Abstract
This study aims to analyze the risk factors associated with delayed postoperative bleeding (DPPB) following colorectal polyp surgery, develop a dynamic nomogram and evaluate the model efficacy, provide a reference for clinicians to identify the patients at high risk of DPPB. Retrospective study was done on patients who underwent endoscopic colorectal polypectomy at the First Hospital of Lanzhou University from January 2020 to March 2023. Differences between the group with and without DPPB were compared, and independent risk factors for DPPB occurrence were identified through univariate analysis and combination LASSO and logistic regression. A dynamic nomogram was constructed based on multiple logistic regression to predict DPPB following colorectal polyp surgery. Model evaluation included receiver operating characteristic (ROC), Calibration curve, Decision curve analysis (DCA). DPPB occurred in 38 of the 1544 patients included. multivariate analysis showed that direct oral anticoagulants (DOACs), polyp ___location in the right hemi colon, polyp diameter, drink, and prophylactic hemoclips were the independent risk factors for DPPB and dynamic nomogram were established. Model validation indicated area under the ROC curve values of 0.936, 0.796, and 0.865 for the training set, validation set, and full set, respectively. The calibration curve demonstrated a strong alignment between the predictions of the column-line diagram model and actual observations. The decision curve analysis (DCA) displayed a significant net clinical benefit across the threshold probability range of 0–100%. The dynamic nomogram aids clinicians in identifying high-risk patients, enabling personalized diagnosis and treatment.
Similar content being viewed by others
Introduction
Colorectal polyps are protruding growths resulting from the excessive proliferation of the intestinal mucosa into the intestinal lumen, which can develop into colorectal cancer through the adenoma-carcinoma sequence pathway1. The prevalence of this condition rises with age, and research indicates that it can reach as high as 12.95% among individuals aged over 40 years2. Endoscopic surgery remains the primary treatment modality for colorectal polyps, However, it is associated with adverse events including bleeding, perforation, and postoperative coagulation syndrome3. Among these, bleeding is particularly common, and postoperative bleeding is classified into immediate postoperative bleeding (IPPB) and DPPB. DPPB is characterized by bleeding occurring within 30 days post-surgery, necessitating additional visits to the emergency room or hospitalization4.The incidence is between 0.3% and 2.8%5,6,7. Clinicians have paid significant attention to the insidious onset of DPPB due to its potential to be life-threatening, prolong hospital stays, and escalate medical costs, particularly in cases of heavy bleeding. Identifying potential independent risk factors for DPPB through screening and conducting individualized assessments for patients are crucial steps in preventing DPPB.
Previous research has identified several risk factors linked to the development of DPPB. However, discrepancies exist between studies regarding the controversy surrounding certain factors, while others have not been sufficiently investigated7,8,9,10,11,12,13. Some studies utilize risk factors and static Nomogram risk maps to achieve quantitative assessment13,14. However, clinicians still encounter challenges in implementing the assessment process. Due to its simplicity, convenience, maneuverability, and high accuracy, the dynamic nomogram finds broad utility in predicting acute respiratory distress syndrome following extracorporeal circulation and community-acquired pneumonia complicating acute kidney injury15,16,17. However, there is a scarcity of studies addressing this issue in the ___domain of DPPB. This study aims to identify risk factors for DPPB, develop a dynamic nomogram for predicting DPPB risk post-colorectal polyp surgery, aid clinicians in identifying high-risk patients, enabling individualized diagnosis and treatment. Moreover, it seeks to implement preoperative interventions and postoperative prophylaxis to mitigate DPPB occurrence.
Patients and methods
Patient data collection
Informed consent was not required for this study because this study is retrospective research. All protocols received approval from the Ethics Committee of the First Hospital of Lanzhou University and were conducted in accordance with its regulations and guidelines.
