Abstract
Functioning pancreatic neuroendocrine neoplasms other than insulinomas and gastrinomas (rf-pNENs) are exceptionally rare tumours. Thus, their characteristics and long-term prognosis have not been well defined. This article aims to present data and experience from a single institution concerning this topic. Twelve of 216 (5.5%) patients with pNENs operated between 2002 and 2022 in the ENETS Centre of Excellence Marburg had rf-pNENs and their data were retrospectively analysed. We identified three vasoactive intestinal polypeptide producing pNENs, four glucagonomas and five calcitoninomas. The tumour could be visualised by preoperative imaging in all 12 patients, and six patients had distant metastases at the time of diagnosis. The tumour was located in the pancreatic tail in nine patients and the median tumour size was 82 (range 12–220) mm. Eleven patients underwent tumour resections (two robotic, nine conventional), nine of which were R0. After a median follow-up of 75 (range 1–247) months, six patients were alive, five of whom had no evidence of disease. All patients who remained disease-free had an initial R0 resection of the primary tumour and no initial liver involvement. This study sheds light on the distinct characteristics and outcomes of these exceedingly rare tumours, offering insights for improved understanding and management.
Similar content being viewed by others
Introduction
Rare functioning pancreatic neoplasms (rf-pNENs) comprise glucagonomas, vasoactive intestinal polypeptide (VIP) producing pNENs (VIPomas), calcitoninomas, somatostatinomas, as well as pNENs secreting parathyroid hormone related peptide, gonadotropin relasing hormone , adrenocorticotropic hormon, renin, luteinising hormone, erythropoietin and insulin-like growth factor II1,2. Rf-pNENs are extremely rare and account for only approximately 5% of functioning pNENs, with an annual incidence of approximately 0.05–0.1/1,000000/year2,3,4,5. The most frequent familial condition associated with rf-pNENs is multiple endocrine neoplasia type 1 (MEN1), with glucagonomas occurring in 5% and VIPomas in 3% of MEN1 patients2,6. Glucagonomas in the setting of MEN1 usually do not cause a syndrome, although glucagon serum levels may be significantly elevated. The diagnosis of an rf-pNEN requires at least the demonstration of an inappropriate elevation of the specific serum hormone (i.e. VIP or glucagon), ideally combined with symptoms caused by over-secretion of the distinct hormone. The diagnosis of rf-pNENs cannot be based solely on positive immunohistochemical results of resected specimens2,5, as these are frequently found also in tumours of asymptomatic patients without elevated hormone levels. As of 2022, approximately 600 glucagonomas7,8,9,10, 100 VIPomas11,12,13 and 60 calcitoninomas14,15 have been reported in case reports or small case series, whereas reports on other rf-pNENs do not exceed 10–25 cases per subtype1,16,17,18,19,20.
Glucagonoma, first described in 1942 by Becker21, is a pancreatic alpha-cell tumour secreting glucagon. It causes glucagonoma syndrome, which includes necrolytic migratory erythema, diabetes, venous thromboembolism, diarrhoea, stomatitis, and anaemia. However, in some series, only half of the patients had necrolytic migratory erythema, and only 20% developed diabetes before the diagnosis10,22. Thus, published cohorts in the literature comprise a heterogenous group of patients suffering from all named features of the syndrome, isolated symtpoms, and sometimes also include asymptomatic patients with glucagon expressing tumors in immunostaining.
VIPomas were first reported by Verner and Morrison in 195823 and are characterised by excessive secretion of VIP, which may cause typical watery diarrhoea, hypokalaemia, and achlorhydria, the so-called WDHA syndrome.
Calcitonin-producing pNENs represent an extremely rare cause of hypercalcitoninemia, which is usually highly suggestive of medullary thyroid carcinoma24. Hormonal excess of calcitoninomas may cause diarrhoea and abdominal pain. Calcitonin producing pNENs appear to have a unique molecular signature compared to other pNEN subtypes15. A recent pathological study found that approximately 10% of all pNENs show a positive calcitonin immunoreactivity, whereas the corresponding serum calcitonin levels are rarely elevated14.
