Abstract
Ecological water replenishment is an important measure for conserving water sources and improving the water environment. To explore the evolution and causes of groundwater chemistry after ecological water replenishment in the Jialu River, this study utilized groundwater monitoring data from 2015 to 2019 following ecological water replenishment. Various methods, including Piper’s trilinear diagram, Gibbs diagram, principal component analysis, and ion ratio analysis, were employed for research purposes. The results indicate that (1) since the implementation of ecological water replenishment in the Jialu River, there has been a general downwards trend in total dissolved solids (TDS) in groundwater. The dominant cation in groundwater is Ca2+, whereas HCO3− is the dominant anion. The concentration of cations in groundwater has generally decreased, with noticeable reductions in SO42− and Cl− concentrations in the upper reaches of the recharge river contributing to improved groundwater quality. (2) A comparison with 2015 reveals a gradual transition at sampling points from chemical types such as HCO3-Ca·Mg and HCO3·Cl–Ca·Mg to an ecological water replenishment chemical type (HCO3-Ca).
Similar content being viewed by others
Introduction
The chemical evolution of groundwater is a complex process that is closely related to the hydrological circulation process1. Studying groundwater chemical characteristics and causes not only helps in understanding regional hydrogeological conditions but also provides support for the rational development, protection, and management of underground water resources. Domestic and foreign scholars often use methods such as the Piper three-line diagram2, Gibbs diagram method3, and ion ratio analysis method4 to study groundwater chemical characteristics and causes. Gao et al.5 used the Piper three-line diagram and other methods to analyse the hydrogeochemical characteristics and causes of underground water in the coastal Yantai Peninsula. The results revealed that the groundwater chemical type in the area can be divided into SO4·Cl–Ca·Mg and SO4·Cl–Na types, and the hydrogeochemical characteristics are affected by both natural and human activities. Wang et al.6 used the Piper three-line diagram, the main ion relationship diagram, and other methods to analyse the chemical characteristics and causes of groundwater in Shuangliao city. The results show that the groundwater chemical type in Shuangliao city is mainly the HCO3-Ca type, and the hydrogeochemical action in the groundwater recharge area is mainly weathering dissolution. Wang et al.7 used the Piper three-line diagram, Gibbs diagram, and other methods to analyse groundwater chemical genesis and influencing factors in the BaiCheng research area. The results show that the groundwater type in the recharge area is mainly the HCO3-Ca·Na type, and it gradually transforms to HCO3-Na-type water along the direction of water flow in the flow area. Zhou et al.8 used Piper’s three-line diagram, Gibbs diagram, etc., to study and analyse the groundwater chemical characteristics of the Hunshui River Basin in Qinghai Province to prevent the deterioration of groundwater chemistry and ensure the sustainable use of groundwater. The results revealed that the main water chemistry type in the Hunshui River Basin of Qinghai Province was HCO3-Ca·Mg and HCO3-SO4-Ca·Mg. Qu et al.9 reported that the groundwater in the northern part of the Ordos Basin was mainly Ca-HCO3 type, and the southern part was mainly Na + K−SO4 type, providing a basis for studying the water chemistry evolution of groundwater pollution caused by coal mining. Gao et al.10 collected 30 sets of groundwater samples from Longkou Bay and analysed the water chemical characteristics and genesis of the area. The results revealed that there were 18 types of groundwater chemistry in the area, and the chemical characteristics of the groundwater were affected mainly by rock weathering.
River ecological water replenishment is the most effective and convenient means to improve the river ecological environment and replenish groundwater sources. Owing to the significant differences in the main water chemical components between ecological water replenishment and local groundwater, the ecological water replenishment entering the aquifer causes changes in hydrochemical conditions, and, under different water‒rock interactions, the groundwater chemical components undergo corresponding changes. Cui et al.11 studied the impact of ecological water replenishment on the water chemical characteristics of the Baiyangdian Basin and reported that, before and after water replenishment, the shallow groundwater water chemistry type transitioned from the Na-HCO3 type to the Ca·Mg-HCO3 type, whereas the deep groundwater did not change. This study provides a scientific basis for the rational allocation of ecological water replenishment and the improvement of the ecological environment of Baiyangdian. Xiong12 and Li13 studied the replenishment of the Chaohe River and the Yongding River with water from the South-to-North Water Transfer Project and local reservoirs and reported that the replenishment process resulted in mixing and dilution effects. The concentrations of K+, Cl−, HCO3−, total hardness, nitrate nitrogen, nitrite nitrogen, and CODMn changed over time and generally showed a downwards trend, significantly improving the groundwater quality. They played a crucial role in the ecological restoration of Beijing’s western groundwater storage area. The influence of river ecological water replenishment on the chemical characteristics of groundwater has become a research hotspot in the field of hydrogeology, but there are currently few studies on the evolution laws and analysis of groundwater chemical characteristics after ecological water replenishment.
Some scholars have studied water environment problems in the study area, most of which have concentrated on the water environment carrying capacity of the Jialu River14 and the current water pollution situation15, and few systematic studies have focused on groundwater chemical characteristics and causes after river ecological water replenishment. Therefore, on the basis of water quality monitoring data from 72 groundwater samples collected from 2015 to 2019 in the Jialu River, Zhengzhou section, this paper uses geospatial analysis, Piper’s three-line diagram, the Stiff diagram, the Gibbs diagram, principal component analysis, ion ratio analysis, etc., to analyse the spatial‒temporal evolution of groundwater chemistry after the ecological water supply, and studies the causative mechanism of groundwater chemistry evolution under the ecological water supply. This research provides a basis for ecological protection in the Jialu River Basin.