The study population comprised patients who underwent endoscopic colorectal polypectomy at the First Hospital of Lanzhou University between January 2020 and March 2023. Data collection encompassed three main aspects: (1) Baseline Characteristics: age, gender, body mass index (BMI), smoking, drink, hypertension, diabetes, chronic liver disease, chronic kidney disease, use of direct oral anticoagulants (DOACs), and history of gastrointestinal surgeries. (2) Polyp and Surgical Details: the number, diameter, ___location, morphology, and pathological staging of the polyps, as well as the use of prophylactic hemoclips, Operative modality and operator experience. (3) Preoperative Examination Parameters: PLT, RBC, HGB, HCT, MCV, MCH, MCHC, WBC, L, N, M, CRP, systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), systemic immune-inflammation response index (SIIRI), TC, TG, HDL-C, and fecal occult blood (FOB). A reference questionnaire was utilized to assess smoking and drink habits. Polyp ___location was classified into three categories: right semicolon (ileocecal junction, ascending colon, and transverse colon), left semicolon (descending colon, sigmoid colon, and rectum) and mixed (indicating polypectomy performed in both the right and left colon). Polyp diameter was estimated by the operator based on the opening width of the biopsy forceps. Polyp pathological staging was divided into adenomatous polyps (tubular adenomas, tubular villous adenomas, and choriocarcinomas) and non-adenomatous polyps (hyperplastic polyps and serrated polyps). Surgical procedures encompassed APC, EMR, ESD, and CSP. Polyps were divided into sessile polyps and pedunculated polyps by morphologic diagnosis. Operator experience was categorized based on the number of gastroenterostomy units operated within a five-year period.
Inclusion and exclusion criteria
Inclusion criteria: (1) Diagnosis of colorectal polyps confirmed by endoscopic examination and pathology; (2) DPPB diagnosis adhering to the European Society of Endoscopy criteria. Exclusion criteria: (1) Concurrent hematologic disorders; (2) History of inflammatory bowel disease or familial adenomatous polyposis; (3) Severe cardiac, pulmonary, or cerebral conditions, or tumors in other locations; (4) Gastrointestinal bleeding from alternate etiologies; (5) Abnormal preoperative coagulation profiles; (6) Current use of antiplatelet or anticoagulant medications, including clopidogrel; (7) Presence of IPPB; (8) Incomplete clinical or pathological data.
Research design
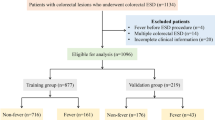
This study collected data from 1591 patients, with 1544 ultimately meeting the inclusion and exclusion criteria. These individuals were divided into the training set (N = 1081) and the internal validation set (N = 463) at a 7:3 ratio. (Fig. 1).
Statistical analysis
Numerical variables were assessed using either the t-test or Mann–Whitney U-test, while categorical variables underwent analysis using either the chi-square test or Fisher's exact test. Initial variable screening employed lasso regression. Subsequently, all variables with non-zero coefficients were incorporated into multifactorial logistic regression to identify independent DPPB risk factors. R software facilitated the creation of Nomogram and supported dynamic network interactions. ROC analysis gauged the predictive model's discrimination, with the Area Under the Curve (AUC) calculated for assessment. Calibration curves evaluated model calibration, and DCA analyzed the predictive model's clinical utility. A two-sided test was utilized, with statistical significance set at P < 0.05.Data were analyzed using IBM SPSS Statistics Version 25.0 (IBM Corp, Armonk, NK, USA) and R version 4.1.3 (http://www.r-project.org).
Results
Over the period from January 2020 to March 2023, our study center conducted a total of 1591 endoscopic colon polypectomies. Among these cases, DPPB was observed in 38 patients (2.39%), aligning with findings from prior research. All individuals experiencing DPPB underwent repeat colonoscopy to identify the bleeding site and received appropriate treatment. Specifically, two patients required a second endoscopic hemostasis, while the remainder achieved successful hemostasis with a single intervention.
Univariate analysis
Baseline information of included patients
Statistically significant differences were observed between patients in the DPPB group and those without DPPB across three factors: drink, diabetes, and DOCAs (P < 0.05), while no significant differences were found for other variables (Table 1).
Analysis of polyp and surgery-related factors
Significant differences were found between patients with DPPB and those without DPPB regarding polyp diameter, polyp ___location, operative modality, and the prophylactic hemoclips (P < 0.05). No other statistically significant differences were observed (Table 2).
Analysis of preoperative tests
Significant differences were observed between patients with DPPB and those without DPPB in PLT, HGB, HCT, TC, HDL-L and FOB(P < 0.05), while no other statistically significant differences were found (Table 3).
The results of univariate analysis revealed associations between the occurrence of DPPB and the following factors: drink, diabetes, DOACs, polyp ___location and diameter, use of prophylactic hemoclips, operative modality, as well as levels of PLT, HGB, HCT, TC, HDL-C, and FOB.