The average age at diagnosis of rf-pNENs is between 50 and 60 years with an equal gender distribution. A significant number of patients with rf-pNENs present with metastatic disease (40–80%) in the liver at initial diagnosis. The management of rf-pNENs is challenging because high-level evidence recommendations are currently lacking, especially for the treatment of symptomatic patients with diffuse metastatic tumours5. Surgical resection of all lesions is considered the only curative option. Symptom and tumour growth control can be achieved either with somatostatin analogues (SSAs), chemotherapy, and targeted therapy with sunitinib or peptide receptor radionucleotide therapy (PRRT)5. Debulking surgery and/or other cytoreductive techniques such as liver transarterial (chemo)embolisation (TACE) or radioembolisation can also be employed5,16. Nevertheless, because of the rarity of these tumours, experiences with more novel and specific therapeutic tools such as PRRT are still sparse. We present a series of 12 patients with rf-pNENs from a tertiary referral centre who were treated for 20 years. Clinicopathological characteristics, therapeutic modalities, and prognosis were evaluated.
Patients and methods
Patient cohort
Patients diagnosed with rf-pNENs were identified from the prospective pancreatic database of the Department of Visceral, Thoracic, and Vascular Surgery, Philipps-University Marburg, which was established in 2002 as a prerequisite for certification as an ENETS Centre of Excellence.
Patients with rf-pNENs were retrieved from this database and their clinical data were retrospectively analysed with special regard to demographics, clinical features, preoperative imaging, operative procedures, pathologic findings, and long-term follow-up.
Selective clinical data from our patients with calcitonin-producing pNENs have been reported previously15,25.
Definition and diagnostics of Rf-pNENs
Rf-pNENs were defined according to the ENETS guidelines, such as VIPoma, glucagonoma, or calcitoninomas5. The diagnosis of a distinct rf-pNEN was confirmed if the pNEN was associated with at least a two-fold elevated serum hormone level and positive immunostaining for the respective hormone.
The fact that initially elevated serum hormone levels dropped to normal levels after potentially curative surgery was considered evidence that the resected pNEN was the source of hormone hypersecretion.
Preoperative imaging routinely includes abdominal ultrasonography, multidetector computed tomography (CT), and/or magnetic resonance imaging (MRI) with gadolinium, and endoscopic ultrasonography (EUS) if the tumor was limited to the pancreas and regional lymph nodes. Some patients also underwent somatostatin receptor scintigraphy (SRS) until 2013, which was then replaced by Gallium-68-positron-emission-tomography combined with CT (Ga68-DOTATOC PET/CT). In the case of resectable disease, and if fit for surgery, patients underwent pancreatic resection with or without metastasectomy. In cases of diffuse metastatic disease without complications (e.g., bowel obstruction), palliative debulking surgery was performed after a multidisciplinary tumour board decision. After potentially curative resection, no adjuvant treatment was administered, in accordance with ENETS guidelines5,26.
Tumours were classified according to the World Health Organization (WHO) classification 201727 and defined as malignant28. All tumours were immunostained for Ki67, synaptophysin and chromogranin A. Ki67 ratio was determined as the percentage of positively stained tumour cells among the total number of malignant cells assessed. Potentially secreted hormones, such as VIP, glucagon, and calcitonin were verified by immunohistochemistry as described in previous publications15,29. Immunohistochemistry for synaptophysin, chromogranin A, and Ki67 was re-evaluated by an experienced pathologist (M.J.) at the time of this study.
Perioperative complications
Complications of surgery were classified according to Clavien-Dindo30. Clinically relevant postoperative pancreatic fistula types B and C were defined according to the International Study Group of Pancreatic Fistula31. The length of hospital stay was not evaluated because several institutional changes in patient demission management over the years would have induced a significant bias.
Follow-up
Follow-up was calculated from the time of the initial surgery to the time of last follow-up or death until the evaluation date of July 31, 2023. Disease-free survival was defined as the time from potentially curative surgery to disease recurrence, last follow-up without evidence of disease recurrence, or death.
An at least annual clinical follow-up was performed at our hospital or an associated physician with a laboratory workup, including the preoperatively increased specific hormone level, MRI of the abdomen, and in case of suspicion of recurrence or metastatic disease, additional somatostatin receptor imaging. Patients were classified as having no evidence of disease if there was no suspicion of symptom recurrence, the respective serum hormone levels were not elevated, and there were no visible tumours on imaging.
In the case of palliative surgical procedures and/or unresectable disease, patients were treated with a variety of modalities such as somatostatin analogues, PRRT, transarterial chemoembolisation, or chemotherapy, during follow-up upon recommendation of the multidisciplinary tumour board.