Materials and methods
Study area
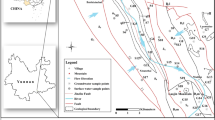
The position of the ecological replenishment section of the Jialu River in Zhengzhou city is shown in Fig. 1. With an average annual precipitation of 625.79 mm, the study area experiences a continental monsoon climate. To solve the problems of drying the Jialu River and water pollution, the government has been using the South-to-North Water Diversion Project to replenish the ecological water of the Jialu River regularly since 2015. The ecological replenishment section of the Jialu River in Zhengzhou starts from the water diversion outlet gate of Zhongyuan West Road in Zhengzhou city and ends at the boundary of the New District of Zhengdong, with an overall length of 39 km. The research ___domain includes three types of groundwater: water in the pore spaces of loose stones, clastic rock, and carbonate rock fractures (karst water). Among them, loose rock hole water is the most abundant, easily exploitable, thick, well-recharged, and readily available. In the research area, it serves as the primary water source for urban, agricultural, and industrial uses. The shallow groundwater in the study area flows from southwest to northeast, with an overall hydraulic gradient of 4.7–2.4%, which is compatible with the topography. The primary origin of the recharge of shallow groundwater in the study area is the penetration of atmospheric precipitation, but lateral recharge from the Yellow River also occurs in certain locations.
Ecological water replenishment section of the Jialu River in the urban area of Zhengzhou. Figure mainly describes the general situation of the research area of Jialu River ecological water replenishment in the Zhengzhou City section and the distribution of groundwater sampling points in the research area. Figure was created using ArcGIS [10.2]
Sample collection and testing
The data were obtained from research results and related data on groundwater monitoring materials and groundwater quality monitoring reports in Zhengzhou city from 2015 to 2019. From 2015 to 2019, an overall of 72 water samples were collected, and the water samples were collected from loose rock. Among them, 15 water samples were collected in 2015, 10 in 2016, 19 in 2017, 12 in 2018, and 16 in 2019. The water samples were collected mainly from urban greening and agricultural irrigation wells. The sampling points are presented in Fig. 1. (Fig. 1 was created using ArcGIS [10.2].)
Indicators of the chemical composition of water include Ca2+, K+, Na+, Mg2+, Cl−, HCO3−, SO42−, NO3−, CO32−16, total dissolved solids (TDS) and pH. According to the relevant specifications, the EDTA titration method was used for the determination of Ca2+ and Mg2+ concentrations17,18; the flame atomic absorption photometric method was used for the determination of Na+ and K+ concentrations19,20; an ICS-1100 ion chromatograph was used for the determination of Cl−, SO42−, and NO3− concentrations via the ion chromatography method21,22,23; the acid‒base titration method was used for the determination of HCO3− and CO32− concentrations24,25; the TDS concentration was measured with a Systronics conductivity TDS metre 30826; and the glass electrode method was used for the determination of the pH. The data in this article was sourced from the authoritative groundwater monitoring institution of Natural Resources Monitoring and Land Consolidation Institute in Henan Province.
Methodology
Inverse distance weighting
The inverse distance weight interpolation method uses the distance between the interpolation point and sample points as weights. The closer a sample point is to the interpolation point, the greater its assigned weight, with the weight contribution inversely proportional to the distance27. Using ArcGIS software, spatial distribution maps of the major components of water chemistry, such as K+, Ca2+, Na+, Mg2+, Cl−, HCO3−, SO42−, and TDS, in groundwater from 2015 to 2019 were drawn via the inverse distance weighting method28. The geographical and chronological distribution characteristics of the groundwater chemical components were analysed.
Piper trilinear diagram
The Piper graph is a graphical representation of groundwater chemistry characteristics29. It is made up of a diamond and two triangles. Each side of the diamond and triangles represents the milliequivalent percentage of all dissolved ions in the water sample, which intuitively shows the relative content of various ions and analyses the characteristics and evolution of groundwater chemistry30. In this research, the properties and development of groundwater chemistry following ecological water replenishment were investigated with a Piper trilinear graph.
Gibbs diagram
The Gibbs diagram uses the logarithm value of TDS as a coordinate and the ratio of cations Na+/(Na+ + Ca2+) or anions Cl−/(Cl− + HCO3−) as the abscissa31. It divides water into precipitation-controlled, weathering-controlled, and evaporation/crystallization-controlled types32,33, reflecting natural influencing factors such as atmospheric precipitation, rock weathering, evaporation/crystallization, and seawater intrusion. On the basis of ecological water replenishment and groundwater monitoring data, this study analysed the mechanism of formation of groundwater chemistry via a Gibbs diagram.
Principal component analysis
One statistical approach to reduce dimensionality is principal component analysis34. By extracting eigenvalues through principal component analysis, the correlations and influencing factors between different chemical components of groundwater can be identified, and the main sources35 and influencing factors of the components of groundwater chemistry after ecological water replenishment in the Jialu River can be determined.
Ion ratio analysis
According to Ma et al.36, ion ratio analysis can indicate the mineral dissolution types of ion sources and highlight the variables that affect the water chemistry components in groundwater. In this work, the ion ratio approach was used to investigate the mechanisms underlying the evolution of groundwater chemistry and to examine the origins and causes of different ions in groundwater.
Results
Statistical features of the chemical components of groundwater
The statistical characteristics of the primary chemical constituents of shallow groundwater in the field of investigation from 2015 to 2019 are shown in Table 1. Figures 2 and 3 depict the fluctuations in primary ion concentrations in groundwater. (Figs. 2 and 3 were created using Origin [2022].)
