Abstract
Abdominal adhesions manifests following abdominal infections triggered by intestinal fistulas. The severity of such adhesions depends on the extent of fiber deposition and peritoneal fibrinolysis following peritoneal injury, which may be influenced by sustained inflammation within the abdominal cavity. In this regard, the visceral-to-subcutaneous fat area (VFA/SFA) ratio has been implicated as a potential marker of inflammation. This study aimed to explore the relationship between VFA/SFA and abdominal adhesions. This multicenter study was conducted across four tertiary institutions and involved patients who had undergone definitive surgery (DS) for intestinal fistula from January 2009 and October 2023. The presence of abdominal adhesions was determined intraoperatively. VFA/SFA was investigated as a potential risk factor for severe adhesions. The study comprised 414 patients with a median age of 50 [interquartile range (IQR) 35–66] years and a median body mass index of 20.0 (IQR 19.2–22.4) kg/m2, including 231 males with a median VFA/SFA of 1.0 (IQR 0.7–1.2) and 183 females a median VFA/SFA of 0.8 (0.6–1.1). VFA/SFA was associated with severe abdominal adhesions in males [odds ratio (OR) = 3.34, 95% CI 1.14–9.80, p = 0.03] and females (OR = 2.99, 95% CI 1.05–8.53, p = 0.04). J-shaped association between VFA/SFA ratio and severe adhesions was revealed in both sex. The increasing trend can be revealed when OR more than 0.8, and 0.6 in males and females respectively. Preoperative VFA/SFA demonstrates predictive value for statues of severe abdominal adhesions in DS for anastomotic fistula after small intestine resection.
Similar content being viewed by others
Introduction
In patients presenting with postoperative small intestinal leakage, the spillover of intestinal fluid can induce chemical and biological damage to the peritoneum1,2,3. This injury prompts a local inflammatory response, characterized by the exudation and precipitation of serous exudate fibers1,2,3, potentially leading to the formation of abdominal adhesions. Particularly in emergency laparotomies, this phenomenon is observed in an increased rate4. These adhesions play a dual role. On one hand, they can inhibit the further spread of intestinal fluid within the abdominal cavity and foster sinus formation, ultimately culminating in fistula. On the other hand, abdominal adhesions can increase the complexity of definitive surgery (DS) for the fistula. Particularly in the presence of severe abdominal adhesions, an increased incidence of postoperative complications such as recurrent fistula, sepsis, and postoperative ileus has been documented5,6.
The existing theory suggests that the peritoneal fibrinolytic system becomes activated following adhesion formation, serving as the primary agent for their dissolution3,7. Thus, the final degree of adhesion reflects a balance between peritoneal fibrinolysis and fiber formation8. Concurrently, the fibrinolysis process is believed to be impeded under chronic inflammatory conditions, which could negatively affect adhesion dissolution9,10. The presence of preoperative adhesions can be determined via diagnostic tools such as ultrasound, magnetic resonance imaging (MRI), and computer tomography (CT) scans11,12,13. However, these imaging techniques often fail to accurately assess the severity of adhesions. Notably, the visceral fat area (VFA) has been associated with chronic inflammation14, whereas the subcutaneous fat area (SFA) is believed to exhibit protective properties against inflammation15. Accordingly, we hypothesize that there is a relationship between VFA/SFA and severe abdominal adhesions in DS for anastomotic fistula after small intestine resection (SIR), in an attempt to provide alternative diagnostic perspectives for the relevant patient population in the future.
Materials and methods
This was a retrospective cohort study conducted at four major centers that specialize in the treatment of enterocutaneous fistula (ECF). All procedures were performed in accordance with the ethical principles of the Declaration of Helsinki and the STROCSS criteria16. All patients were referred from external hospitals to our centers for ECF treatment. All experimental protocols were approved by Ethics Committee of Jinling Hospital (2023DZKY029-02).