Ethical standards
All patients provided written informed consent to register their data. While the treatment was conducted as part of routine clinical care, additional ethical approval was obtained for the retrospective analysis during this study from the local ethics committee of the University of Marburg (no. RS 22-51). All research was performed in accordance with relevant guidelines/regulations and in accordance with the Declaration of Helsinki.
Statistics
Descriptive statistics were performed whenever applicable. Due to the small cohort size, analytical statistics were not reasonable.
Results
In the study period from January 2002 to December 2022, 216 patients with pNENs underwent surgery at the ENETS Centre of Excellence Marburg. They included 156 patients with sporadic and 60 with MEN1-associated pNENs. Overall, 79 (36.6%) patients had functioning tumours, of which 12 (12/216, 5.5%) had rf-pNENs according to the actual ENETS definition5.
The demographic characteristics and symptoms of the 12 patients are summarised in Table 1. Five patients had a calcitoninoma, four patients had a glucagonoma and three patients had a VIPoma. Except for one patient with MEN1-associated VIPoma, the other 11 tumours were sporadic. Five patients were female. The median age at the time of surgery was 60 years (range 28–73 years). All 12 patients had at least two-fold elevated serum levels of the specific tumour-released hormone (median 31-fold, range 2–189-fold). CgA was measured preoperatively in ten patients and was elevated in eight cases (median 707ng/ml, range 211 – 15,174). All but three patients with calcitoninomas had specific symptoms. These three patients with calcitoninomas had unspecific symptoms (abdominal pain, weight loss) in combination with hypercalcemia, which where cured after surgery and therefore considered to be linked to the hormonal excess. Six patients had all above described features of a hormone-associated syndrome (three VIPomas, two glucagonomas, and one calcitonin producing pNEN). Diarrhoea was the most common symptom, presenting in six of 12 patients (three VIPomas, one calcitoninoma, and two glucagonomas).
The time between diagnosis and the first surgery varied between one and 137 months. Preoperative imaging revealed the rf-pNENs in all 12 patients (Table 2). All but one tumour had a size > 20mm. For the visualisation of the primary tumour, EUS and MRI were the most sensitive methods, with 100% detection and accuracy. Four patients underwent SRS until 2013, but after 2014, Ga68-DOTATOC PET/CT was performed on another five patients. SRS imaging showed the primary tumour in eight of nine patients, lymph node metastases in none of the patients, but liver metastases in all six patients with histologically confirmed distant metastases. It is of note, that only EUS and MRI detected a small (12 mm) glucagonoma in the pancreatic head. EUS-guided fine needle aspiration (FNA) was performed in four patients and confirmed the presence of pNENs in all four cases. The tumour was located in the pancreatic tail in nine patients and in the pancreatic head in three patients.
Eleven of the 12 rf-pNENs were considered completely resectable based on preoperative imaging. Eight patients underwent distal splenopancreatectomies, one patient underwent pylorus preserving pancreaticoduodenectomy, one patient underwent total pancreatectomy, and one patient underwent enucleation of a 12mm glucagonoma in the pancreatic head. The remaining patient with a large VIPoma with far advanced diffuse metastatic disease in the liver and the peritoneum had only an exploratory laparotomy with palliative ileocecal resection for bowel obstruction without removal of the primary tumour. Ten patients underwent conventional open surgery, and the remaining two patients underwent robotic-assisted procedures. In addition to the pancreatic resection, five patients also underwent resection of liver metastases. In nine of the 12 patients, complete (R0) resection of the primary tumour and, if present, liver metastases was achieved, so that serum hormone levels dropped to normal levels postoperatively.
Postoperative complications (Clavien-Dindo > III) occurred in four patients (all three with VIPoma and one with glucagonoma), all of whom had to be reoperated. One of them was due to peritonitis, with a suspected anastomotic leak. However, this has not been confirmed. The second patient required open surgical abdominal lavage and drainage of a subphrenic and paracolic abscess. The third patient underwent metachronous splenectomy because of haemorrhage. The last patient underwent two reoperations because of tumour-associated perforation of the small intestine. He died on the 30th postoperative day after the initial surgery due to multi-organ failure. None of the patients developed postoperative pancreatic fistulas type B or C. The operations and postoperative complications of all patients are summarised in Table 3.
