Abstract
Da Vinci robot-assisted pancreaticoduodenectomy offers advantages, including minimal invasiveness, precise, and safe procedures. This study aimed to investigate the clinical effectiveness of implementing enhanced recovery after surgery (ERAS) concepts in Da Vinci robot-assisted pancreaticoduodenectomy. A retrospective analysis was conducted on clinical data from 62 patients who underwent Da Vinci robot-assisted pancreaticoduodenectomy between January 2018 and December 2022. Among these patients, 30 were managed with ERAS principles, while 32 were managed using traditional perioperative management protocols. Surgical time, intraoperative blood loss, postoperative oral intake time, time to return of bowel function, time to ambulation, visual analog scale (VAS) pain scores, fluid replacement volume, length of hospital stay, total hospital expenses, complications, and patient satisfaction were recorded and compared between the two groups. Postoperative follow-up included assessment of postoperative functional scores, reoperation rates, SF-36 quality of life scores, and survival rates. The average follow-up time was 35.6 months (range: 12–56 months). There were no statistically significant differences in general characteristics, including age, surgical time, intraoperative blood loss, and preoperative medical history between the two groups (P > 0.05). Compared to the control group, the intervention group had an earlier postoperative oral intake time, faster return of bowel function, rapid ambulation, and shorter hospital stays (P < 0.05). The intervention group also had lower postoperative VAS scores, lower fluid replacement volume, lower total hospital expenses, and a lower rate of complications (P < 0.05). Patient satisfaction was higher in the intervention group (P < 0.05). There were no statistically significant differences between the two groups in two-year functional scores, reoperation rates, quality of life scores, and survival rates (P > 0.05). Implementing ERAS principles in Da Vinci robot-assisted pancreaticoduodenectomy substantially expedited postoperative recovery, lowered pain scores, and diminished complications. However, there were no notable differences in long-term outcomes between the two groups.
Similar content being viewed by others
Introduction
Enhanced recovery after surgery (ERAS), introduced by the ERAS Research Society in 2001, emerged from the early 1990s concepts of rapid postoperative recovery, also called fast-track surgery1. This approach was introduced by Kehlet H and colleagues from the University of Copenhagen in Denmark. They were among the initial contributors to the ERAS collaborative group and prominent researchers in the field of ERAS studies1,2,3. Recently, the ERAS concept has gained widespread application in gastrointestinal surgery.
Pancreaticoduodenectomy is primarily employed for tumors located in the lower part of the bile duct, such as periampullary cancer, pancreatic head cancer, primary tumors of the duodenum, malignant tumors involving the pancreaticoduodenal area, gastrointestinal tumors, pancreatic and duodenal injuries, and the surgical treatment of rare diseases in the pancreatic head4,5. It is also used for the surgical management of chronic refractory pancreatitis. Pancreaticoduodenectomy is a complex surgery characterized by significant disruption of normal anatomical structures and relatively slow postoperative recovery, which significantly benefits from minimally invasive surgery and enhanced recovery6,7,8. Da Vinci Xi robot-assisted surgery facilitates precise identification of anatomical sites, accurate removal of lesions, and reduced damage to surrounding structures, thereby effectively minimizing surgical trauma9,10,11. Although ERAS protocols have been widely implemented in pancreaticoduodenectomy6,12,13, there have been no reports on applying ERAS protocols in Da Vinci robot-assisted pancreaticoduodenectomy. Therefore, this study aimed to retrospectively analyze clinical data from patients who underwent Da Vinci robot-assisted pancreaticoduodenectomy and assess the clinical value of implementing ERAS protocols in this surgery.