From 2015 to 2019, Ca2+ was the dominant cation in groundwater, with a growing trend interspersed with a declining trend. The dominant anion was HCO3−, which initially decreased but then increased. The coefficients of variation for Na+, Ca2+, Mg2+, Cl−, SO42−, and HCO3− were less than 1, indicating relatively small differences in their regional distributions. However, the coefficients of variation for K+ and NO3− were greater than 1, indicating relatively large differences in their regional distributions37. The average TDS values of groundwater from 2015 to 2019 showed a fluctuating downwards trend, with TDS2018 > TDS2015 > TDS2017 > TDS2016 > TDS2019 (TDS2018, TDS2015, TDS2017, TDS2016, and TDS2019 represent the average TDS contents in 2018, 2015, 2017, 2016, and 2019, respectively.) With the implementation of ecological water replenishment in 2015, the concentrations of calcium and magnesium ions, which are related to groundwater hardness, showed an overall decreasing trend. The concentrations of chloride and nitrate ions, as anions, also tended to decrease.
Features of the spatiotemporal evolution of the main ions and TDS
Characteristics of the spatial‒temporal evolution of essential ions
The main cations in the groundwater in the study area are K+, Na+, Ca2+, and Mg2+. From a temporal perspective, compared with 2015, the high-concentration area of K+ in groundwater gradually decreased in 2019, and the overall concentration of K+ showed a decreasing trend. (Fig. 4a,b,c,d,e) respectively represent the geographical distribution of the K+ concentration in the research area in 2015, 2016, 2017, 2018, and 2019.) From 2015 to 2019, the Na+ concentration in groundwater generally decreased, especially in the upper reaches of river water, where the Na+ concentration decreased significantly. (Fig. 5a,b,c,d,e) respectively represent the spatial distribution of the Na+ concentration in the research area in 2015, 2016, 2017, 2018, and 2019.) From 2015 to 2019, the Ca2+ concentration in groundwater showed an overall decreasing trend, with the low-concentration area of Ca2+ increasing and the high-concentration area decreasing. (Fig. 6a,b,c,d,e) respectively represent the geographical distribution of the Ca2+ concentration in the research area in 2015, 2016, 2017, 2018, and 2019.) From 2015 to 2019, the concentration of Mg2+ in groundwater generally decreased, but in the lower reaches of the river, the concentration of Mg2+ increased in some water samples. (Fig. 7a,b,c,d,e) respectively represent the spatial distribution of the Ca2+ concentration in the research area in 2015, 2016, 2017, 2018, and 2019.) (Figs. 4, 5, 6 and 7 were created using ArcGIS [10.2].)
Geographical distribution of the K+ concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of K+ in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of K+ in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
Spatial distribution of the Na+ concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of Na+ in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of Na+ in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
Geographical distribution of the Ca2+ concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of Ca2+ in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of Ca2+ in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
Spatial distribution of the Mg2+ concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of Mg2+ in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of Mg2+ in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
Geographically speaking, the central and lower sections of river water replenishment usually contain groundwater with a K+ concentration of more than 6–8 mg/L but are mainly below 2 mg/L in the upper reaches (Fig. 4). There was some spatial distribution in the Na+ concentration. In the upper reaches of the river, where water replenishment occurs, the concentration is often less than 30 mg/L, whereas in the lower parts of the river, the proportion is greater. Considering the local hydrogeological conditions and field surveys, the depth of groundwater near the upper reaches of the Jialu River is greater than that of the lower reaches, evaporation is weaker, salt accumulation is relatively lower, and groundwater flows from the southwest (higher reaches of the Jialu River) to the northeast (lower sections of the Jialu River) (Fig. 5). Uneven spatial fluctuations were observed in the Ca2+ concentration in groundwater, with higher concentrations on the right bank of the Jialu River than on its left bank, particularly in 2016 and 2018 (Fig. 6). The increase in Ca2+ concentration is generally caused by the discharge of domestic sewage and industrial wastewater and the dissolution of gypsum to produce [SO42−] and [Ca2+ + Mg2+]. However, according to hydrogeological conditions and field studies, there is no large amount of gypsum minerals in the aquifer in the study area, and the concentrations of Mg2+ and SO42− also do not show zonal distribution (Fig. 7, Fig. 9). Additionally, the urban development areas and human activities are distributed mainly on the right bank of the Jialu River in the city of Zhengzhou. Therefore, the abnormal distribution of Ca2+ concentrations is likely due to human activities (Fig. 6). The Mg2+ concentration in groundwater near the upper reaches of the Jialu River is lower than that in the middle and lower reaches (Fig. 7), similar to the variation characteristics of Na+ and K+ concentrations, which are related to the flow conditions and groundwater levels (Fig. 7d,e).
In summary, since the ecological replenishment of Jialu River water, the cation concentrations in the surrounding groundwater have shown an overall decreasing trend between 2015 and 2019. Good-quality ecologically replenished water enters the river and infiltrates shallow groundwater, improving groundwater quality through dilution and mixing.
The Cl− concentration of groundwater in different years exhibited different spatial distribution characteristics. The upper reach of the river recharge area was home to most of the low-value groundwater Cl− area in 2019, whereas before 2019, it was irregularly distributed. The Cl concentration of groundwater from 2015 to 2019 generally tended to first increase but then decrease over time. (Fig. 8a,b,c,d,e) respectively represent the spatial distribution of the Cl− concentration in the research area in 2015, 2016, 2017, 2018, and 2019. Figure 8 was created using ArcGIS [10.2].)
Spatial distribution of the Cl− concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of Cl− in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of Cl− in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
The spatial distribution of the SO42− concentration was uneven, and the high-value area changed from the upper reach to the lower reach between 2015 and 2019. In 2019, a segmented distribution pattern similar to that of Cl− was observed. Generally, the SO42− concentration in groundwater near the upper reach of a river gradually decreases, whereas in the lower reach, it remains stable or tends to increase. (Fig. 9a,b,c,d,e) respectively represent the spatial distribution of the SO42− concentration in the research area in 2015, 2016, 2017, 2018, and 2019. Figure 9 was created using ArcGIS [10.2].)
