refinery wastewater sample was first filtered through Wattsman filter paper to remove suspended solids.
Conventional synthesis of N-hexadecylchitosan
Chitosan (0.5 g, 0.3 mmol) and 1-bromo-hexadecane (0.92 g, 0.3 mmol) were dissolved in 2% glacial acetic acid (40 mL) in a clean reaction flask (250 mL) and stirred at 40 °C for 4 h. The precipitates were isolated by changing pH with a 1 M sodium hydroxide solution. After the precipitates were filtered, thoroughly rinsed with distilled water and then treated with ethyl acetate, they produced a 75% yield when dried. The product was obtained as a brown solid.
Microwave-assisted synthesis of N-hexadecylchitosan
Chitosan (0.1 g, 0.6 mmol) and 1-bromo-hexadecane (0.18 g, 0.6 mmol), 2% glacial acetic acid (7 mL) were taken in a 10 mL sealed microwave tube (Pyrex) and irradiated in microwave synthesizer (CEM Discover System, Model: 908010) by optimizing the condition (power 100 W, pressure 80 bar and temperature 35 °C for 5 min). The precipitates were formed by adding the mixture to a 1 M solution of sodium hydroxide. The precipitates were filtered, rinsed with distilled water, and then followed by ethyl acetate which was provided by 84% after drying. Subsequently, the product was isolated as a brown solid.
Characterization
The structure of synthesized N-hexadecyl chitosan was characterized using a PerkinElmer spectrometer for FTIR analysis. FTIR facilitates the analysis of both organic and inorganic materials by revealing specific information on the distribution of functional groups and vibrations of the chemical bonding states. JEOL [JDX-3532] X-ray diffractometer was used to investigate the structural properties of NHDC. Additionally, SEM JEOL [JSM-5910 JAPAN] was used to investigate surface topography.
COD determination of wastewater sample
A sample (3 mL) of refinery wastewater was taken in a COD vial and 3 mL of strong oxidants were added (Acidified K2Cr2O7) and placed in a COD reactor (COD-571-1) at 120 °C for 120 min. After cooling, the digested sample was diluted with distilled water, 2–3 drops of ferroin indicator were added and titrated against a 0.1 N solution of ferrous ammonium sulfate (NH₄)₂ Fe(SO₄)₂·6H₂O). Distilled water was used for blank reading. The volume of titrant consumed was recorded.
Adsorption experiments
One of the best techniques to remove contaminants from wastewater is the batch adsorption method. In this project, batch experiments (in triplicate) were carried out using dose 0.08 g of pure chitosan and N-hexadecychitosan. The material was dispersed in 20 mL of real refinery wastewater in a 100 mL conical flask. The flask was placed on a temperature-controlled magnetic stirrer and the stirring rate was set to 350 rpm for all adsorption tests. Initially, the procedures were carried out at ambient temperature, and the optimized contact time was found to be 60 min. The mixture was filtered after adsorption and 3 mL of the filtrate was placed in a vial together with 3 mL of strong oxidants (acidified K2Cr2O7) and 0.5 mL of Ag2SO4 added as a catalyst. The mixture was then digested for 120 min at 120 °C in a COD reactor. After cooling the digested sample to room temperature, it was then diluted with 20 mL distilled water. After dilution, 2–3 drops of ferroin indicator were added and titrated against a 0.1 N solution of ferrous ammonium sulfate (NH₄)₂Fe(SO₄)₂·6H₂O). Distilled water was used for blank reading. The volume of titrant consumed was recorded and the % removal of chemical oxygen demand (COD) was measured using Eq. (1) formula.
where “Ci” is the COD content before treatment (mg/L) and “Ct” is the COD content after treatment (mg/L). The same procedure as above was repeated for evaluating the best optimized conditions, for instance, adsorbent dosage, contact time, temperature, and pH.
Results and discussion
Synthesis of NHDC
In the current study, N-hexadecylchitosan was synthesized by treating chitosan with long chain alkylhalide (bromohexadecane) both conventionally and via microwave-assisted microwave- assisted irradiation. The synthesis scheme is shown in Fig. 2. The microwave-assisted synthesized product had a higher yield (84%) compared to the results of the conventional procedure (75%). Microwave-assisted organic synthesis exploits dielectric volumetric heating as an alternative heat source, which results in faster and more selective reactions due to the uniform heat distribution. The crude products obtained using a microwave-assisted technique is significantly purer than those obtained using a conventional reaction process. The main advantage of the microwave-assisted procedure over the conventional protocol in this study is the reduction in reaction time (4 h to 5 min) and higher yield. The desired material was identified and characterized through analytical characterization techniques, i.e. FTIR, SEM, and XRD analysis.