Factor selection for the predictive model and dynamic nomogram developing
The Lasso regression initially screened 13 variables. Shrinkage coefficients for these variables were plotted (Fig. 2), and cross-validated graphs were generated (Fig. 2). From cross-validation results, seven variables (Drink, DOACs, Polyp Location, Polyp Diameter, prophylactic hemoclips, HGB, FOB) exhibited non-zero coefficients in the Lasso regression analysis, minimizing the model error to 0.0048 (Fig. 2). Furthermore, the correlation heatmap indicated no multicollinearity among these seven factors (Fig. 2). Subsequently, multifactorial logistic analysis (Table 4) identified five independent predictors (Drink, DOACs, Polyp Location, Polyp Diameter, Prophylactic hemoclips). These five risk factors were then used to construct visualized nomogram (Fig. 3) and dynamic interactions, accessible via a web page. (http://ldyy-xhnj.shinyapps.io/dynnomapp) (Fig. 3).
(a) The nomogram constructed from the full model was used to predict DPPB.(b) A dynamic nomogram is deployed on the shinyapps.io platform http://ldyy-xhnj.shinyapps.io/dynnomapp.Users can calculate the probability of a poor prognosis for DPPB patients by submitting five related traits online via mobile phone or compute.
Evaluation of the model
ROC curves were employed to assess the discriminative abilities of the model (Fig. 4). The AUC values for the model were 0.936, 0.796, and 0.865 in the training, testing and entire cohorts, respectively, surpassing those of the individual variables. Calibration curves were utilized to confirm the model's consistency (Fig. 4), demonstrating that the predictions from the column-line plot model aligned well with actual observations across all cohorts. Additionally, DCA curves illustrated the net clinical gain (Fig. 4), indicating favorable clinical net gains across all cohorts. Moreover, the overall model exhibited superior performance compared to the individual variable model across all threshold probabilities from 0–100%.
(a) ROC curves depicting the occurrence of DPPB for individual risk factors and the entire model in the training cohort. (b) ROC curves depicting the occurrence of DPPB for individual risk factors and the entire model in the testing cohort. (c) ROC curves depicting the occurrence of DPPB for individual risk factors and the entire model in the entire cohort. (d) Calibration curves in the training cohort. (e) Calibration curves in the testing cohort. (f) Calibration curves in the entire cohort. (g) DCA curves illustrating the occurrence of DPPB for individual risk factors and the entire model in the training cohort. (h) DCA curves illustrating the occurrence of DPPB for individual risk factors and the entire model in the testing cohort. (i) DCA curves illustrating the occurrence of DPPB for individual risk factors and the entire model in the entire cohort.
Ethics statement
Informed consent was not required for this study because this study is retrospective research. All protocols received approval from the Ethics Committee of the First Hospital of Lanzhou University and were conducted in accordance with its regulations and guidelines.
Discussion
DPPB represents a significant adverse event following colorectal polypectomy, with most cases occurring within 1 to 6 days postoperatively3. This analysis identified several independent risk factors for DPPB, including DOACs, polyp diameter, polyp ___location, prophylactic hemoclips, and drink. Furthermore, it developed a dynamic nomogram prediction model for DPPB, demonstrating robust predictive efficacy. This model facilitates individualized clinical diagnosis and treatment, thereby enhancing clinical decision-making.
This study identified DOACs as a significant risk factor for the development of DPPB following colorectal polypectomy, aligning with findings from previous studies10,18,19.Whereas Yu et al. found no significant increase in gastrointestinal bleeding (GIB) after polypectomy in patients taking DOACs in a retrospective analysis of the Clinformatics Data Mart Database20.Current guidelines are unclear on the discontinuation time and postoperative recovery time for taking DOACs21,22,23. The occurrence of DPPB might relate to inadequate perioperative discontinuation time or early postoperative medication resumption. Future investigations are necessary to delve into the underlying mechanisms and ascertain the optimal perioperative dosage and timing for medication resumption in high-risk patients.
The diameter of polyps has long been acknowledged as an independent risk factor for DPPB. In a retrospective study, Lu et al. discovered a 4.035-fold higher risk of hemorrhage associated with polyps larger than 10 mm compared to those smaller than 10 mm13. Bae and colleagues discovered that the risk of delayed post-polypectomy bleeding (DPPB) escalated by 11.6% with each 1 mm increment in polyp size8. In our investigation, polyps in the DPPB group exhibited a larger diameter compared to those in the non-DPPB group. This phenomenon is likely attributable to the increased vascularization associated with larger polyps, thereby enhancing the susceptibility to vascular injury during manipulation14.