Rf-pNENs were confirmed by histopathological examination in all patients. All 12 primary tumours were immunohistochemically positive for chromogranin A, synaptophysin, and their respective secreted hormones. All primary tumours were well differentiated and graded G1 in three patients and G2 in the remaining nine patients. The tumour was classified as T1 in three patients, T2 in three, T3 in three, and T4 in two patients. Six patients had locoregionally limited, Stage I-III rf-pNENs, and the other six patients had Stage IV disease. The median tumour size was 82 (range 12–220) mm. Five of the 12 patients had lymph node metastasis. A median of 13 (range 5–28) lymph nodes were removed. In nine of the 12 patients, resection was defined as R0 resection. Histopathology results and hormone levels after initial surgery are summarised in Table 4.
Follow-up
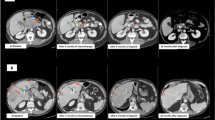
The median follow-up period was 75 months (range 1–247 months). Four of the six patients with resected localised disease (stages I-III) remained disease-free for a median of 61 (range 6–108) months. In this group, one patient experienced local recurrence six months after glucagonoma enucleation from the pancreatic head, which required subsequent pylorus-preserving pancreatic head resection. Following this reoperation, the patient remained disease-free until the last follow up 90 months after surgery. Another patient in this group had local tumor recurrence 84 months after resection of a 22 cm-sized initially locoregionally metastasised VIPoma in the pancreatic tail. This patient underwent a total of seven redo-surgeries and more than 40 cycles of multimodal therapy (SSA, interferon, PRRT, chemotherapy) due to tumour recurrence and/or progression. All the treatment modalities are summarised in Fig. 1. The sequence of multimodal treatment led to a survival of 247 months after initial diagnosis.
In the six patients with stage IV disease, multimodal treatments were performed, including somatostatin analogues, chemotherapy regimens with dacarbazine, doxorubicin, streptozotocin, capecitabine, targeted therapy with sunitinib, radiofrequency ablation or TACE of liver metastases, recurrent PRRTs, and reoperations. One patient with a Stage IV calcitoninoma developed a local lymph node recurrence 11 months after the initial surgery, and excision of this metastasis was performed. He was alive with the disease after 49 months of follow-up. The remaining five patients died of disease 1, 20, 48, 52, 114, and 247 months after the initial surgery. See Table 5.
Among five patients who experienced recurrence after initially normalised hormone levels, three patients had biochemical evidence of disease synchronously with tumour recurrence on imaging, the remaining patients developed tumours on imaging three, 14 and 36 months after evidence of increased hormone levels.
One-year survival in our cohort was 91%, and the true five-year survival rate was 55%. In three patients follow-up within this study was less than five years.
The characteristics, treatments, and follow-up of the individual tumour subtypes are summarised in Table 6.
Discussion
In our series, patients with rf-pNENs had a median age of 61.5 (range 28–73 years), which is in the range reported in the literature7,14,22,32,33. In addition, no sex predominance was noted in either our series or in the literature. All three patients with VIPoma presented with the classic symptomatology of watery diarrhoea, which is typical in these patients11,12,32. However, only two of four patients with glucagonoma in the present series had necrolytic migratory erythema, and only one patient had diabetes. This is in line with a previous Swedish study of 23 patients22. Only 22% of these patients had developed diabetes before the diagnosis of glucagonoma, and necrolytic migratory erythema was diagnosed in only 52% of patients. In a recent clinicopathological study of 25 pNENs with calcitonin expression in immunohistochemistry, none of the patients had symptoms of calcitonin excess, eight had an insulinoma and one patient had symptoms of a somatostatinoma14. Elevated serum calcitonin levels were not reported in the clinical records of any of these 25 cases. The five patients presented here all had significantly elevated calcitonin serum levels up to 150-fold. However, only the patient with a 150-fold elevated calcitonin had diarrhoea, which is typical for excessive hypercalcitonemia. Abdominal pain due to tumour size was a leading symptom in three of our 12 (25%) patients with rf-pNEN, which was reported to be present in 19.8%-33% of other case series7,10,34,35.
In the present cohort, there was a predominant tumour ___location in the left pancreas (75%), which is in line with previous case series of VIPomas, glucagonomas, and calcitonin producing pNENs7,14,22,32,33. The median diameter in our cohort was 82 (range 12–220), and only one patient with a glucagonoma had a tumour size < 20mm. In an older literature review of 407 patients with glucagonoma, 80 (29%) patients had small tumours of 20mm or less and 8.8% of these patients had metastases7. The median tumour size in the present cohort was larger than that previously reported for VIPoma, with a median diameter of 32–57.5 mm33, glucagonoma with a median size from 50 to 55 mm22,36, and calcitonin producing pNENs with a median size of 48 mm14.