Fourier transform infrared analysis of chitosan and NHDC
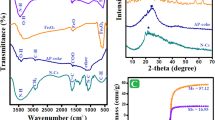
The FTIR spectrum of raw chitosan and NHDC are given in Fig. 3. The FTIR spectra showed characteristic bands in the frequency range between 4000 and 650 cm−1. The spectrum of chitosan illustrates an intense broad band at 2994 and 3612 cm−1 due to stretching vibration of hydrogen bonded −OH present in the structure. Two peaks positioned at 3294 and 3357 cm−1 attributed to the symmetric and asymmetric and the stretching of −NH2 in chitosan unit34. Peak appear at 2866 cm−1 corresponds to the stretching of CH2 present in side chain. The grade of chitosan used in the present study was calculated as 85%, an amide bond peak was present in the spectra and the vibrational mode of amide C═O stretching was observed at 1646 cm−1 and peak at 1594 cm−1 were assigned to strong N–H bending vibrations of amide. The spectrum was also compared with the standard chitosan and correlations were observed in the spectra35. A significant peak at 1374 cm−1 of chitosan was observed corresponding to C–O stretching in the primary OH group36. The peak at 1149 cm–1 indicated the beta 1,4-glycosidic bond of chitosan unit. In NHDC, two sharp stretching bands observed in the range of 2918–2855 cm−1 which indicates aliphatic CH2- asymmetric and symmetric stretching vibrational modes, clarified a significant change that confirms alkylation. Similarly, the emerged peak at 1668 cm−1 displays stretching vibration mode of amide C = O present in the parent structure of chitosan polymer. The characteristic band at 1463 cm−1 due to CH2 bending vibration was also observed in the synthesized NHDC. Furthermore, the broad band in 3400–3100 cm−1 due to the hydrogen-bonded OH groups that contribute to the complex vibrational stretches associated with the free inter- and intramolecular bound hydroxyl group37.
XRD analysis
The XRD diffractogram of chitosan and NHDC is shown in Fig. 4. In case of native chitosan, XRD profile exhibited a very broad peak at 2θ = 10.2 and 2θ = 20°. Similar results were obtained in earlier research reported elsewhere38. The broad peaks at 10.2 and 20° for chitosan in the present study are evidence that chitosan has a certain degree of crystallinity. However, since it incorporates molecules rather than only atoms or ions, it is possible to determine that the crystalline structure of the chitosan polymer is significantly dependent on the arrangement of the atoms and is more complicated39. Generally, the crystalline phase of chitosan polymer is often defined as the packing of polymeric chains that are connected by intermolecular hydrogen bonds. The amorphous area of chitosan is related to a higher reflection between 30 and 35°. Similarly, in NHDC, the characteristic peaks at 2θ = 10.2° was completely disappeared and the very broad peak at 2θ = 20° became weak in the synthesized NHDC. These results suggest that the semicrystalline phase of chitosan becomes weak, which leads to the formation of a new amorphous network. The XRD pattern also revealed that NHDC exhibit an amorphous arrangement, which can be attributed to the intermolecular interaction of the chitosan and long chain alkyl group. Therefore, as the alkyl group was introduced, the relative crystallinity of chitosan became significantly lower, implying that the intra and intermolecular hydrogen bonds of the chitosan network have been broken during synthesis.
SEM analysis of chitosan and N-hexadecyl chitosan
Figure 5 illustrates SEM micrographs of chitosan and synthesized NHDC. These images revealed that NHDC had a distinct morphology compared to that of raw chitosan. Examination of the SEM images of native chitosan revealed a fluffy exterior with a little folding over its surface. On the other hand, the synthesized NHDC has a structured morphology that has microfibers. The results of the SEM micrographs indicated that N-alkylation has improved porosity and resulted in a rougher surface in comparison to that of raw chitosan. The length of the carbon chain to the chitosan unit gave an irregular surface topography with enhanced porosity. The rough appearance showed that the synthesized NHDC has heterogeneous networks in its structure.