Polyps located in the right semicolon were an independent risk factor for DPPB in this study, consistent with previous studies8,9,13. A plausible explanation for this phenomenon is the susceptibility of the thin, high-tension wall of the cecum to vascular injury within the deeper submucosal layers during manipulation. Additionally, the influx of digestive enzymes and bile acids from the ileal fluid into the cecum could dissolve blood clots that cover post-polypectomy trauma, thereby elevating the risk of DPPB9,24, Moreover, the constrained space and challenging maneuverability within the right half of the colon invariably result in mucosal damage when polyps are abundant.
The association between prophylactic hemoclips and the onset of DPPB remains contentious. Interestingly, in our investigation, the utilization of prophylactic hemoclips was associated with a heightened incidence of DPPB, aligning with findings reported by Okinawa et al25. Several findings indicate that the prophylactic use of tourniquets is not correlated with the incidence of DPPB26,27. While the majority of studies advocate for the use of prophylactic hemoclips to decrease the incidence of DPPB28. Potential explanations include ineffective clamping positions or dislodgement of titanium clips29. Furthermore, the decision to clip relies on the discretion of the overseeing endoscopist. In cases where the risk of DPPB is deemed substantial, there may be a heightened reliance on titanium clamps, resulting in inevitable mucosal damage and an escalated DPPB risk. Limited research exists on this topic, and future inquiries could delve deeper into evaluating the efficacy of titanium clamping in wound closure for DPPB prevention, as well as its susceptibility to individual variables within endoscopic practice.
Previous correlation studies that included drink did not identify statistically significant associations7,12,30,Our study identified drink as an independent risk factor for DPPB. Some studies have demonstrated that chronic heavy alcohol consumption can impair the intestinal mucosal barrier, resulting in heightened intestinal permeability and microbial translocation31.Nonetheless, the precise mechanism remains elusive. Asquith et al. observed in an animal experiment that chronic drinking (CD) could substantially disturb mucosal homeostasis via microRNA expression32. Barr et al. identified region-specific alterations in the microbial composition of mucosal tissues in individuals with chronic alcohol consumption. These alterations included a reduction in beneficial bacteria, such as Brucella. in the jejunum and Ruminococcaceae. in the ileum, and an elevation in inflammation-associated bacteria, such as Porphyromonas and Prevotella33. The inflammatory response in the intestines exacerbates pathological damage34. Hence, we hypothesized that the onset of DPPB might correlate with inflammatory responses, impairment of the intestinal mucosal barrier, and alterations in intestinal flora due to alcohol consumption. Future investigations are warranted to elucidate the relationship and mechanisms linking DPPB and alcohol consumption.
One of the strengths of this study is establishing a dynamic nomogram prediction model with strong predictive performance based on DPPB risk factors. The identified predictive parameters are routine in the examination of patients with colorectal polyps, making the model user-friendly and widely applicable. Accessible via computers, cell phones, and other electronic devices, it serves as a convenient navigational tool for preoperative tendency diagnosis, personalized therapeutic measures, and surgical modality selection. Additionally, the study identifies alcohol consumption as an independent risk factor for DPPB, warranting further investigation in future studies.
However, this study has several limitations. Firstly, it is a retrospective single-center study, necessitating future multicenter and prospective studies to validate the predictive accuracy and clinical utility of dynamic columnar mapping. Secondly, the study primarily included multiple small polyps and did not assess the impact of specific locations on DPPB occurrence.
Conclusion
This study developed a straightforward and effective online prediction model for DPPB, providing clinicians with a tool to identify high-risk patients and enabling personalized diagnosis and treatment.
Data availability
Part of the dataset generated and/or analyzed during the current study is included in this published article. Remaining data is available from the corresponding author on reasonable request.