Spatial distribution of the SO42− concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of SO42− in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of SO42− in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
The spatial distribution pattern of the HCO3− concentration is similar to that of the K+, Na+, and Mg2+ cations. The concentration is lower in the upper reach of the river and higher in the lower reach. The reasons are similar to those for cations such as K+, Na+, and Mg2+. The depth of groundwater near the upper Jialu River is greater than that of the lower Jialu River, and the evaporation is weaker, resulting in relatively less salt accumulation. In addition, the direction of groundwater flow is from the southwest (upper reach of the Jialu River) to the northeast (lower section of the Jialu River), and HCO3− accumulates continuously with the flow of groundwater. The HCO3− concentration varies irregularly over time, showing an overall trend of first decreasing and then increasing. (Fig. 10a,b,c,d,e) respectively represent the spatial distribution of the HCO3− concentration in the research area in 2015, 2016, 2017, 2018, and 2019. Figure 10 was created using ArcGIS [10.2].)
Spatial distribution of the HCO3- concentration in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of HCO3− in the research area from 2015 to 2019. (a), (b), (c), (d), and (e) respectively represent the spatial concentration changes of HCO3− in the research area in 2015, 2016, 2017, 2018, and 2019. Figure was created using ArcGIS [10.2].
In summary, the spatial distribution of the HCO3− concentration between the anions is similar to that of the K+, Mg2+, and Na+ cations. In the upper reach of the river, the Cl− and SO42− concentrations decreased markedly from 2015 to 2019, which was related to the regular ecological replenishment of the Jialu River. The supply of high-quality water to the shallow groundwater aquifer causes mixing and dilution effects, resulting in improved groundwater quality.
To more clearly show how the chemical makeup of groundwater evolved in the research area from 2015 to 2019, this paper plots a stiff diagram on the basis of the average concentration of major ions in the study region38 (Fig. 11). From the diagram, it can be visually observed that the Ca2+ and Mg2+ cations in groundwater show a fluctuating decreasing trend, whereas K+ and Na+ show fluctuating changes without a clear pattern. Among the anions, HCO3− and SO42− show fluctuating changes with no clear pattern, whereas Cl− shows a decreasing trend. (Fig. 11 was created using Origin [2022].)
Stiff diagram from 2015 to 2019. Fig. is a stiff diagram drawn based on the average concentration of major ions in groundwater in the research area, which is intended to more intuitively show the changes of chemical components of groundwater in the research area from 2015 to 2019. Figure was created using Origin [2022].
Characteristics of the spatiotemporal evolution of the TDS
The geographical dispersion of TDSs is shown in Fig. 12. (Fig. 12 was created using ArcGIS [10.2]. (a), (b), (c), (d), and (e) respectively represent the spatial distribution of TDS concentrations in the research area in 2015, 2016, 2017, 2018, and 2019.) On a spatial scale, most of the shallow groundwater in the upper course of the Jialu River has TDS levels lower than 600 mg/L, whereas in the middle reach, it ranges between 600 and 900 mg/L, and in the lower reach, it is mostly above 900 mg/L. The combination of vertical evaporation and groundwater runoff is the primary cause of this phenomenon. Salt accumulates continually along the direction of groundwater flow. Furthermore, according to regional hydrogeological conditions, the groundwater level is approximately 10 m deep in the lower Jialu River reach and 20 m deep in the upper reach. Evaporation intensifies salt accumulation, resulting in an increasing trend of TDS in groundwater along the river flow direction. From 2015 to 2019, the TDS of the surrounding groundwater in the upper Jialu River range showed a decreasing trend, whereas the TDS in the lower range remained stable or increased. The possible reason for this is that groundwater in the upper reaches receives more ecological replenishment from the river and is affected by the combined effect of groundwater runoff in the southwest to northeast direction.
Spatial distribution of TDS concentrations in the research area from 2015 to 2019. Figure mainly describes the spatial concentration changes of TDS in the research area from 2015 to 2019. (a), (b), (c), (d) and (e) respectively represent the spatial concentration changes of TDS in the research area in 2015, 2016, 2017, 2018 and 2019. Figure was created using ArcGIS [10.2].
Discussion
Changes in groundwater chemical types
Figure 13 shows the Piper graph of groundwater samples from different years between 2015 and 2019. According to the findings of the study, most of the cations in the water samples are dominated by Ca2+ and most of the anions are dominated by HCO3−. The main chemical types are HCO3-Ca·Mg and HCO3·Cl–Ca·Mg. The chemical type of the ecological replenishment water is HCO3-Ca. An analysis of the 2015 anomaly points that fell in Zone 2 of the cation plot in the diagram reveals a clear influence of human activities (field investigations revealed that these samples came from wells used for domestic purposes), with relatively high Na+ concentrations. (Fig. 13 was created using Origin [2022].)
Compared with Fig. 14(a,b,c) with 2015, in 2016, 2017, 2018, and 2019, the water samples gradually transitioned to the chemical type of ecological replenishment water (HCO3-Ca type), characterized by a decreasing proportion of (SO42− + Cl−) and Mg2+. According to Fig. 14d, in the early stage of ecological replenishment in 2015, groundwater was mainly of the types HCO3−Ca·Mg and HCO3·Cl–Ca·Mg. Compared with that in 2018, although there was not much change in the chemical type of water in 2019, it was mainly the HCO3-Ca·Mg type but had a reduced proportion of Cl−. HCO3·Cl–Ca·Mg-type water was rarely observed, indicating a general transition to the HCO3-Ca type of ecological replenishment. This suggests that, after the implementation of ecological replenishment, the proportions of Cl− and Mg2+ in groundwater decreased. (Fig. 14 was created using Origin [2022]. (a), (b), and (c) respectively represent the piper diagrams of groundwater in the research area in 2016, 2017, and 2018, and (d) represents the comparison of piper diagrams of groundwater in the research area in 2015 and 2019.)