Investigation of COD removal using synthesized NHDC
The COD of refinery wastewater calculated before treatment was 1253 mg/L. NHDC was evaluated for the % decrease in COD of refinery wastewater. Initially, 0.06 g dose was optimized and treated with 20 mL of refinery wastewater at ambient temperature for 1 h. The % removal COD was calculated, and the data obtained was compared with that of raw chitosan as shown in Table 1. NHDC showed higher % removal with a value of 63% as compared to raw chitosan where it was only 21%.
Effect of adsorbent dose
The adsorbent dosage (g/L) is a significant parameter that affects the adsorption system. The impact of adsorbent dose on the removal of organic contaminants was investigated by varying the dose of NHDC from 0.02 to 0.12 g/20 mL of sample at ambient temperature. The results are presented in Fig. 6 and indicate that a higher % decrease (75%) of COD is achieved for adsorbent dose (0.08 g), but the % removal decreases with increasing the dose from 0.08 to 0.12 g of absorbent. This can be attributed to a variety of factors, including binding site saturation, electrostatic interactions, and decreased mixing due to the high concentrations of adsorbents in the solution. As a result of the restricted accessibility of the adsorbed species, a significant number of adsorption sites remain unsaturated, lowering the uptake and adsorption efficiency. The figure also shows that increasing the dose beyond 0.08 g/20 mL had little effect on the reduction in COD, and therefore this value was considered the optimal dose and used for the rest of the experimental work.
Effect of contact time
Contact time plays a very important role in the adsorption procedure. The effect of time on the removal efficiency of COD for the adsorbent (NHDC) was investigated by optimizing the time from 0 to 120 min at 25 °C temperature and selecting the dose 0.08 g. Figure 7 shows that the % COD removal efficiency increased with increasing contact time. Before reaching equilibrium, the adsorption proceeded through two significant stages: the initial fast uptake stage was preceded by a comparatively slow uptake stage. This can be described by the existence of a large number of accessible sites on the surface for adsorption; moreover, as the period of time passed, very few accessible sites on the surface of the adsorbent for adsorption became challenging to occupy as a result of the intense repulsions between the organic pollutant molecules on the surface of the adsorbent. Adsorption reached equilibrium within approximately 90 min with a maximum COD removal efficiency of 76%, and no significant changes were observed after 90 min. This pattern might initially be ascribed to a large number of active sites available on the surface of the adsorbent initially15. After a contact time of 90 min, the majority of active sites present in the NHDC have been occupied and equilibrium is attained.
Effect of temperature
Figure 8 shows the % COD removal efficiency of NHDC from wastewater determined at different temperatures of 25, 40 and 60 °C. According to previous reports, increasing the temperature has resulted in lower COD40. Increasing the temperature from 25 to 60 °C resulted in an improvement in the % removal of COD by 96%. This is due to the increase in the kinetic energy of adsorbate with temperature that led to an overall improvement in the accessibility of adsorbate towards adsorbent's active sites15.
Effect of initial pH
The effect of the initial pH on the removal of % COD was evaluated at optimized temperature (60 °C) for an initial COD concentration of 1253 mg/L and different initial pH values were optimized in the range of 2–10. The initial pH of the refinery wastewater was approximately 5.3, and it was adjusted to the desired value by adding a few drops of 0.1 M HCl or 0.1 M NaOH. Figure 9 shows the adsorption efficiency at different pH levels for the adsorbent. The results show that a higher percentage of COD removal (95%) was observed for the initial pH 6. This figure states that increasing the pH of the solution to 6 increases COD removal and after that the COD removal efficiency of NHDC decreases. A higher concentration of H+ ions in the acidic solution neutralizes the negative charge of the NHDC surface and reduces the COD removal efficiency by ion exchange. On the other hand, high OH- ion concentration in the alkaline/basic solution prevents the diffusion of organic materials into the NHDC pores and decreases the COD removal. Similar results have been reported for the adsorption of COD41.