Patients
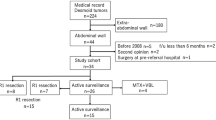
Consequently, the focus was on 995 non Inflammatory Bowel Disease patients undergone DS for anastomotic fistula after SIR between January 2009 and October 2023. The study excluded 8 individuals aged less than 18 years, 208 patients with enteroatmospheric fistula which artificially inflates the potential for abdominal adhesions, or 336 individuals with incomplete medical records (it was reasonable indicated that the abdominal contamination condition when SIR might also have influence on the subsequent abdominal adhesion, and most of the 336 individuals were excluded mainly due to the lack of the surgical records on the resection process, which reflecting the abdominal contamination). In addition, 29 patients who were considered to have severe adhesion before SIR according to their surgical records reporting that all the intestines could not be dissected were also excluded from the study. Thus, the final cohort consisted of 414 eligible (Supplementary Fig. 1). The digestive tract was gradually dissociated and abdominal adhesions were assessed during DS. For every DS, resection of the bowel segment with the ECF and latero-lateral anastomosis was performed using a linear stapler. Among these, 54 (13%) patients were diagnosed with severe adhesions.
Status of abdominal adhesions
Abdominal adhesion status was evaluated according to Hobson et al. during DS17. The abdominal adhesions were classified as follows: Grade I = no adhesions; Grade II = minimal adhesions localised to one or two areas; Grade III = diffuse but not extensive adhesions; Grade IV = diffuse extensive adhesions that are easily lysed; Grade V = diffuse extensive, dense adhesions that are difficult to lyse. Grades IV and V abdominal adhesions were defined as severe abdominal adhesions.
Preoperative management
The treatment of fistulas involved six aspects: sepsis control, optimization of nutritional status, wound care, determination of fistula anatomy, timing of surgery, and formulation of a surgical strategy18. The decision to proceed with DS was deferred until the patient’s condition had stabilized for at least a month and met specific criteria: a body mass index (BMI) of ≥ 18.0 kg/m2 coupled with normal physical strength, hemoglobin levels of ≥ 100 g/L, and albumin levels of ≥ 30 g/L. Furthermore, the interval since the occurrence of the fistula had to be a minimum of three months. During this process, some patients achieved spontaneous closure, and were not scheduled for surgery.
Evaluation of body composition
Enhanced CT scans were performed one week before DS as part of the preoperative assessment. VFA, SFA, and total abdominal muscle area index (TAMAI) were evaluated using abdominal CT scans and processed using Image J software (NIH, Bethesda, MD, USA) according to the different densities under CT scan19. For each patient, two consecutive axial CT images at the level of the inferior endplate of the L3 lumbar vertebra were selected and averaged. Sex-specific TAMAI cut-off values were used to define sarcopenia: 52.4 cm2/m2 for men and 38.5 cm2/m2 for women20.
Data collection, outcomes, and statistical analysis
The surgical record on the SIR was collected when admitted in our centers and recorded in the admission records. The degree of abdominal contamination is evaluated based on the amount of intestinal fluid/pus recorded during the resection. If there is no intestinal rupture, the intestinal fluid/pus is considered to be 0 ml. Patients underwent enhanced CT scans and gastrointestinal X-ray series one week before the DS for investigation of adjacent structures and the ___location of the fistula. Preoperative laboratory examination was conducted at least every four days throughout the treatment duration. Demographic data, preoperative laboratory examination results, ___location and output of fistula, as well as body composition data (TAMAI, VFA, SFA, etc.), were documented based on observations within the week before surgery. Etiology, the time interval between fistula occurrence and admission, the duration from fistula occurrence to DS, and comorbidities were all examined based on available records. The possibility of abdominal contamination during the early stages of abdominal infection was believed to be related to subsequent abdominal adhesions. Therefore, the characteristics of abdominal infections during the early stages of fistula development were investigated as potential risk factors. This included laboratory results, the presence of sepsis at admission, and the duration of abdominal infection. Sepsis was defined as per The Third International Consensus Definitions for Sepsis and Septic Shock21. The above mentioned variables were divided into seven parts (Supplementary Table 1): (1) demographic data, (2) fistula characteristics, (3) preoperative laboratory test results, (4) comorbidity, (5) body composition, (6) abdominal contamination during the SIR and (7) characteristics of abdominal infections in the early stage of fistula. We defined parts 1–5 as preoperative characteristics.