Piper diagram of groundwater in the research area from 2015 to 2019. Figure is a refinement of the piper diagram of groundwater chemistry in the research area from 2015 to 2019. (a), (b) and (c) represent the piper diagrams of groundwater in the research area in 2016, 2017, and 2018 respectively, and (d) represent the comparison of piper diagrams of groundwater in the research area in 2015 and 2019. Figure was created using Origin [2022].
Analysis of the causes of the chemical evolution of groundwater
Analysis of the mechanisms of groundwater chemical formation
The Gibbs graph of groundwater39 from the study region between 2015 and 2019 is shown in Fig. 15. (Fig. 15 was created using Origin [2022]. (a) and (b) respectively represent the analysis of major cations and anions in groundwater under different ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−). Most sampling points fall within the Na+/(Na+ + Ca2+) ratio range below 0.5, with Ca2+ being the dominant cation, and all groundwater sampling points fall within the range of Cl−/(Cl− + HCO3−) ratios below 0.5, with HCO3− being the dominant anion. This suggests that most groundwater in the study ___domain is formed by rock weathering40, and a small portion is influenced by evaporation and concentration. The source of water for ecological replenishment is influenced mainly by rock weathering, which is closely related to the hydrogeological circumstances of the source region of the ecological replenishment water. Compared with the early stage of replenishment in 2015, some water samples in 2019 moved from the evaporation and concentration area to the rock weathering area, demonstrating the impact of ecological replenishment on the groundwater environment.
Gibbs plot of groundwater in the research area. Figure is the Gibbs diagram of groundwater samples and ecological water recharge in the research area from 2015 to 2019, which is used to analyze the causes of groundwater and ecological water recharge. (a) and (b) respectively represent the analysis of major cations and anions in groundwater under different ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−), to analyze the origin of groundwater. Figure was created using Origin [2022].
Evolutionary research on chemicals in groundwater
Ion correlation analysis
The Pearson correlation coefficient of the chemical makeup of groundwater from 2015 to 2019 was calculated via the statistical software SPSS 26. By analysing the Pearson correlation coefficient between the nine indicators of groundwater, the hydrochemical relationship in the formation and variation process of the main ions in groundwater is analysed.
The graph of the matrix of correlation coefficients of hydrochemical components from 2015 to 2019 (Fig. 16) reveals that the correlation coefficients between each indicator and pH are relatively small, indicating a weak correlation. The TDS in groundwater had a strong correlation (values ranging from 0.77 to 0.90) with HCO3−, Mg2−, Ca2−, Cl−, and SO42− in 2015. Furthermore, notable associations were identified among Ca2+, Cl−, SO42−, and HCO3− and Mg2+. In 2016, groundwater TDS was significantly correlated with Cl−, SO42−, and Mg2+, with correlation values ranging from 0.77 to 0.90. Notable associations between Cl−, SO42−, and Ca2+ were also found. In 2017, the TDS in groundwater was significantly correlated with HCO3−, Mg2+, Ca2+, Cl−, SO42−, and Na+, with correlation values ranging from 0.65 to 0.93. Additionally, noteworthy associations were observed between HCO3−, Na+, and Mg2+ and between Ca2+, Cl−, and SO42−. In 2018, TDS was significantly correlated with Mg2+, SO42−, HCO3−, Cl−, and Na+, with correlation coefficients ranging from 0.74 to 0.94. Furthermore, notable connections were found between SO42−, Mg2+, and Cl− and between HCO3−, Mg2+, and Na+. In 2019, TDS was significantly correlated with Na+, Cl−, Mg2+, HCO3−, and SO42−, with correlation coefficients ranging from 0.71 to 0.93. Notable correlations were also observed between HCO3−, Mg2+, and Na+. In summary, the correlation coefficients between the ions did not differ much over time, but with the implementation of ecological replenishment, the correlation coefficients of the main ions underwent a trend change. The effects of rock weathering characterized by HCO3−, Ca2+, and Mg2+ continued to strengthen. (Fig. 16 was created using Origin [2022].)
Correlation coefficient matrix of the chemical composition from 2015 to 2019. Figure shows the correlation coefficient matrix of hydrochemical components in 2015, 2016, 2017, 2018, and 2019, which is used to analyze the hydrochemical relationship during the formation and change of major ions in groundwater. Figure was created using Origin [2022].
By comparing the differences in the correlations of the main ions in groundwater between the initial ecological replenishment stage in 2015 and 2019, in 2015, the correlation coefficients between SO42−, Cl−, and Ca2+, representing the effect of evaporative concentration, were between 0.77 and 0.80, whereas the correlations between HCO3−, Na+, and Mg2+, representing the effect of rock weathering, were not significant, with correlation values ranging from 0.59 to 0.80. In contrast, in 2019, the values for the correlations between SO42− and Ca2+ or Mg2+ were 0.70 and 0.67, respectively, whereas the correlation coefficients between HCO3− and Na+ or Mg2+ were 0.90 and 0.93, respectively. This indicates that, during the five years of ecological replenishment, the weathering of the rock has strengthened. The analysis results are consistent with the Gibbs diagram analysis results.