As in other studies, patients with rf-pNENs frequently show liver metastases at the time of initial diagnosis as in 50% of patients in our cohort. This is in the range of reported rates for VIPoma of 36–75%32,33,34,37 and glucagonoma of 50–78%8,22,36. It is of note, that in the present series of patients who underwent surgery, two of five calcitonin producing pNENs presented with liver metastases, whereas in a previous series only four of 23 (17%) tumours did so14. None of our patients with rf-pNENs showed initial bone metastases, whereas a rate of 6–15% has been reported previously7,8,32. This might in part be due to the lower sensitivity of SRS scintigraphy imaging used in the early 2000s.
All 12 rf-pNENs in the present cohort were G1 (n = 3) or low G2 (n = 9) tumours with a Ki67 index in the primary tumour of at most 10%. This is in line with the Ki67 index in the majority of reported glucogonomas22,36 and calcitonin producing pNENs14. For VIPoma, however, G3 tumours have been described in up to 27% of cases37.
In the present cohort, all rf-pNENs could be visualised by preoperative imaging with either CT or MRI because of the large tumour size, as reported in the previous series37,38,39,40. EUS was used in only half of our patients and had an additional value in only one patient with 12 mm glucagonoma in the pancreatic head. According to recent guidelines3,5, imaging with 68Ga-labelled somatostatin analogues with PET/CT is more sensitive and highly specific for rare pNENs and is therefore recommended as the first-line diagnostic imaging method for staging in patients with rf-pNENs. Our cohort supports this recommendation since eight of nine patients who underwent SRS imaging showed positive primary tumours and in all five metastatic patients who underwent SRS imaging, metastasis could be visualised. In patients with rapid tumour growth and/or high G2 or G3 tumours, 18-FDG-PET/CT can also be considered to assess tumour burden41,42. In our cohort, this was not applied because we had only G1 and low G2 tumours.
The indications for surgery are influenced by clinical symptom control, the technical possibility of local R0 resection, and the presence and extent of metastatic spread3,5,43. Curative intended surgery should always be indicated, even in the presence of metastatic disease, if a complete resection can be achieved and the patient is fit for surgery5,34. In the present cohort, we could achieve a potential curative R0 resection, documented by postoperative normal serum hormone levels, in nine of 12 (75%) patients of whom three also had liver metastases. The surgery rate as well as the R0 resection rate are comparable to other series, ranging from 28 to 63%11,34,37,39,44. In two patients with calcitonin producing pNENs with liver metastases, R0 resection was attempted based on preoperative imaging, but only an R1 resection could be achieved. However, this fact is not considered to indicate failure since elevated hormone levels dropped to the normal range postoperatively. In addition, according to ENETS guidelines, debulking surgery can be considered in rf-pNEN if at least 80% of the gross tumour is thought to be resectable5,43. The type of surgery for rf-pNENs depends on the ___location of the primary tumour. Because of the usually large rf-pNEN size and high prevalence of liver metastases, curative surgery usually requires formal pancreatic resection with lymphadenectomy2,5,43. This was performed in 10 of 11 patients in the present series, as well as in the majority of patients in other series11,37. Even for small rf-pNEN, parenchyma-sparing resection, such as enucleation, might be insufficient. One of our patients had a robotic-assisted enucleation of 12 mm-sized glucagonoma out of the pancreatic head with negative lymph node sampling and developed local recurrence six months later. Consequently, the patient underwent partial pancreaticoduodenectomy, which has resulted in a disease-free survival of 90 months so far.