Methods
Patient selection
A retrospective analysis was conducted on the clinical data of 62 patients who underwent Da Vinci robot-assisted pancreaticoduodenectomy at our hospital from January 2018 to December 2022. The studies involving humans received approval from the Ethics Board of the Affiliated Hospital of Zunyi Medical University and Guizhou Provincial People’s Hospital. They were conducted following clinical guidelines and institutional requirements. Written informed consent for participation was waived by the Ethics Board of the same hospital due to the retrospective nature of the study. Patients were divided into the ERAS group and the control group based on whether they followed the concept of accelerated surgical recovery. Patients with surgical indications for pancreaticoduodenectomy, including lower common bile duct cancer, ampulla of Vater cancer, pancreatic head cancers, duodenal cancers, and periampullary cancers, were included in the study. Among these patients, indications for Da Vinci robot-assisted surgery included the following: (1) Benign and low-grade pancreatic tumors that can maintain the pancreas after surgery; (2) lower common bile duct tumors (benign or malignant) without metastasis; (3) early or mid-stage pancreatic head cancers without metastasis; (4) periampullary cancers requiring resection; (5) patients or families required to perform ERAS and minimize surgical trauma. The exclusion criteria were as follows: (1) Presence of other site tumors or severe metastatic cancer; (2) intraoperative or postoperative death; (3) difficulty in implementing robot-assisted surgery leading to conversion to open surgery during the procedure; (4) poor compliance, interruption of ERAS protocol, or low execution rate, or being lost to follow-up in the early postoperative period. Eligible patients were informed about the ERAS protocol. If the patient declined to participate in the ERAS protocol, routine rehabilitation protocols were used. The control group was matched by demographic data with patients who underwent Da Vinci robot-assisted pancreaticoduodenectomy during the same period, ensuring no chronological difference between ERAS and control groups.
Surgical procedure
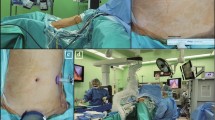
The patient underwent general anesthesia, the establishment of pneumoperitoneum, and the installation of robot equipment using a five-port method. The abdominal cavity was explored to confirm the resectability of the tumor. Subsequently, a Kocher incision was made to mobilize the duodenum and pancreatic head. The gallbladder was removed, the common bile duct was separated, and the lower edge of the pancreas was dissected through the retropancreatic tunnel. A closing device was used to cut off the stomach near the gastric antrum, and the pancreas was dissected using an ultrasonic scalpel. The jejunum was divided at the distal end of Treitz ligament, gradually freeing and dissecting the uncinate process of the pancreas. The specimen was removed in one piece, followed by pancreaticojejunostomy and common bile duct anastomosis, and tube jejunostomy and gastrointestinal anastomosis in sequence. After surgery, all patients received standard treatments, including hepatoprotective, acid suppression, analgesic, antiemetic, anti-infection, and rehydration therapies. There were no significant differences in surgical procedures or surgeon proficiency between the two groups.
ERAS protocol
The ERAS protocol, conducted collaboratively by multiple disciplines, included perioperative nutritional support, anesthesia and pain management, and postoperative care. (1) Preoperative nutritional support: Individualized dietary plans were formulated based on the patient’s mental state, disease type, underlying conditions (diabetes, hypertension, and heart disease), nutritional status, and dietary habits. The approach involved frequent small meals. Fasting time was 6 h before surgery, without water allowed for 2 h before surgery. Within 2 h before surgery, 400 mL of 10% glucose solution was administered. In cases where patients had difficulty eating due to gastrointestinal obstruction or decreased appetite, enteral or parenteral nutrition support was utilized. (2) Intraoperative anesthesia protocol: The intervention group underwent epidural anesthesia with strict control of fluid volume during surgery. (3) Postoperative urinary catheter removal time: Urinary catheters were removed within 6 h after surgery. In cases of severe prostate enlargement or urinary retention after catheter removal, the indwelling time could be extended or the catheter reinserted. (4) Postoperative fluid management: Strict control of postoperative fluid intake was maintained with daily input and output recording. Adequate postoperative pain management included patient-controlled analgesia (PCA) for the first three days, followed by oral pain medication based on the patient’s pain level. (5) Postoperative nutritional support: Enteral nutrition was provided within 24–48 h after surgery. If the patient could not tolerate oral intake, parenteral nutrition was provided within two weeks based on daily nutritional requirements, primarily using lipid emulsions and amino acids, with an increased proportion of carbohydrates. (6) Drain removal time: Drain removal times were similar between the two groups and were determined primarily based on postoperative recovery. The criteria for removal of drains were less than 20 mL bloody drainage for three consecutive days and exclusion of intestinal or anastomotic fistulas. (7) Early postoperative mobilization: Early postoperative mobilization was encouraged, with patients able to mobilize as early as 6 h after surgery, depending on their mental state and lower limb strength. (8) Postoperative pain management: The intervention group received patient-controlled intravenous analgesia (PCIA) supplemented with intramuscular injection of diclofenac.