Principal component analysis
By calculating and obtaining the principal component load matrix of the hydrochemical components of groundwater in 2015 and 2019, refer to Tables 2 and 3. In 2015, the first major component was influenced by ions such as Ca2+, Mg2+, Cl−, and SO42−, with factor loadings of 0.94, 0.84, 0.92, and 0.84, respectively, contributing 44.91% of the variation. Ions such as HCO3− and Na+ had an impact on the second significant component, which had factor loadings of 0.80 and 0.75, respectively, and contributed 27.07% of the variation. In 2019, the first major factor was influenced mainly by ions such as HCO3−, Mg2+, and Na+, with factor loadings of 0.91, 0.92, and 0.97, respectively, contributing 38.36% of the variance. For the second factor, the factor loadings of Cl−, Ca2−, and SO42− ions were 0.89, 0.92, and 0.84, respectively, contributing 33.48% of the variance. In conclusion, the effect of the evaporative concentration influenced by Cl−, Ca2+, and SO42− in groundwater was stronger in the initial stage of ecological replenishment, with a contribution rate of 44.91%, but this contribution rate decreased to 33.48% in 2019. On the other hand, the rock weathering effect influenced by HCO3−, Mg2+, and Na+ increased, with a contribution rate of 38.36% in 2019. The study findings show that certain groundwater in the area has changed from having an evaporative concentration effect to having a rock-weathering effect as a result of the influence of ecological replenishment.
Analysis of the ion ratio
Figure 17 shows a graph of the coefficient of the ion ratio for groundwater in the study ___domain. (Fig. 17 was created using Origin [2022].)When γ(Na+)/γ(Cl−) ≈ 1, the main origin of Na+ in groundwater is dissolved rock salt37. As shown in Fig. 17a, when γ(Na+)/γ(Cl−) > 1, cation exchange and sodium silicate dissolution may occur in addition to rock salt dissolution. A decrease in Na+ may result from the dissolution of silicate and cation exchange, as most data points are above the 1:1 line and only a small number are below it. This suggests that Na+ is not only derived from the breakdown of rock salt. (Fig. 17a represents the ratio of Na+ to Cl−).
Figure of the proportional coefficient. Figure shows the proportional coefficient of groundwater ion concentration from 2015 to 2019, (a) represents the ratio of Na+ to Cl−; (b) represents the ratio of Ca2+ to SO42−; (c) represents the ratio of Ca2+ to HCO3−; (d) represents the ratio of Mg2+ + Ca2+ to HCO3−; (e) represents the ratio of Ca2+ + Mg2+ to SO42− + HCO3− and (f) represents the ratio of (Ca2+ + Mg2+)–(SO42− + HCO3−) to Na+–Cl−. Figure was created using Origin [2022].
The groundwater replenishment section of Fig. 17b shows that the ratio of γ(Ca2+) to γ(SO42−) is greater than 1, suggesting that the surplus Ca2+ is produced by the dissolution of other mineral carbonate rocks in addition to gypsum. Carbonate rocks, such as dolomite and calcite, dissolve and release Ca2+, Mg2+, and HCO3−41,42. Most of the γ(Ca2+) to γ(HCO3−) ratios, as shown in Fig. 17c, are between 1:1 and 1:2, revealing that the dissolution of calcite is the single origin of Ca2+ and HCO3−. (Fig. 17b represents the ratio of Ca2+ to SO42−; (c) represents the ratio of Ca2+ to HCO3−.)
When γ(Ca2+ + Mg2+)/γ(HCO3−) > 1, Ca2+, Mg2+, and HCO3− typically originate from the dissolution of carbonate rocks, including calcite and dolomite43,44. As shown in Fig. 17d, most of the γ(Ca2+ + Mg2+) to γ(HCO3−) ratios are above the 1:1 line, confirming that Mg2+, Ca2+, and HCO3− originate from the disintegration of dolomite and calcite. Furthermore, according to Fig. 17e, most of the data points are located near the γ(Ca2+ + Mg2+)/γ(SO42− + HCO3−) = 1:1 line, demonstrating that the main sources of Ca2+, Mg2+, SO42−, and HCO3− are the dissolution of dolomite, gypsum, and calcite5,10. (Fig. 17d represents the ratio of Mg2+ + Ca2+ to HCO3−; (e) represents the ratio of Ca2+ + Mg2+ to SO42− + HCO3−).
When γ(Na+–Cl−) and γ[(Ca2+ + Mg2+)–(SO42− + HCO3−)] = − 1, it indicates cation exchange in groundwater45. The relationship is shown in Fig. 17f, and the linear fit equation is y = − 1.11x + 0.05, with a slope of − 1.11, which is close to the theoretical value of − 1, and the correlation coefficient R2 = 0.94. This indicates that cation exchange, mostly between Na+, Ca2+, and Mg2+, occurs in the groundwater of the studied area. (Fig. 17f represents the ratio of (Ca2+ + Mg2+)–(SO42− + HCO3−) to Na+–Cl−). The reaction equations are as follows:
(Positive ion exchange, with Ca2+ and Mg2+ as the dominant ions)
(Positive ion exchange, with Na+ as the dominant ion)
In conclusion, the primary sources of groundwater ions are rock salt, gypsum, plagioclase feldspar, calcite, and dolomite, which dissolve and precipitate, as well as undergo cation exchange with rock and soil media. In addition to Eqs. (1) to (4), the following equations are also involved:
Conclusions
-
1.
From 2015 to 2019, the groundwater in the ecological replenishment section remained weakly alkaline, and the overall total dissolved solids showed a decreasing trend. The dominant cation in the groundwater was Ca2+, and the dominant anion was HCO3−. Compared with the initial stage of ecological replenishment in 2015, the concentration of cations in groundwater revealed a declining pattern from 2015 to 2019. The concentrations of SO42− and Cl− in the anions in the upper section of the replenishment river significantly decreased, which was related to the ecological replenishment of the Jialu River. The replenishment of high-quality water to the shallow groundwater aquifer caused mixing and dilution, improving groundwater quality. In the upper portion of the replenishment, the TDS of the shallow groundwater was primarily less than 600 mg/L; in the middle section, it varied between 600 and 900 mg/L; and in the lower area, it was primarily more than 900 mg/L. The primary cause of this is the interaction between vertical evaporation and groundwater runoff. According to the Piper trilinear diagram, the chemical types of groundwater in the initial stage of ecological replenishment in 2015 were mainly HCO3-Ca·Mg and HCO3·Cl–Ca·Mg. Compared with those in 2015, from 2016 to 2019, the total groundwater samples gradually transitioned to the chemical type of ecological replenishment (HCO3-Ca).