Five of the six patients with recurrent disease and one patient with an unresectable VIPoma underwent several cycles of multimodal treatment, including redo surgery, somatostatin analogues, chemotherapy, targeted therapy with sunitinib, local ablative therapies with TACE and/or radiofrequency ablation and PRRT (see Table 5). Although more therapies have become available for pNENs during the last decade, including targeted therapies with e.g. sunitinib, new chemotherapy regimens (e–g. Tem/Cap), and PRRT, no significant data has yet been compiled on the oncological outcomes in patients with rf-pNEN. Thus, the discussion of the possible advantages and disadvantages of individual treatments and their sequence is somewhat vague and is beyond the scope of this article. However, somatostatin analogues were used as the mainstay treatment in several other series11,32,37,40 when curative surgery was not an option. They can control symptoms caused by excessive hormone secretion and prolong progression-free survival45. This was the case in six of seven patients with recurrent or persistent disease in our cohort. Since the majority of rf-pNEN somatostatin receptor-positive grade 1/2 tumours, 8 of 9 investigated in the presented series, PRRT is currently a very good option to control symptoms of hormonal excess as well as tumour growth progression11,46,47. In a previous series, PRRT was the most efficacious second-line treatment in patients with VIPoma who had refractory WDHA syndrome despite receiving the maximum doses of SSA32. It is also noteworthy that repeated surgery for disease recurrence should always be considered in the multimodal concept. One of our patients with a 22cm sized VIPoma underwent—embedded in SSA treatment, chemotherapy, and PRRT—a total of seven reoperations, which resulted in a survival of over 20 years, as summarised in Fig. 1.
Since the treatment armamentarium is complex, all patients with advanced rf-pNENs should be discussed in a multidisciplinary tumour board, ideally in centres of excellence.
In the present study, the eight patients with potentially curative surgery had a five-year survival of 63%, which is similar to that reported by others8,34,44,48,49.
The study from Azizian et al. showed that patients who underwent surgery had a longer overall survival than patients who were treated with other therapeutic modalities (44 vs. 33 months)11. Five (42%) patients remained free of disease for a median of 74 months after initial surgery. This outcome is similar to that of previous studies of rf-pNENs ranging from 15 to 180 months39,44,50,51.
A study by Sakurai et al. reported a disease-free interval after an initial surgery of 180 months. After a 180-month disease-free interval, this patient underwent a second curative surgery for the locally recurrent VIPoma. The patient is alive with no relapse 14 months after the second surgery51. These cases demonstrate the importance of long-term observation of patients.
The study from Murakami et al. showed, that the median survival time for patients with VIPoma was 71 months33. In the present study, the three patients with VIPoma showed a five-year tumour survival of 33% and a median survival time of 100 months.
It is crucial to distinguish between cases in which all metastases and the primary tumour are resectable and cases in which complete resection of all lesions is not possible (debulking surgery). The survival of the palliatively operated three patients in the present study was 48, 49, and 52 months. Brugel et al. showed in a retrospective analysis of four cases, a debulking surgery with palliative intent. The median progression-free survival was 21 months34.
It should be mentioned that calcitonin producing pNENs might be less aggressive than VIPoma or glucagonoma since according to a recent study of 25 calcitonin producing pNENs, 60% of patients were alive with no evidence of disease and 20% survived with disease after five years of follow-up14. This tendency can be confirmed by the present study.
The present study has three major limitations. First, the collective size is very small, as is usual for these very rare tumour entities. Second, the retrospective design implies bias and missing data. _ Considering the large variety of therapeutic options and the small number of patients, prospective registration in international databases (e.g., the ENETS-Database) is required to better understand the characteristics and outcomes of these extremely rare tumours.
Conclusion
In conclusion, rf-pNENs are a heterogeneous group of tumours with a good long-term prognosis, if initially radically resected. Long-term survival can even be achieved in metastasised tumours using multimodal treatment. As these tumours are extremely rare, treatment in expert centres is recommended. Through a comprehensive analysis of these extremely rare tumours, valuable insights into the management of these tumours are provided.
Data availability
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants but are available from one of the authors [M.S.] upon reasonable request.
References
O’Toole, D. et al. Frascati consensus conference; European neuroendocrine tumor society Rare functioning pancreatic endocrine tumors. Neuroendocrinology. 84, 189–195 (2006).
Jensen, R. T. et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 95, 98–119 (2012).
Falconi, M. et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 103, 153–171 (2016).
de Herder, W. W., Hofland, J. Glucagon & Glucagonoma Syndrome in Endotext (ed. Feingold K. R., et al.) (MDText.com, 2013).
Hofland, J. et al. European neuroendocrine tumor society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. Neuroendocrinology. 35, 13318. https://doi.org/10.1111/jne.13318 (2023).
Halfdanarson, T. R., Rabe, K. G., Rubin, J. & Petersen, G. M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann Oncol. 19, 1727–1733 (2008).