The primary outcome of the study was the degree of intraoperative adhesion. Three models were used to evaluate the influence of the VFA/SFA on adhesion formation. Model 1 involved adjusting the VFA/SFA with variables related to preoperative characteristics, excluding the ratio itself. In Model 2, adjustments were made to the VFA/SFA using characteristics related to abdominal contamination during the SIR and abdominal infections during the early stages of fistula development. In Model 3, the VFA/SFA was adjusted with variables from both Model 1 and Model 2. Univariate analysis was used to screen potential risk factors for severe adhesion. Subsequently, multivariate logistic regression was performed for each Model. The performance of each model was assessed using the area under the receiver operating characteristic curve analysis. The model with the highest Area Under Curve (AUC) was considered the most suitable.
To perform internal validation of the points system within the most suitable model, 500 bootstrap samples from the original sample were taken (random samples with replacement of the same number of elements as the original sample), and AUC values were calculated for each bootstrap sample. In the most suitable model, the association between VFA/SFA and severe abdominal adhesions was assessed using restricted cubic splines with three knots placed at the 10th, 50th, and 90th centiles. Statistical analysis included Mann–Whitney U tests for continuous variables and Fisher’s exact test for categorical variables. Statistical analyses were performed using SPSS version 26.0 for Windows (IBM, Armonk, NY, USA) and The R Project for Statistical Computing version 4.3.1.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All experimental protocols were approved by Ethics Committee of Jinling Hospital (2023DZKY02-0129).
Informed consent
Informed consent was waived by the ethics committee of Jinling Hospital.
Results
Clinical characteristics of the overall patients
The median age of 414 patients (231 males, 183 females) was 50 [interquartile range (IQR) 35–66] years, and the median BMI was 20.0 (IQR 19.2–22.4) kg/m2. The median interval from fistula occurrence to DS was 4 (IQR 3.0–5.0) months. The causes for the resection included rupture (n = 270, including 54 cases caused by trauma, 171 cases of rupture discovered during the postoperative period after abdominal surgery, 41 cases detected during the treatment of obstruction due to prior abdominal surgery, and 4 patients with unknown reason), obstruction due to prior abdominal surgery (n = 116), intestinal necrosis due to mesenteric thrombosis (n = 28) (Table 1). The VFA, SFA, and VFA/SFA were 84.3 (IQR: 58.2–106.3) cm2, 93.2 (IQR: 74.7–109.2) cm2, and 0.88 (IQR: 0.62–1.21), respectively (Table 2). Abdominal infection was diagnosed on admission in each patient with a WBC count of 12.8 × 109 /L (IQR 10.3 × 109/L–15.4 × 109/L) and a CRP level of 57.6 (IQR 47.4–69.2) mg/L. The median duration from admission to infection control was 26 (IQR 17–33) days. In addition, 133 (32.1%) patients exhibited sepsis at admission (Table 3).
Population distribution of VFA/SFA
Out of the 414 patients, 235 had a VFA/SFA of < 1, 174 had a VFA/SFA of ≥ 1 but < 2, and 5 had a VFA/SFA of ≥ 2. The distribution of various VFA/SFA ratios among patients with normal BMI and those classified as overweight showed comparable proportions (Supplementary Fig. 2A, p = 0.36).
The proportion of patients with different VFA/SFA varied between genders (Supplementary Fig. 2B, p = 0.004). The proportion of patients with VFA/SFA < 1 was higher (p = 0.001), and correspondingly, the proportion of patients with VFA/SFA ≥ 1 but < 2 was lower (p = 0.001) in males.
Clinical characteristics between genders
Notably, differences were observed only in variables related to body composition (Table 2). Specifically, TAMAI [38.0 (IQR 33.3–47.5) cm2 vs. 28.2 (IQR 23.4–36.1) cm2; p < 0.001] and VFA/SFA [1.0 (IQR 0.7–1.2) vs. 0.8 (IQR 0.6–1.1); p < 0.001]were higher in males, whereas VFA/TAMAI [2.2 (IQR 1.5–2.8) vs. 2.8 (IQR 1.9–3.6); p < 0.001] and SFA [88.2 (IQR 72.2–99.1) cm2 vs. 103.4 (IQR 81.4–118.2) cm2; p < 0.001] were lower.