Adsorption isotherms
Empirical equilibrium data were verified using different isotherm models. In this study, synthetic wastewater of different initial concentrations (that is, 200–1300 ppm) was used to investigate the removal capacity of synthesized NHDC. Two well-known adsorption isotherms shape the adsorption process, Table 2 shows that the RL values were 0 < RL < 1 and the values of n were more significant than 1, demonstrating the favorable adsorption of the hydrocarbons on NHDC (Fig. 10). This value suggests that the synthesized NHDC is a suitable adsorbent for the removal of organic contaminants from wastewater. Also, the R2 values for NHDC copolymers were higher than 0.99 for both the Langmuir and Freundlich isotherms, describing that monolayer and multilayer adsorption played a crucial role in the uptake of hydrocarbon on alkylated chitosan. The maximum monolayer adsorption capacity was obtained to be 315.5 mg/g for NHDC.
Adsorption kinetics
To understand the reaction pathways and kinetics of the adsorption performance of NHDC for oily waste, we further fitted the experimental data of synthetic wastewater using pseudo-first order and pseudo-second order kinetic models. A correlation of the experimental and calculated values of the adsorption capacity is presented in Table 3. It was deduced that the values of R2 (0.999) of the pseudo-second-order kinetic model are very close to 1 while the pseudo-first order (0.743) exhibited a prominent difference (Fig. 11). The reaction fits well to a pseudo-second-order model as it indicates a trend towards chemisorption. In this kind of adsorption, the chemical reaction seems significant in the rate control step and the pseudo-second order chemical reaction kinetics provides the best correlation of the experimental data and the mechanism of the adsorption is chemically rate controlling, and because of this, it is called chemisorption15. According to15, the removal from a solution is due to physicochemical interactions between the two phases. This observation is consistent with the available literature42.
Adsorption thermodynamic parameters
The spontaneity of the adsorption process can be considered by the thermodynamic parameters, the enthalpy (ΔH°), the entropy (ΔS°) and the free energy change (ΔG°) where ∆G° and ∆H° are in kJ/mol, ∆S° is in J/mol K, T is the adsorption temperature in K and R is the universal gas constant (8.314 J/mol). In this study, the initial concentration of synthetic wastewater (400 ppm) was studied at different temperatures 25, 40 and 60 °C. Thermodynamic parameters (Table 4) were investigated from the slope and intercept of the lnKL plot vs 1/T as given in Fig. 12. The negative ΔG° values confirmed that adsorption was indeed a spontaneous process. The positive values for the ΔH° designate that the adsorption process is endothermic, which describes the increase in adsorption capacity by increasing temperature. It was also approved that the adsorption was complex with simultaneous chemical and physical reactions. However, the positive values of ∆S° intend the increase in randomness at the adsorbent/adsorbed interface during the adsorption process.
Regeneration and recycling studies
Regeneration and recycling of the adsorbent should always be considered in scale-up operations to make adsorption more economical and environmentally beneficial technique for wastewater treatment. Solvent washing was used to investigate the removal efficiency of adsorbed organic contaminants from NHDC regeneration. Various desorption solvents such as ethanol, Methanol, acetone, and n-hexane were tested. After drying, the sample was applied to evaluate the % COD reduction in the refinery wastewater. Acetone showed promising results among the selected desorption solvents. The process was repeated for five consecutive cycles washing with acetone. As observed in Fig. 13, the NHDC adsorbent did not show any notable loss of its adsorption capacity through five cycles, where it changed from 92% in first cycle to 71.5% in the fifth cycle. This shows that NHDC can be used effectively for potential industrial scale wastewater treatment processes.
Comparison with previous works
Table 5 shows a comparison between our results and the results of familiar works. As can be observed, the current system performed better in terms of COD removal. The reason behind these results could be the higher turbulence promotion observed by using the batch recirculation mode of operation. Therefore, adopting batch recirculation in the removal of COD considers a promising step in the application of such a mode of operation for the treatment of different types of wastewater generated from different industrial activities.