Abbreviations
- DPPB:
-
Delayed post-polypectomy bleeding
- IPPB:
-
Immediate postoperative bleeding
- DOACs:
-
Direct-acting oral anticoagulants
- BMI:
-
Body Mass Index
- PLT:
-
Platelet count
- RBC:
-
Red blood cell count
- HGB:
-
Hemoglobin
- HCT:
-
Hematocrit
- MCV:
-
Mean corpuscular volume
- MCHC:
-
Mean corpuscular hemoglobin concentration
- WBC:
-
White blood cell
- N:
-
Neutrophils
- L:
-
Lymphocyte
- M:
-
Monocyte
- CRP:
-
C-reactive protein
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL-C:
-
High density lipoprotein
- SII:
-
Systemic immune-inflammation index
- SIR:
-
Systemic inflammation response index
- SIIRI:
-
Systemic immune-inflammation response index
- FOB:
-
Fecal occult blood
- EMR:
-
Endoscopic mucosal resection
- ESD:
-
Endoscopic submucosal dissection
- CSP:
-
Cold Snare Polypectomy
- APC:
-
Argon plasma coagulation
- GIB:
-
Gastrointestinal bleeding
- ROC:
-
Receiver operating characteristic
- DCA:
-
Decision curve analysis
- CD:
-
Chronic drinking
References
Shaukat, A. et al. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 369, 1106–1114. https://doi.org/10.1056/NEJMoa1300720 (2013).
Koirala, D. et al. Detection of Colonic Polyps During Colonoscopy in a Tertiary Care Center of Nepal. J. Nepal Health Res. Counc. 19, 596–602. https://doi.org/10.33314/jnhrc.v19i3.3678 (2021).
Reumkens, A. et al. Post-colonoscopy complications: A systematic review, time trends, and meta-analysis of population-based studies. Am. J. Gastroenterol. 111, 1092–1101. https://doi.org/10.1038/ajg.2016.234 (2016).
Ferlitsch, M. et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 49, 270–297. https://doi.org/10.1055/s-0043-102569 (2017).
Kim, S. Y., Kim, H. S. & Park, H. J. Adverse events related to colonoscopy: Global trends and future challenges. World J. Gastroenterol. 25, 190–204. https://doi.org/10.3748/wjg.v25.i2.190 (2019).
Amato, A. et al. Early and delayed complications of polypectomy in a community setting: The SPoC prospective multicentre trial. Dig. Liver Dis. 48, 43–48. https://doi.org/10.1016/j.dld.2015.09.007 (2016).
Jaruvongvanich, V. et al. Risk factors for delayed colonic post-polypectomy bleeding: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 32, 1399–1406. https://doi.org/10.1007/s00384-017-2870-0 (2017).
Bae, G. H. et al. Risk factors of delayed bleeding after colonoscopic polypectomy: Case-control study. Korean J. Gastroenterol. 59, 423–427. https://doi.org/10.4166/kjg.2012.59.6.423 (2012).
Buddingh, K. T. et al. Location in the right hemi-colon is an independent risk factor for delayed post-polypectomy hemorrhage: A multi-center case-control study. Am. J. Gastroenterol. 106, 1119–1124. https://doi.org/10.1038/ajg.2010.507 (2011).
Kubo, T. et al. Heparin bridge therapy and post-polypectomy bleeding. World J. Gastroenterol. 22, 10009–10014. https://doi.org/10.3748/wjg.v22.i45.10009 (2016).
Furuhashi, H. et al. Blood group O is a risk factor for delayed post-polypectomy bleeding. Surg. Endosc. 35, 6882–6891. https://doi.org/10.1007/s00464-020-08195-y (2021).
Wu, X. R., Church, J. M., Jarrar, A., Liang, J. & Kalady, M. F. Risk factors for delayed postpolypectomy bleeding: how to minimize your patients’ risk. Int. J. Colorectal Dis. 28, 1127–1134. https://doi.org/10.1007/s00384-013-1661-5 (2013).
Lu, Y., Zhou, X., Chen, H., Ding, C. & Si, X. Establishment of a model for predicting delayed post-polypectomy bleeding: A real-world retrospective study. Front. Med. (Lausanne) 9, 1035646. https://doi.org/10.3389/fmed.2022.1035646 (2022).
Seo, M. et al. A risk-scoring model for the prediction of delayed bleeding after colorectal endoscopic submucosal dissection. Gastrointest. Endosc. https://doi.org/10.1016/j.gie.2018.11.029 (2019).
Wang, Y. et al. Early plasma proteomic biomarkers and prediction model of acute respiratory distress syndrome after cardiopulmonary bypass: a prospective nested cohort study. Int. J. Surg. 109, 2561–2573. https://doi.org/10.1097/JS9.0000000000000434 (2023).
Bou Kheir, G., Khaldi, A., Karam, A., Duquenne, L. & Preiser, J. C. A dynamic online nomogram predicting severe vitamin D deficiency at ICU admission. Clin. Nutr. 40, 5383–5390. https://doi.org/10.1016/j.clnu.2021.08.024 (2021).