Univariate analysis of VFA/SFA for severe abdominal adhesions
The incidence of severe abdominal adhesions was 12.5% (n = 29) in males and 13.7% (n = 25) in females (p = 0.64). Among males, 86 cases of grade II, 118 cases of grade III,17 cases of grade IV, and 12 cases of grade V adhesions were observed. In females, the numbers were 64, 95, 15, and 10, respectively.
Univariate analysis of the VFA/SFA was performed by genders (Supplementary Table 2 and 3). In males with severe adhesions, the ratio was 1.2 (IQR 0.9–1.6), which was higher than that in patients without severe adhesions [0.9 (IQR 0.7–1.2), p = 0.01]. The AUC for VFA/SFA showed a predictive value of 0.66 [95% confidence interval (CI) 0.54–0.76; p = 0.01, Supplementary Fig. 3A] for severe adhesions in males. Similarly, in females, the VFA/SFA was higher in those with severe adhesions [1.0 (IQR 0.7–1.4) vs. 0.7 (IQR 0.5–1.1); p = 0.001]. The AUC for VFA/SFA in females was 0.68 (95% CI 0.58–0.78, p = 0.005; Supplementary Fig. 3B).
Association between VFA/SFA and severe abdominal adhesions
The VFA/SFA was included in the three predictive models for further analysis. The AUC values for each model are shown in Fig. 1A. Model 3 (AUC = 0.821) was considered the most suitable model, compared to Model 1 (AUC = 0.682, p = 0.02) and Model 2 (AUC = 0.745, p = 0.04). The VFA/SFA exhibited an odds ratio (OR) of 3.34 (95% CI 1.14–9.80, p = 0.03; Table 4). Bootstrap validation of Model 3 yielded consistent parameters, with an AUC of 0.812 (95% CI 0.791–0.824, Fig. 1B). In addition, when the VFA/SFA was excluded, the AUC of Model 3 decreased to 0.756 (95% CI 0.654–0.858), compared to the initial Model 3 (p = 0.04). A J-shaped association between VFA/SFA and severe adhesions was revealed (Fig. 2A). Using a median VFA/SFA of 1.0 as a reference, the probability of severe adhesions increased when the VFA/SFA was more than 1.0 (Fig. 2A). This trend persisted for patients with VFA/SFA between 0.8 and 1.0.
In females, Model 3 had a higher AUC (Fig. 1C, AUC = 0.805, 95% CI 0.734–0.876) compared to Model 1 (AUC = 0.681, p = 0.02) and Model 2 (AUC = 0.717, p = 0.04). In this model, the VFA/SFA was significantly associated with severe abdominal adhesions (OR = 2.99; 95% CI 1.05–8.53, p = 0.04; Table 5). When the VFA/SFA was excluded, the resultant AUC was 0.715 (95%CI: 0.618–0.812), which was lower than that of initial Model 3 (p = 0.04). Bootstrap validation of Model 3 yielded consistent parameters, with an AUC of 0.792 (95% CI 0.774–0.801; Fig. 1D). A J-shaped association between VFA/SFA and severe adhesions was revealed (Fig. 2B). Using a median VFA/SFA of 0.8 as reference, the probability of severe adhesions increased when the VFA/SFA was more than 0.8 (Fig. 2B). Furthermore, this trend persisted for patients with VFA/SFA between 0.6 and 0.8.
Discussion
This study investigated the predictive value of preoperative VFA/SFA on predicting severe abdominal adhesions in DS. Abdominal adhesions are recognized as an outcome of inflammatory reactions1,2,3,4,5,6. This condition is commonly initiated owing to the exposure of the basal membrane of the mesothelial layer following peritoneal injury, often resulting from surgical procedures, infection, or irritation1,2,3. This leads to the activation of acute inflammatory responses1,2,3.