Conclusions
Chitosan as an abundant natural biopolymer was modified to enhance its hydrophobic/lipophilic properties with high adsorption capacity toward hydrocarbon pollutants from refinery wastewater. The amino group in the chitosan was substituted with long chain alkyl halide (16 carbon atoms) by alkylation using both conventional and microwave-assisted synthetic procedures. The test materials were evaluated using different characterization techniques, i.e. FTIR, XRD, and SEM, which showed important perspectives related to physicochemical properties of the synthesized material. The prepared NHDC demonstrated excellent scavenging ability of oily hydrocarbons from wastewater using real refinery wastewater samples by measuring % COD. Batch adsorption experiments show that the % COD removal efficiency was optimal, i.e., 96% at the contact time of 60 min at 60 °C using 0.08 g adsorbent dosage. On the other hand, regeneration studies demonstrated that NHDC can be effectively reused for wastewater treatment up to five consecutive cycles. These results showed the real possibility of NHDC as an alternative to native chitosan with enhanced properties for sustainable refinery wastewater treatment systems.
Data availability
All the data is available within the manuscript.
References
Chen, Y. C. Evaluating greenhouse gas emissions and energy recovery from municipal and industrial solid waste using waste-to-energy technology. J. Clean. Prod. 192, 262–269. https://doi.org/10.1016/j.jclepro.2018.04.260 (2018).
Younis, S. A., Maitlo, H. A., Lee, J. & Kim, K. H. Nanotechnology-based sorption and membrane technologies for the treatment of petroleum-based pollutants in natural ecosystems and wastewater streams. Adv. Coll. Interface Sci. 275, 102071. https://doi.org/10.1016/j.cis.2019.102071 (2020).
Diya’uddeen, B. H., Daud, W. M. A. W. & Aziz, A. A. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Prot. 89(2), 95–105. https://doi.org/10.1016/j.psep.2010.11.003 (2011).
Ahmed, S. N. & Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 29(34), 342001. https://doi.org/10.1088/1361-6528/aac6ea (2018).
Al-Shamrani, A., James, A. & Xiao, H. Destabilisation of oil–water emulsions and separation by dissolved air flotation. Water Res. 36(6), 1503–1512. https://doi.org/10.1016/S0043-1354(01)00347-5 (2002).
Yan, L. et al. Comparative study of different electrochemical methods for petroleum refinery wastewater treatment. Desalination 341, 87–93. https://doi.org/10.1016/j.desal.2014.02.037 (2014).
Singh, B., Singh, J. P., Kaur, A. & Singh, N. Bioactive compounds in banana and their associated health benefits–A review. Food Chem. 206, 1–11. https://doi.org/10.1016/j.foodchem.2016.03.033 (2016).
Ishak, W. W. et al. Quality of life in patients suffering from insomnia. Innov. Clin. Neurosci. 9(10), 13 (2012).
Sudhakar, S., Moondra, N. & Christian, R. A. A comparative study on treatment of CETP wastewater using SBR and SBR-IFAS process. Water Conserv. Manag. 6(1), 51–54 (2022).
Nwankwoala, H. O., Harry, M. T. & Warmate, T. Assessing aquifer vulnerability and contaminant plume at artisanal refining sites in parts of Okrika and Ogu-Bolo local government areas, rivers state Nigeria. Water Conserv. Manag. 4(2), 68–72. https://doi.org/10.26480/wcm.02.2020.68.72 (2020).
Nguyen, T. H., Le, T. H., Le, V. V. & Dong, T. M. H. A Study on selection of ballast water treatment technologies to meet BWM 2004 convention. Water Conserv. Manag. 5(1), 53–59. https://doi.org/10.26480/wcm.01.2021.53.59 (2021).
Sakthivadivel, M., Nirmala, A., Sakthivadivel, J., Mukhilan, R. R. & Tennyson, S. Physicochemical and biological parameters of water at industrial sites of metropolitan city of Chennai, Tamil Nadu India. Water Conserv. Manag. 4(2), 90–98. https://doi.org/10.26480/wcm.02.2020.90.98 (2020).
Wan, Q., Wu, X., Hou, Z., Ma, Y. & Wang, L. Organophotoelectrocatalytic C(sp2)–H alkylation of heteroarenes with unactivated C(sp3)–H compounds. Chem. Commun. https://doi.org/10.1039/D4CC01335B (2024).
Tomoki, S. et al. Development of carbon nanotube as highly active photocatatlytic adsorbent for treatment of acid red 88 dye. Water Conserv. Manag. 5(1), 26–29 (2021).
Ilyas, M. et al. Removal of anthracene from vehicle-wash wastewater through adsorption using eucalyptus wood waste-derived biochar. Desalin. Water Treat. https://doi.org/10.1016/j.dwt.2024.100115 (2024).