Conventional intervention
The control group received conventional perioperative care, which included fasting for 12 h before surgery. Postoperative analgesics were the same as those administered to the ERAS group, including PCIA, supplemented with intramuscular pain medication based on postoperative pain assessment. Urinary catheters were removed on the third day after surgery. Early postoperative parenteral nutrition was initiated, and early oral intake was discouraged, adhering to standard fluid replacement protocols.
Outcome measures
Baseline patient characteristics compared between the two groups included age, gender, body mass index (BMI), underlying diseases (hypertension, diabetes, and heart disease), hemoglobin levels, the Charlson comorbidity index, and American Society of Anesthesiologists (ASA) classification. Data recorded for both groups included surgical duration, intraoperative blood loss, intraoperative transfusion, daily average postoperative fluid replacement, postoperative complications, time to postoperative ambulation, time to first anal gas passage, pain scores, length of postoperative hospital stay, total hospitalization cost, and patient satisfaction (rated on a scale of 1–10). Long-term follow-up was conducted for at least one year, assessing postoperative functional scores, reoperation rates, SF-36 quality of life scores, and survival rates for both groups. Patient satisfaction ranged from 1 to 10, with 1 representing dissatisfaction and 10 representing high satisfaction. The SF-36 score comprises eight health-related quality of life questionnaires: physical functioning, quality of life, importance of life, mental health, emotional health, social functioning, fatigue, and general health. A comprehensive score was calculated after weighing these elements.
Statistical analysis
Statistical analysis was conducted using the R software. Continuous variables are presented as mean ± standard deviation. Normality and homogeneity of variances were assessed for all continuous variables. Normally distributed and homogenous data were analyzed using independent sample t-tests. Non-parametric tests were applied when assumptions were not met. Categorical data are expressed as proportions. The group comparisons were performed using the chi-squared or Fisher’s exact test when necessary. Rank-sum tests were employed for comparisons of ordinal data. A significance level of P < 0.05 was considered statistically significant.
Results
General Information
A total of 62 patients who underwent Da Vinci robot-assisted pancreaticoduodenectomy and were discharged after recovery were included in the study. The intervention group comprised 30 patients (17 males and 13 females) with an average age of 65.27 ± 13.64 years. In the control group, there were 32 patients, including 18 males and 14 females, with an average age of 69.06 ± 11.48 years. There were no statistically significant differences in demographic data between the two groups, including age, gender, BMI, and underlying diseases (hypertension, diabetes, and heart disease) (P > 0.05; Table 1). The two groups had similar surgical risk classifications, including the Charlson comorbidity index and ASA classification, with no statistically significant differences (P > 0.05; Table 1). Additionally, there were no statistically significant differences in surgical-related data between the two groups, including surgical duration, intraoperative bleeding volume, the number of intraoperative blood transfusions, and the volume of intraoperative blood transfusions (P > 0.05). The baseline data of the two groups were comparable (Table 1). Pathological diagnosis identified six pancreatic head cancers, 22 cholangiocarcinomas, 15 ampullary cancers, seven duodenal papilla cancers, and 12 periampullary cancers.