During these inflammatory responses, an extensive amount of fibrous tissue is secreted and deposited as part of the tissue repair process3,7. If the injury persists, acute inflammation metamorphoses into chronic inflammation, wherein fibrin provides a matrix for invading fibroblasts and new blood vessels3. Concurrently, the peritoneal fibrinolytic system is activated, and timely dissolution of fibrin is essential for restoring the preoperative non-inflamed state8. Inflammatory mediators, such as cytokines and acute-phase proteins, can upregulate the expression of plasminogen activator inhibitor-1 (PAI-1), thereby suppressing fibrinolysis. Additionally, the inflammatory process can promote the deposition of fibrin, while also affecting the activity of plasminogen activators, such as tissue plasminogen activator (tPA) and urokinase type plasminogen activator (uPA), shifting the balance between fibrin formation and fibrinolysis22. Essentially, the process of peritoneal fibrinolysis is inhibited by persistent abdominal inflammation. Thus, in patients with fistula who have insufficient drainage, persistent damage to the peritoneum caused by intestinal fluid exacerbates chronic inflammation, leading to an increased likelihood of developing abdominal adhesions.
VFA is associated with metabolic diseases, chronic inflammation, and the production of proinflammatory cytokines such as tumor necrosis factor and interleukin-614. In contrast, SFA is typically linked to a low-grade inflammatory condition and a favorable metabolic profile of glucose and lipids15. Chronic inflammation exerts a greater influence on VFA than on SFA15. The relative distribution of body fat is considered more significant than VAT or SAT individually23. The VFA/SFA is proposed as a superior predictor of the impact of inflammation on postoperative outcomes in gastrointestinal surgery23, acute24 or chronic diseases25, and cancer26.
The observed association between VFA/SFA and abdominal adhesions in this study could be viewed as a consequence of persistent abdominal inflammation influencing fat distribution, thereby contributing to the exacerbation of abdominal adhesions. The occurrence of abdominal sepsis on admission could influence the formation of postoperative abdominal adhesions. This is because abdominal sepsis upon admission represents the severity of abdominal inflammation. In addition, the duration of abdominal infections represents the duration of inflammation. Thus, these two variables offer insights into the continuum of persistent abdominal inflammation from different angles.
Other individual inflammatory indicators at admission might be easily affected by treatment interventions (e.g., use of antibiotics and human serum albumin supplementation), and their relationship with infection severity and inflammatory indicators might not follow a linear pattern. Therefore, other indicators could be easily interfered with in predicting the degree of inflammation and subsequently, adhesion formation. Preoperative inflammatory indicators were also included and analyzed in this study.
Even after multivariate regression, albumin continued to show predictive value for adhesions. This suggests that preoperative inflammatory indicators, such as albumin, along with VFA/SFA ratio, have a predictive effect on adhesion formation. However, for patients with good preoperative preparation, traditional inflammatory indicators such as C-reactive protein (CRP) and white blood cell (WBC) have limited predictive value. This could be attributed to the fact that only a small number of patients presented with both normal WBC count and elevated CRP levels before undergoing DS, making it challenging to establish statistical significance.
In addition, preoperative inflammatory indicators lack specificity, and after several months of recovery before DS, they might even return to baseline normal levels. Therefore, we believe that relying only on individual predictors without combining other indicators may have little significance. However, it is important to note that our research was in preliminary stages in terms of detecting preoperative inflammation, and the absence of data on certain inflammatory factors such as Interleukin- 6 and Tumor necrosis factor—α is a limitation. We intend to address this issue in future research.
The study revealed that the VFA/SFA could influence abdominal adhesions across different genders. Distinct patterns of body fat distribution are evident between men and women27. Males typically exhibit higher VFA levels27, which are readily influenced by inflammation or hormones27. The present study suggests that the impact of chronic inflammation on VFA appears to be similar across genders. SFA, in contrast, is more stable28, and tends to be more abundant in females than in males26. Accordingly, in the present study, females had higher SFA, leading to a gender discrepancy in VFA/SFA ratios. Both male and female participants showed the significance of the VFA/SFA in predicting abdominal adhesions. Notably, the cutoff value was lower in females, mirroring the results of other studies related to chronic diseases27,29,30.