Abdrashitova, R. N. et al. Synthesis of ZNO doped multi walled carbon nanotubes (MWNTS) for dyes degradation and water purification. Water Conserv. Manag. 7(1), 01–05. https://doi.org/10.26480/wcm.01.2023.01.05 (2023).
Abdulwahid, K. D. Phytoremediation of cadmium pollutants in wastewater by using Ceratophyllum demersum L. as an aquatic macrophytes. Water Conserv. Manag. 7(2), 83–88. https://doi.org/10.26480/wcm.02.2023.83.88 (2023).
Peters, T. Membrane technology for water treatment. Chem. Eng. Technol. 33(8), 1233–1240. https://doi.org/10.1002/ceat.201000139 (2010).
Karabach, Y. Y., Kopylovich, M. N., Mahmudov, K. T. & Pombeiro, A. J. Microwave-assisted catalytic oxidation of alcohols to carbonyl compounds. In Advances in Organometallic Chemistry and Catalysis: The Silver/Gold Jubilee Organometallic Chemistry Celebratory Book (ed. Pombeiro, A. J. L.) (Wiley, 2013).
Vilardi, G., Di Palma, L. & Verdone, N. Heavy metals adsorption by banana peels micro-powder: Equilibrium modeling by non-linear models. Chin. J. Chem. Eng. 26(3), 455–464. https://doi.org/10.1016/j.cjche.2017.06.026 (2018).
Prasad, R. & Yadav, K. D. Use of response surface methodology and artificial neural network approach for methylene blue removal by adsorption onto water hyacinth. Water Conserv. Manag. 4(2), 83–89. https://doi.org/10.26480/wcm.02.2020.83.89 (2020).
Bullo, T. A. & Bayisa, Y. M. Optimizing the removal efficiency of chromium from tanning plant effluent by adsorption method with activated carbon chat stems (catha edulis) using response surface methodology. Water Conserv. Manag. 6(1), 15–21. https://doi.org/10.26480/wcm.01.2022.15.21 (2022).
Dibaba, W. T. & Ebsa, D. G. Identifying erosion hot spot areas and evaluation of best management practices in the toba watershed ethiopia. Water Conserv. Manag. 6(1), 30–38. https://doi.org/10.26480/wcm.01.2022.30.38 (2022).
Dotto, G. L. & McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 8(4), 103988. https://doi.org/10.1016/j.jece.2020.103988 (2020).
Bubanale, S. & Shivashankar, M. History, method of production, structure and applications of activated carbon. Int. J. Eng. Res 6, 495–498 (2017).
Wang, Y., Peng, C., Padilla-Ortega, E., Robledo-Cabrera, A. & López-Valdivieso, A. Cr (VI) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. J. Environ. Chem. Eng. 8(4), 104031. https://doi.org/10.1016/j.jece.2020.104031 (2020).
Lutzu, G. A. et al. Latest developments in wastewater treatment and biopolymer production by microalgae. J. Environ. Chem. Eng. 9(1), 104926. https://doi.org/10.1016/j.jece.2020.104926 (2021).
Zhu, S. et al. Near-complete recycling of real mix electroplating sludge as valuable metals via Fe/Cr co-crystallization and stepwise extraction route. J. Environ. Manag. 358, 120821. https://doi.org/10.1016/j.jenvman.2024.120821 (2024).
Debela, S. K. & Feyessa, F. F. Rainfall-runoff modeling and its prioritization at sub-watershed level using swat model: A case of Finca’aa, Oromia Western Ethiopia. Water Conserv. Manag. 6(1), 22–29 (2022).
Shen, Y., Sun, P., Ye, L. & Xu, D. Progress of anaerobic membrane bioreactor in municipal wastewater treatment. Sci. Adv. Mater. 15(10), 1277–1298. https://doi.org/10.1166/sam.2023.4531 (2023).
Periayah, M. H., Halim, A. S. & Saad, A. Z. M. Chitosan: A promising marine polysaccharide for biomedical research. Phcog. Rev. 10(19), 39. https://doi.org/10.4103/0973-7847.176545 (2016).
Aranaz, I. et al. Chitosan: An overview of its properties and applications. Polymers 13(19), 3256. https://doi.org/10.3390/polym13193256 (2021).