Postoperative recovery
The average time to ambulation after surgery in the intervention group was 3 (2, 4) days, while in the control group, it was 5 (4, 6) days. The intervention group had an earlier time to ambulate postoperatively compared to the control group, and the difference was significantly different (P < 0.05). The time to first anal gas passage in the intervention group was 2 (1.75, 4) days, whereas in the control group, it was 4.5 (3, 6) days. The intervention group had an earlier return of bowel function, with the difference being statistically significant (P < 0.05). The visual analog scale pain score in the intervention group was 4 (3, 5.25), while in the control group, it was 5 (4, 7). The postoperative pain score in the intervention group was lower than that in the control group, and the difference was significantly different (P < 0.05). The intervention group also had a lower volume of postoperative fluid intake compared to the control group, with the difference being statistically significant (P < 0.05; Table 2). There was no significant difference in the timing of the start of walking between the two groups (2 days versus 3 days, P > 0.05).
Postoperative complications
After excluding surgical complications directly related to the operation, there were 9 cases of postoperative complications in the intervention group (30%) and 18 cases in the control group (56.25%). In the intervention group, 2 patients experienced deep vein thrombosis in the lower limbs (6.67%), 1 had a lung infection (3.33%), 2 had postoperative urinary retention (6.67%), and 5 had postoperative abdominal distension and nausea or vomiting (16.67%). In the control group, 5 patients had deep vein thrombosis in the lower limbs (15.63%), 4 had lung infections (12.5%), 6 had postoperative urinary retention (18.75%), and 10 had postoperative abdominal distension and nausea or vomiting (31.25%). Some patients experienced more than one complication. The incidence of postoperative complications was significantly lower in the intervention group compared to the control group, with a statistically significant difference (P < 0.05; Table 3).
Postoperative discharge time and hospitalization costs
The average length of postoperative hospital stay in the intervention group was 8 (6.75, 9) days, whereas in the control group, it was 11 (9, 13) days. The postoperative hospital stay in the intervention group was significantly shorter than that in the control group, indicating a statistically significant difference (P < 0.05). The total hospitalization cost in the intervention group was 43,000 Yuan (Renminbi) (39,000–48,000 Yuan), while in the control group, it was 51,500 Yuan (45,000–57,500 Yuan). Surgical costs in the intervention group were significantly lower than those in the control group, demonstrating a statistically significant difference (P < 0.05). Patient satisfaction in the intervention group was rated at 8.5 (6.5, 9.75) points, whereas in the control group, it was 6.5 (5, 8.75) points. Patient satisfaction in the intervention group was higher than that in the control group, demonstrating a statistically significant difference (P < 0.05; Table 3). The readmission rate within 30 days after discharge was 6.7% in the ERAS group and 15.6% in the control group, with no significant differences observed (P > 0.05).
Two-year postoperative outcomes
Two years of postoperative follow-up revealed a reoperation rate of 10% in the intervention group and 15.6% in the control group. There was no statistically significant difference between the two groups (P > 0.05). The rate of pancreatic fistula was 13.33% in the intervention group and 15.63% in the control group. The two-year overall survival rate in the intervention group was 76.7%, while in the control group, it was 68.8%, indicating a statistically non-significant difference (P > 0.05). Among the surviving patients (23 in the intervention group and 22 in the control group), there were no statistically significant differences in gastrointestinal function score, SF-36 quality of life assessment scale, Karnofsky score, or Eastern Cooperative Oncology Group Performance Status (ECOG) score (P > 0.05; Table 4).
Discussion
In this study, it was observed that implementing the ERAS protocol in Da Vinci robot-assisted pancreaticoduodenectomy effectively reduces postoperative complications and facilitates postoperative recovery without impacting long-term clinical outcomes.