This study has some limitations. First, potential biases might stem from retrospective design and the relatively small sample size. However, given that cases of small intestinal fistula with extensive abdominal adhesions are relatively rare, the number of patients enrolled in this study may be adequate. Second, all patients had previously undergone emergency SIR at other hospitals due to rupture. As such, obtaining accurate information about the abdominal condition during such emergency surgeries proved difficult. The degree of contamination from these emergencies might have influenced the formation of abdominal adhesions after surgery, possibly affecting the research findings. However, this potential contamination might be reflected through the presence of sepsis on admission or the duration of abdominal infections after admission. In addition, deciliter of intestinal fluid/pus in overall patients reflecting the contamination was included for analysis, as well. Consequently, even patients with severe contamination and adhesion during emergency surgery could still be assessed using the VFA/SFA ratio. Third, differing viewpoints may exist, such as the argument that even if abdominal adhesions can be determined, they cannot affect the surgical process and do not have a predictive effect on prognosis. However, determining abdominal adhesions can make surgeons more alert and design potential bail-out procedures or even refer the patient to a more specialized center. Another limitation is that the immunologic response differs among each individual, and this is an important confounding factor for adhesion formation that is difficult to assess. As a result, future research should adopt a prospective design with a larger sample size to mitigate potential biases and enhance the robustness of findings. Additionally, future in-depth laboratory research on the immunologic response following abdominal infections is needed. Finally, developing standardized protocols for assessing and managing abdominal adhesions could validate the predictive utility of the VFA/SFA ratio and its impact on surgical outcomes and prognosis.
Conclusion
The preoperative VFA/SFA demonstrates predictive value for status of severe abdominal adhesions in definitive surgeries for anastomotic fistula after SIR. This ratio could serve as a predictive index for severe adhesions, aiding preoperative assessments and surgical planning. Implementing the VFA/SFA ratio in routine evaluations may help identify high-risk patients, optimize preoperative management, and improve surgical outcomes. Future research should validate this index across diverse populations and explore interventions to reduce VFA and adhesion severity, enhancing clinical utility.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CT:
-
Computer tomography
- DS:
-
Definitive surgery
- ECF:
-
Enterocutaneous fistula
- IQR:
-
Interquartile range
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- PN:
-
Parenteral nutrition
- SFA:
-
Subcutaneous fat area
- SIR:
-
Small intestine resection
- TAMAI:
-
Total abdominal muscle area index
- VFA:
-
Visceral fat area
- WBC:
-
White blood cell
References
Capobianco, A., Cottone, L., Monno, A., Manfredi, A. A. & Rovere-Querini, P. The peritoneum: Healing, immunity, and diseases. J. Pathol. 243(2), 137–147 (2017).
van Baal, J. O. et al. The histophysiology and pathophysiology of the peritoneum. Tissue Cell. 49(1), 95–105 (2017).
Hellebrekers, B. W. & Kooistra, T. Pathogenesis of postoperative adhesion formation. Br. J. Surg. 98(11), 1503–1516 (2011).
Lasithiotakis, K. et al. The Hellenic Emergency Laparotomy Study (HELAS): A prospective multicentre study on the outcomes of emergency laparotomy in Greece. World J. Surg. 47(1), 130–139 (2023).
Tian, W. et al. Chyme reinfusion reducing the postoperative complications after definitive surgery for small intestinal enteroatmospheric fistula: A cohort study. Front. Nutr. 9, 708534 (2022).
Tian, W., Yan, M., Xu, X., Yao, Z. & Zhao, R. Risk factors and outcomes for postoperative ileus after small intestinal fistula excision in patients with diffuse extensive abdominal adhesions. Front. Surg. 8, 632241 (2021).
Vipond, M. N., Whawell, S. A., Thompson, J. N. & Dudley, H. A. Peritoneal fibrinolytic activity and intra-abdominal adhesions. Lancet. 335(8698), 1120–1122 (1990).
ten Broek, R. P. et al. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ. 347, f5588 (2013).
Levi, M. & van der Poll, T. Inflammation and coagulation. Crit. Care Med. 38(2 Suppl), S26–S34 (2010).
Koutroubakis, I. E. The relationship between coagulation state and inflammatory bowel disease: Current understanding and clinical implications. Expert Rev. Clin. Immunol. 11(4), 479–488 (2015).
Lang, R. A. et al. Cine-MRI detection of intraabdominal adhesions: Correlation with intraoperative findings in 89 consecutive cases. Surg. Endosc. 22(11), 2455–2461 (2008).
Fenner, J. et al. Towards radiological diagnosis of abdominal adhesions based on motion signatures derived from sequences of cine-MRI images. Phys. Med. 30(4), 437–447 (2014).