Chen, D. et al. Dynamic nomogram for predicting acute kidney injury in patients with community-acquired pneumonia. BMJ Open Respir. Res. https://doi.org/10.1136/bmjresp-2022-001495 (2023).
Valvano, M. et al. Risk of colonoscopic post-polypectomy bleeding in patients on single antiplatelet therapy: Systematic review with meta-analysis. Surg. Endosc. 36, 2258–2270. https://doi.org/10.1007/s00464-021-08975-0 (2022).
Yasuda, R. et al. Multicenter study of the hemorrhage risk after endoscopic mucosal resection associated with direct oral anticoagulants. Gastroenterol. Res. Pract. 2019, 5743561. https://doi.org/10.1155/2019/5743561 (2019).
Yu, J. X. et al. Patients Prescribed Direct-Acting Oral Anticoagulants Have Low Risk of Postpolypectomy Complications. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2018.11.051 (2019).
Fujimoto, K. et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig. Endosc. 26, 1–14. https://doi.org/10.1111/den.12183 (2014).
Committee, A. S. et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 83, 3–16. https://doi.org/10.1016/j.gie.2015.09.035 (2016).
Veitch, A. M. et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Gut 70, 1611–1628. https://doi.org/10.1136/gutjnl-2021-325184 (2021).
Sorbi, D. et al. Postpolypectomy lower GI bleeding: descriptive analysis. Gastrointest. Endosc. 51, 690–696. https://doi.org/10.1067/mge.2000.105773 (2000).
Okugawa, T. et al. Effect of Instruction on Preventing Delayed Bleeding after Colorectal Polypectomy and Endoscopic Mucosal Resection. J. Clin. Med. https://doi.org/10.3390/jcm10050928 (2021).
Feagins, L. A. et al. Efficacy of Prophylactic Hemoclips in Prevention of Delayed Post-Polypectomy Bleeding in Patients With Large Colonic Polyps. Gastroenterology 157, 967 (2019).
Matsumoto, M. et al. Multicenter randomized controlled study to assess the effect of prophylactic clipping on post-polypectomy delayed bleeding. Dig. Endosc. 28, 570–576. https://doi.org/10.1111/den.12661 (2016).
Xu, J. H. et al. Clip-assisted endoloop ligation of the mucosal defect after resection of colorectal polyps decreased postprocedural delayed bleeding. Ther. Adv. Gastroenterol. 15, 17562848221131132. https://doi.org/10.1177/17562848221131132 (2022).
Zhang, Q. et al. Assessment of risk factors for delayed colonic post-polypectomy hemorrhage: A study of 15553 polypectomies from 2005 to 2013. PLoS One https://doi.org/10.1371/journal.pone.0108290 (2014).
Watabe, H. et al. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: Polyp-related factors and patient-related factors. Gastrointest Endosc. 64, 73–78. https://doi.org/10.1016/j.gie.2006.02.054 (2006).
Molina, P. E., Gardner, J. D., Souza-Smith, F. M. & Whitaker, A. M. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 29, 203–215. https://doi.org/10.1152/physiol.00055.2013 (2014).
Asquith, M. et al. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol. Clin. Exp. Res. 38, 980–993. https://doi.org/10.1111/acer.12325 (2014).
Barr, T. et al. Concurrent gut transcriptome and microbiota profiling following chronic ethanol consumption in nonhuman primates. Gut. Microb. 9, 338–356. https://doi.org/10.1080/19490976.2018.1441663 (2018).
Jia, M. et al. Carbon dots induce pathological damage to the intestine via causing intestinal flora dysbiosis and intestinal inflammation. J. Nanobiotechnol. 21, 167. https://doi.org/10.1186/s12951-023-01931-1 (2023).
Author information
Authors and Affiliations
Contributions
LTX, YMW and HL conceived and designed the project and wrote the manuscript, LTX, NZ and YXZ were involved in the collection of the data. DL and YZ analyzed data and established a dynamic online nomogram about DPPB. QL and YMW analyzed data and provided important suggestions regarding this project. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, L., Zhang, N., Zhang, Y. et al. A dynamic online nomogram for predicts delayed postoperative bleeding after colorectal polyp surgery. Sci Rep 14, 19728 (2024). https://doi.org/10.1038/s41598-024-70635-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70635-9