Fan, L. et al. Fabrication of magnetic chitosan nanoparticles grafted with β-cyclodextrin as effective adsorbents toward hydroquinol. Coll. Surf. B Biointerfaces 95, 42–49. https://doi.org/10.1016/j.colsurfb.2012.02.007 (2012).
Pinto, E. P. et al. Copaiba essential oil loaded-nanocapsules film as a potential candidate for treating skin disorders: Preparation, characterization, and antibacterial properties. Int. J. Pharm. https://doi.org/10.1016/j.ijpharm.2023.122608 (2023).
de Queiroz, A. R. S. C. M. et al. Preparation and characterization of chitosan obtained from shells of shrimp (litopenaeus vannamei Boone). Mar. Drugs 15(5), 141. https://doi.org/10.3390/md15050141 (2017).
Divya, K., Rebello, S., Jisha, M. A simple and effective method for extraction of high purity chitosan from shrimp shell waste. In Proc. of the international conference on advances in applied science and environmental engineering-ASEE, 10.15224/ 978-1-63248-004-0-93 (2014).
Vimal, S. et al. Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquaculture 358, 14–22. https://doi.org/10.1016/j.aquac.2012.06.012 (2012).
Rasti, H., Parivar, K., Baharara, J., Iranshahi, M. & Namvar, F. Chitin from the mollusc chiton: Extraction, characterization and chitosan preparation. Iran. J. Pharm Res. 16(1), 366 (2017).
Song, R., Xue, R., He, L. H., Liu, Y. & Xiao, Q. L. The structure and properties of chitosan/polyethylene glycol/silica ternary hybrid organic-inorganic films. Ch. J. Polym. Sci. 26(05), 621–630 (2008).
Ahmad, W. et al. Batch mode and continuous flow adsorption of hydrocarbon pollutants from refinery wastewater using graphene oxide derived from fish scales. Environ. Sci. Water Res. Technol. 9(8), 2089–2098 (2023).
Parande, A. K., Sivashanmugam, A., Beulah, H. & Palaniswamy, N. Performance evaluation of low cost adsorbents in reduction of COD in sugar industrial effluent. J. Hazard. Mater. 168(2–3), 800–805. https://doi.org/10.1016/j.jhazmat.2009.02.098 (2009).
Omer, A. M. et al. Kinetic and thermodynamic studies for the sorptive removal of crude oil spills using a low-cost chitosan-poly (butyl acrylate) grafted copolymer. Desalin. Water Treat. 192, 213–225. https://doi.org/10.5004/dwt.2020.25704 (2020).
El-Naas, M. H., Al-Zuhair, S. & Alhaija, M. A. Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 173(1–3), 750–757 (2010).
Nekoo, S. & Fatemi, S. Experimental study and adsorption modeling of COD reduction by activated carbon for wastewater treatment of oil refinery. Iran. J. Chem. Chem. Eng. (IJCCE) 32(3), 81–89. https://doi.org/10.30492/ice.2013.5834 (2013).
Devi, M. G., Al-Moshrafi, S. M. K., Al Hudaifi, A. & Al Aisari, B. H. Treatment of refinery waste water using environmental friendly adsorbent. J. Inst. Eng. India Ser. E https://doi.org/10.1007/s40034-017-0105-0 (2017).
Khader, E. H. et al. Removal of organic pollutants from produced water by batch adsorption treatment. Clean Technol. Environ. Policy https://doi.org/10.1007/s10098-021-02159-z (2021).
Acknowledgements
The authors acknowledge the support of the Centralized Resources Laboratories (CRL), University of Peshawar.
Author information
Authors and Affiliations
Contributions
R, Khan. (Conceptualization, Methodology, Investigation, Validation, Data Curation, Visualization, Writing—Original Draft, Supervision); Z, Haram. (Methodology, Writing—Review & Editing; W, Ahmad. (Writing—Review & Editing); S, Sohni, J. Xu & M, Ilyas. (Review & Editing, Visualization).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, R., Haram, Z., Ahmad, W. et al. Removal of hydrocarbon pollutants from refinery wastewater using N-hexadecylchitosan as an efficient adsorptive platform. Sci Rep 14, 17236 (2024). https://doi.org/10.1038/s41598-024-66429-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66429-8