Gerner-Rasmussen, J., Donatsky, A. M. & Bjerrum, F. The role of non-invasive imaging techniques in detecting intra-abdominal adhesions: A systematic review. Langenbecks Arch. Surg. 404(6), 653–661 (2019).
Landskron, G., De la Fuente, M., Thuwajit, P., Thuwajit, C. & Hermoso, M. A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 149185 (2014).
Kim, J. M. et al. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin. Nutr. 40(11), 5631–5638 (2021).
Mathew, G., Agha, R., For the STROCSS Group. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case–control studies in Surgery. Int. J. Surg. 2021(96), 106165 (2021).
Hobson, K. G. et al. Expression of transforming growth factor beta1 in patients with and without previous abdominal surgery. Arch. Surg. 138(11), 1249–1252 (2003).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9(7), 671–675. https://doi.org/10.1038/nmeth.2089 (2012).
Visschers, R. G., Olde Damink, S. W., Winkens, B., Soeters, P. B. & van Gemert, W. G. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J. Surg. 32(3), 445–453 (2008).
Pecorelli, N. et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br. J. Surg. 103(4), 434–442 (2016).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Tang, J., Xiang, Z., Bernards, M. T. & Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 116, 84–104 (2020).
Bocca, G., Mastoridis, S., Yeung, T., James, D. R. C. & Cunningham, C. Visceral-to-subcutaneous fat ratio exhibits strongest association with early post-operative outcomes in patients undergoing surgery for advanced rectal cancer. Int. J. Colorectal Dis. 37(8), 1893–1900 (2022).
Pisitsak, C. et al. Increased ratio of visceral to subcutaneous adipose tissue in septic patients is associated with adverse outcome. Crit. Care Med. 44(11), 1966–1973 (2016).
Jung, C. H. et al. Visceral-to-subcutaneous abdominal fat ratio is associated with nonalcoholic fatty liver disease and liver fibrosis. Endocrinol. Metab. (Seoul) 35(1), 165–176 (2020).
Wada, M. et al. Visceral-to-subcutaneous fat ratio is a possible prognostic factor for type 1 endometrial cancer. Int. J. Clin. Oncol. 27(2), 434–440 (2022).
White, U. A. & Tchoukalova, Y. D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta 1842(3), 377–392 (2014).
Kim, E. H. et al. Sex differences of visceral fat area and visceral-to-subcutaneous fat ratio for the risk of incident type 2 diabetes mellitus. Diabetes Metab. J. 46(3), 486–498 (2022).
MacCannell, A. D. V. et al. Sexual dimorphism in adipose tissue mitochondrial function and metabolic flexibility in obesity. Int. J. Obes. (Lond). 45(8), 1773–1781 (2021).
Delaney, K. Z. & Santosa, S. Sex differences in regional adipose tissue depots pose different threats for the development of type 2 diabetes in males and females. Obes. Rev. 23(3), e13393 (2022).
Acknowledgements
We would like to thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
Data curation, Weiliang Tian, Yunzhao Zhao and Wuhan Li; Formal analysis, Tao Tian and Zheng Yao; Investigation, Weiliang Tian, Guoping Zhao, Tao Tian, Wuhan Li and Zheng Yao; Methodology, Yunzhao Zhao, Guoping Zhao, Tao Tian and Zheng Yao; Project administration, Zheng Yao; Resources, Risheng Zhao, Weiliang Tian and Tao Tian; Software, Guoping Zhao and Zheng Yao; Supervision, Qianhuang, Risheng Zhao and Guoping Zhao; Visualization, Risheng Zhao; Writing—original draft, Shikun Luo, Fan Yang, and Weiliang Tian. Shikun Luo, Fan Yang, and Weiliang Tian were the first authors. Guoping Zhao, Zheng Yao, Qian Huang and Risheng Zhao were the corresponding author.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, F., Tian, W., Luo, S. et al. Visceral to subcutaneous fat area ratio predicts severe abdominal adhesions in definitive surgery for anastomotic fistula after small intestine resection. Sci Rep 14, 19063 (2024). https://doi.org/10.1038/s41598-024-69379-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69379-3