Soga, J. & Yakuwa, Y. Glucagonomas/diabetico-dermatogenic syndrome (DDS): A statistical evaluation of 407 reported cases. J. Hepatobiliary Pancreat. Surg. 5, 312–319 (1998).
Eldor, R. et al. Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin. Endocrinol. (Oxf). 74, 593–598 (2011).
Wei, J. et al. Glucagonoma and glucagonoma syndrome: one center’s experience of six cases. J. Pancreat. Cancer. 4, 11–16 (2018).
Song, X. et al. Glucagonoma and the glucagonoma syndrome. Oncol Lett. 15, 2749–2755 (2018).
Azizian, A., König, A. & Ghadimi, M. Treatment options of metastatic and nonmetastatic VIPoma: A review. Langenbecks Arch Surg. 407, 2629–2636 (2022).
Ichimura, T., Kondo, S., Okushiba, S., Morikawa, T. & Katoh, H. A calcitonin and vasoactive intestinal peptide-producing pancreatic endocrine tumor associated with the WDHA syndrome. Int. J. Gastrointest. Cancer. 33, 99–102 (2003).
Nguyen, H. N. et al. Long-term survival after diagnosis of hepatic metastatic VIPoma: Report of two cases with disparate courses and review of therapeutic options. Dig Dis. Sci. 44, 1148–1155 (1999).
Uccella, S. et al. Calcitonin-Producing Neuroendocrine Neoplasms of the Pancreas: Clinicopathological Study of 25 Cases and Review of the Literature. Endocr. Pathol. 28, 351–361 (2018).
Döring, C. et al. Whole-exome sequencing of calcitonin-producing pancreatic neuroendocrine neoplasms indicates a unique molecular signature. Front. Oncol. 13, 1160921. https://doi.org/10.3389/fonc.2023.1160921 (2023).
Fountas, A. et al. Cushing’s syndrome due to CRH and ACTH Co-secreting pancreatic tumor-presentation of a new case focusing on diagnostic pitfalls. Endocr Pathol. 26, 239–242 (2015).
Maragliano, R. et al. ACTH-secreting pancreatic neoplasms associated with Cushing syndrome: Clinicopathologic study of 11 cases and review of the literature. Am. J. Surg. Pathol. 39, 374–382 (2015).
Al-Toubah, T., Pelle, E., Hallanger-Johnson, J., Haider, M. & Strosberg, J. ACTH-secreting pancreatic neuroendocrine neoplasms: A case-series. J Neuroendocrinol. 35, 13336. https://doi.org/10.1111/jne.13336 (2023).
de Herder, W.W., Hofland, J. Somatostatinoma in Endotext (ed. Feingold K. R., et al.) (MDText.com, 2023).
Elangovan, A., Zulfiqar, H. Somatostatinoma in StatPearls [Internet] (ed. Smith, R.) (StatPearls Publishing, 2023).
Becker, S. W., Kahn, D. & Rothman, S. Cutaneous manifestations of internal malignant tumors. Arch. Dermatol. Syphilol. 45, 1069–1080 (1942).
Kindmark, H. et al. Endocrine pancreatic tumors with glucagon hypersecretion: a retrospective study of 23 cases during 20 years. Med Oncol. 24, 330–337 (2007).
Verner, J. V. & Morrison, A. B. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am. J. Med. 25, 374–380 (1958).
Viola, D. & Elisei, R. Management of medullary thyroid cancer. Endocrinol Metab Clin North Am. 48, 285–301 (2019).
Schneider, R. et al. Calcitonin-secreting pancreatic endocrine tumors: systematic analysis of a rare tumor entity. Pancreas. 40, 213–221 (2011).
Perren, A. et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pathology: Diagnosis and prognostic stratification. Neuroendocrinology. 105, 196–200 (2017).
Klöppel, G. Neuroendocrine neoplasms: Dichotomy Origin and Classifications. Visc. Med. 33, 324–330 (2017).
Rindi, G. et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 33, 115–154 (2022).
Pezzilli, R. et al. Ki-67 prognostic and therapeutic decision driven marker for pancreatic neuroendocrine neoplasms (PNENs): A systematic review. Adv. Med. Sci. 61, 147–153 (2016).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213 (2004).
Chong, E. et al. Systematic review and meta-analysis of risk factors of postoperative pancreatic fistula after distal pancreatectomy in the era of 2016 International Study Group pancreatic fistula definition. HPB (Oxford). 23, 1139–1151 (2021).