-
2.
Research on the mechanism of groundwater chemistry development reveals that rock weathering is the main source of groundwater in most research domains, with the evaporative concentration having a minor influence. Compared with the initial stage of replenishment in 2015, some water samples in 2019 shifted from the evaporative concentration area to the rock weathering area, demonstrating the replenishment effect of ecological replenishment on groundwater. The principal component analysis and the Gibbs diagram results are consistent, showing that with the implementation of ecological replenishment, rock weathering increased in the research area. During the ecological replenishment process, the major changes in ion composition in groundwater are due to the disintegration and precipitation of salt, gypsum, sodium feldspar, dolomite, and calcite, as well as the alternating adsorption of cations with rock and soil media.
-
3.
Groundwater from the studied region has a complicated chemical origin. This study analysed only the characteristics of groundwater chemical changes and the water‒rock interaction mechanism under ecological replenishment conditions. The groundwater in some sections is also influenced by factors such as lateral replenishment from the Yellow River and human activities. Therefore, in future research, the analysis of chemical changes and genesis mechanisms should consider the inclusion of other influencing factors.
Data availability
All the data generated or analysed during this study are included in this published article.
References
Jia, H. et al. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 717, 137170 (2020).
Lu, T., Huo, A., Wang, J., Lu, Y. & Zhou, W. Hydrodynamic behaviors and geochemical evolution of groundwater for irrigation in Yaoba Oasis, China. Water 14, 3924–3924 (2022).
Wei, H., Liang, X., Liu, S., Liu, M. & Xiao, C. Hydrochemical evolution of groundwater in Dehui, China. Water 12, 3378–3378 (2020).
Chen, Z. et al. Groundwater chemistry and isotope for interpreting the hydrogeological conditions and hydrochemical evolution of multilayer aquifer system of Donghai island, China. Appl. Geochem. 159, 105833 (2023).
Gao, Z., Li, Q., Liu, J. & Su, Q. Hydrochemical characterizations and groundwater quality assessment in the coastal region of the Jiaodong peninsula, North China. Mar. Pollut. Bull. 196, 115596–115596 (2023).
Wang, M., Liang, X. & Xiao, C. Analysis on chemical characteristics and genesis of groundwater in Shuangliao City. Water Resour. Hydropower Eng. 50, 124–131 (2019).
Wang, X., Liang, X., Xiao, C. & Lu, H. Chemical characteristics and causes of phreatic water in Baicheng city. Water Resour. Power 37, 37–41 (2019).
Zhou, B., Wang, H. & Zhang, Q. Assessment of the evolution of groundwater chemistry and its controlling factors in the Huangshui River basin of Northwestern China, using hydrochemistry and multivariate statistical techniques. Int. J. Environ. Res. Public Health 18, 7551–7551 (2021).
Qu, S., Shi, Z., Liang, X., Wang, G. & Jin, X. Origin and controlling factors of groundwater chemistry and quality in the Zhiluo aquifer system of northern Ordos Basin, China. Environ. Earth Sci. https://doi.org/10.1007/s12665-021-09735-y (2021).
Gao, Z. et al. Assessment of groundwater hydrogeochemistry, controlling factors, water quality, and nitrate-related health risks in the Longkou Bay, North China. Water Air Soil Pollut. https://doi.org/10.1007/s11270-024-07154-w (2024).
Cui, X. et al. Impacts of ecological water replenishment on the hydrochemical characteristics of surface water and groundwater in Lake Baiyangdian watershed. J. Lake Sci. 33, 1675–1686 (2021).
Li, H. et al. Analysis on the effect of ecological water replenishment of Yongding River in spring 2020 on groundwater conservation in Beijing. Urb. Geol. 16, 133–138 (2021).
Xiong, X., Liu, L. & Guo, M. Analysis of the influence of Chaobai River Water Source on groundwater quality in South-to-North Water Transfer Project. Beijing Water Affairs 13–16 (2018).
Ren, W. et al. Water environment carrying capacity evaluation of Jialu River (Zhengzhou section)and research on pollution source traceability. Henan Sci. Technol. 40, 132–136 (2021).
Zhou, Y., Li, H. & Li, D. Water pollution analysis and treatment countermeasures of Jialu river in Zhengzhou. Henan Sci. 33, 1823–1827 (2015).
Wang, P. et al. Impacts of environmental flow controls on the water table and groundwater chemistry in the Ejina Delta, northwestern China. Environ. Earth Sci. 64, 15–24 (2011).
Aboubakar, S. & Cissé, O. C. A. Hydrogeochemical characterization and natural background level determination of selected inorganic substances in groundwater from a semi-confined aquifer in Midwestern Burkina Faso, West Africa. Environ. Monit. Assess. 195, 519–519 (2023).
Ranjan, H. S., Nath, H. R. & Shreerup, G. Statistical appraisal of major ion chemistry of groundwater: A Case study from a river-bounded rural area. J. Geol. Soc. India 99, 1253–1262 (2023).
Li, P., Wu, J. & Qian, H. Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ. Earth Sci. 69, 2211–2225 (2013).
Pallavi, N., Kumar, M. A., Priyajit, S., Somnath, K. & Patitapaban, M. Groundwater quality, hydrogeochemical characteristics, and potential health risk assessment in the Bhubaneswar City of eastern India. Water Air Soil Pollut. https://doi.org/10.1007/s11270-023-06614-z (2023).
Han, G. & Liu, C.-Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 204, 1–21 (2004).
Yang, L., Liu, L., Liu, Y., Chen, G. & Liang, L. Hydrodynamic groundwater modeling and hydrochemical conceptualization of the closure mining area of the WuMa River watershed of China. ACS Omega 9, 520–537 (2024).