Angelousi, A. et al. Diagnostic and management challenges in vasoactive intestinal peptide secreting tumors: A series of 15 patients. Pancreas. 48, 934–942 (2019).
Murakami, M. et al. A clinical analysis on functioning pancreatic neuroendocrine tumors (focusing on VIPomas): A single-center experience. Endocr. J. 69, 1201–1209 (2022).
Brugel, M. et al. Groupe d’Étude des tumeurs endocrines (GTE) efficacy of treatments for VIPoma: A GTE multicentric series. Pancreatology. 21, 1531–1539 (2021).
Rodríguez, G., Vargas, E., Abaúnza, C. & Cáceres, S. Necrolytic migratory erythema and pancreatic glucagonoma. Biomedica. 36, 176–181 (2016).
John, A. M. & Schwartz, R. A. Glucagonoma syndrome: A review and update on treatment. J. Eur. Acad. Dermatol. Venereol. 30, 2016–2022 (2016).
Nikou, G. C. et al. VIPomas: An update in diagnosis and management in a series of 11 patients. Hepato-gastroenterol. 52, 1259–1265 (2005).
Kann, P. H. Is endoscopic ultrasonography more sensitive than magnetic resonance imaging in detecting and localizing pancreatic neuroendocrine tumors?. Rev. Endocr. Metab. Disord. 19, 133–137 (2018).
Schizas, D. et al. Clinicopathological data and treatment modalities for pancreatic vipomas: A systematic review. J. BUON. 24, 415–423 (2019).
Giampatzis, V. et al. Pancreatic vasoactive intestinal peptide-producing tumor as a rare cause of acute diarrhea and severe hypokalemia. J Med Cases. 14, 307–316 (2023).
Calabrò, D., Argalia, G. & Ambrosini, V. Role of PET/CT and therapy management of pancreatic neuroendocrine tumors. Diagnostics (Basel). 10, 1059 (2020).
Magi, L. et al. Role of [18F]FDG PET/CT in the management of G1 gastro-entero-pancreatic neuroendocrine tumors. Endocrine. 76, 484–490 (2022).
Partelli, S. et al. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br. J. Surg. 104, 34–41 (2017).
Smith, S. L. et al. Vasoactive intestinal polypeptide secreting islet cell tumors: A 15-year experience and review of the literature. Surgery. 124, 1050–1055 (1998).
Caplin, M. E. et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl. J. Med. 371, 224–233 (2014).
Camus, B. et al. Indications of peptide receptor radionuclide therapy (PRRT) in gastroenteropancreatic and pulmonary neuroendocrine tumors: An updated review. J. Clin. Med. 10, 1267 (2021).
Zandee, W. T. et al. Symptomatic and radiological response to 177Lu-DOTATATE for the treatment of functioning pancreatic neuroendocrine tumors. J. Clin. Endocrinol. Metab. 104, 1336–1344 (2019).
Câmara-de-Souza, A. B. et al. Insulinoma: A retrospective study analyzing the differences between benign and malignant tumors. Pancreatology 18, 298–303 (2018).
Soga, J. & Yakuwa, Y. Vipoma/diarrheogenic syndrome: a statistical evaluation of 241 reported cases. J Exp Clin Cancer Res. 17, 389–400 (1998).
Ghaferi, A. A., Chojnacki, K. A., Long, W. D., Cameron, J. L. & Yeo, C. J. Pancreatic VIPomas: Subject review and one institutional experience. J Gastrointest Surg. 12, 382–393 (2008).
Sakurai, M. et al. A case of vasoactive intestinal peptide-secreting tumor (VIPoma) arising from MEN1 inactivation which recurred 15 years after the initial resection. Endocr. J. 70, 573–579 (2023).
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by Open Access funding provided by the Open Access Publication Fund of the Philipps University Marburg, Germany with support of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.K.B., M.B.A..; methodology and software: M.S., collecting patient data: M.S., J.M., M.B.A., D.W., D.K.B, writing-original draft preparation: M.S., M.B.A.; review and editing: D.K.B., M.B.A., J.M., M.J., A.R., D.W.; supervision: D.K.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albers, M.B., Sevcik, M., Wiese, D. et al. Characteristics, therapy, and outcome of rare functioning pancreatic neuroendocrine neoplasms. Sci Rep 14, 18507 (2024). https://doi.org/10.1038/s41598-024-68290-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68290-1