Adomako, D., Osae, S., Akiti, T. T., Faye, S. & Maloszewski, P. Geochemical and isotopic studies of groundwater conditions in the Densu River Basin of Ghana. Environ. Earth Sci. 62, 1071–1084 (2011).
Pastukhov, M. V., Poletaeva, V. I. & Hommatlyyev, G. B. Hydrochemical characteristics and water quality assessment of Irkutsk reservoir (Baikal Region, Russia). Water 15(23), 41–42 (2023).
Lal, M. P. Study on the hydrogeochemical processes regulating the groundwater chemistry in the Southeast Rajasthan. J. Geol. Soc. India 98, 1455–1465 (2022).
Masilamani, P. et al. Geochemical characterisation and geostatistical evaluation of groundwater suitability: A case study in Perambalur District, Tamil Nadu, India. Environ. Sci. Pollut. Res. Int. 30, 62653–62674 (2023).
Zheng, L., Yang, C., Huang, L. & Li, C. Distribution of water quality in a pumped storage reservoir based on inverse distance weighted interpolation. Ningxia Eng. Technol. 317, 270–273 (2018).
Ma, G., Qu, Z., Wang, L., Jia, Y. & Liu, Z. Research on soil water and salt movement and groundwater dynamics in Hetao Irrigation District based on ArcGIS spatial interpolation. J. Soil Water Conserv. 35, 208–216 (2021).
Deshpande, S. M. et al. Appraisal of groundwater quality in parts of Ranjangaon Shenpunji area of Aurangabad district, Maharashtra. Bull. Pure Appl. Sci. Geol. 41(1), 38–50 (2022).
Ohwoghere-Asuma, O., Aweto, K. E. & Ugbe, C. F. Lithofacies identification and multivariate analysis of groundwater chemistry in coastal aquifers in Koko Area of the Western Niger Delta. Hydrology 6(2), 31 (2019).
Chidambaram, S., Anandhan, P., Prasanna, M. V., Srinivasamoorthy, K. & Vasanthavigar, M. Major ion chemistry and identification of hydrogeochemical processes controlling groundwater in and around Neyveli Lignite Mines, Tamil Nadu, South India. Arab. J. Geosci. 6, 3451–3467 (2013).
Chen, J. et al. Hydrochemical genesis and ecological environment influence of groundwater in dezhou city at lower Yellow River Basin. Environ. Chem. 42, 125–137 (2023).
Wang, L., Zhang, Q. & Wang, H. Rapid urbanization has changed the driving factors of groundwater chemical evolution in the large groundwater depression funnel Area of Northern China. Water 15(16), 2917 (2023).
Wang, W. et al. Spatial-temporal characteristics and influencing factors of lake water and groundwater chemistry in Hulun Lake, Northeast China. Water 15, 937–937 (2023).
Liu, C., Hou, Q., Chen, Y. & Huang, G. Hydrogeochemical characteristics and groundwater quality in a coastal urbanized area, South China: Impact of land use. Water 14, 4131–4131 (2022).
Ma, J. et al. Hydrochemical characteristics and genetic analysis of shallow groundwater in the North Shore of Jiaozhou Bay. J. China Hydrol. 41, 103–108 (2021).
Ye, F. & Zuo, T. Hydrochemical characterstics and quality evaluation of groundwater in Baoding Yimu Spring water source. Ground Water 45, 20–23 (2023).
Meenakshi, A. et al. The influence of stormwater infiltration on downslope groundwater chemistry. Environ. Geochem. Health 45, 8489–8501 (2023).
Lauric, T. et al. Geochemical characteristics and quality of groundwater in a rural sub-Saharan context. A case study of Bafou–Baranka on Bambouto Mountain West Cameroon. Arab. J. Geosci. https://doi.org/10.1007/s12517-023-11287-3 (2023).
Wang, K., Xie, W., Liu, W., Wang, W. & Zhang, T. Chemical characteristics and evolution of shallow groundwater of Yellow River and Tuhai River basin in Huimin area, Shandong Province, China. IOP Conf. Ser. Earth Environ. Sci. https://doi.org/10.1088/1755-1315/804/2/022052 (2021).
Krishna, K. S. et al. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ. Monit. Assess. 159, 341–351 (2009).
Liu, Y. et al. Groundwater chemical characteristics and circulation mode in the Suixiao coal-mining district. Q. J. Eng. Geol. Hydrogeol. 53, 227–235 (2020).
Ma, R. et al. Geochemical evolution of groundwater in carbonate aquifers in Taiyuan, northern China. Appl. Geochem. 26, 884–897 (2011).
Wang, Y. & Jiao, J. J. Origin of groundwater salinity and hydrogeochemical processes in the confined Quaternary aquifer of the Pearl River Delta, China. J. Hydrol. 438–439, 112–124 (2012).
Dorleku, M. et al. Spatio-temporal variation of groundwater chemistry in the Upper Oti Basin of Ghana. Environ. Monit. Assess. https://doi.org/10.1007/s10661-022-10760-y (2022).
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFC3006603).
Funding
This work was supported by the National Key R&D Program of China (2023YFC3006603).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study. The design and conception of the study were completed by J.Q., J.L., J.W., and T.Y. The methodology was completed by J.Q., J.L., and K.R. Data collection was performed by J.Q., J.L., J.W., and K.R. Data analysis was performed by T.Y. and J.Z. J.Q., J.L., and Y.L. prepared the figures. The first draft of the manuscript was written by J.Q., J.L., and J.W. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qu, J., Lin, J., Wang, J. et al. Analysis of the evolution and causes of groundwater chemistry after ecological water replenishment of the Jialu River, China. Sci Rep 14, 18759 (2024). https://doi.org/10.1038/s41598-024-69704-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69704-w