The ERAS protocol aims to minimize perioperative stress, reduce postoperative complications, and enhance postoperative recovery to the greatest extent possible. An ideal ERAS program should involve multidisciplinary collaboration, encompassing nutrition, surgery, anesthesia, pain management, rehabilitation, nursing, and hospital administration14,15,16. A comprehensive ERAS protocol includes perioperative nutritional support, fluid management, anesthesia management, pain management, postoperative care, and recovery17,18. Patient and family cooperation are crucial factors in the successful implementation of ERAS2,19,20. This study is the first to implement the ERAS protocol in Da Vinci robot-assisted pancreaticoduodenectomy. Da Vinci robot-assisted surgery offers advantages such as precision, speed, and safety and has been widely utilized in the surgical treatment of various general surgical diseases21. Its flexible robotic arms can effectively eliminate tremors, enhance surgical precision, provide a clear and broad surgical field of view, significantly improve surgical accuracy and safety, and reduce surgical trauma, particularly in tumor resection surgery. Combining Da Vinci robot-assisted surgery and the ERAS protocol can effectively reduce surgical trauma and facilitate postoperative recovery.
This study carefully matched clinical cases, ensuring significant comparability regarding gender, age, BMI, pre-existing medical conditions, hemoglobin levels, and preoperative risk stratification. It also balanced surgical-related data between the two groups, including surgical duration, intraoperative bleeding volume, the number of intraoperative blood transfusions, and transfusion volume, thus ensuring comparability in demographic and surgical-related data between the two groups. However, related biases and errors are unavoidable. Given the high surgical difficulty and risk associated with pancreaticoduodenectomy, which requires precision in surgical operations, Da Vinci robot-assisted surgery can notably improve surgical accuracy and reduce postoperative complications. Several clinical studies have reported on the application of Da Vinci robot-assisted pancreaticoduodenectomy.
Shyr et al. discovered that the robotic pancreaticoduodenectomy significantly reduced delayed gastric emptying, blood loss, postoperative stay duration, and wound infection rates when compared to open pancreaticoduodenectomy22. In their literature review, Kornaropoulos et al. concluded that Da Vinci robot-assisted pancreaticoduodenectomy can be safely performed in institutions with a high volume of surgeries, experienced surgeons, and well-trained support staff, highlighting the challenges of robotic-assisted surgery for pancreaticoduodenectomy23. Notably, the cost of Da Vinci’s Xi-assisted surgery cannot be ignored. Di Franco et al. compared the costs of Da Vinci Xi surgery with open pancreaticoduodenectomy and observed that higher material costs were the primary contributor to the increased medical expenses associated with robotic-assisted surgery24. However, these higher costs can be partially offset by shorter postoperative hospital stays and comparable personnel expenses. Furthermore, this study combined the ERAS protocol with Da Vinci Xi-assisted pancreaticoduodenectomy, resulting in enhanced postoperative recovery, including fewer complications, shorter hospital stays, and reduced medical costs. When considering two-year survival rates and functional outcomes, our study observed that the ERAS protocol yielded clinical outcomes similar to traditional perioperative management.
The patients in this study underwent pancreaticoduodenectomy for periampullary cancers, considering the malignant nature of these tumors, with most pancreatic cancer cases having a limited life expectancy of 3–5 years25. It is important to acknowledge that the comparison of functional scores can be influenced by the general condition of surviving patients and any postoperative anti-cancer treatments they may receive. However, our study revealed that the two-year survival rate and reoperation rate were comparable between the intervention and traditional management groups, indicating that the short-term benefits did not impact the long-term clinical outcomes. Despite these considerations, applying ERAS protocol in Da Vinci Xi-assisted pancreaticoduodenectomy significantly enhanced postoperative rehabilitation, further emphasizing its potential clinical benefits.
In this study, the intervention group uniformly employed epidural anesthesia, followed by PCA combined with intramuscular injections for postoperative pain management. This approach effectively reduces complications associated with general anesthesia, including postoperative nausea, vomiting, pneumonia, atelectasis, and central nervous system symptoms26. Our research indicated a significant reduction in postoperative anesthesia-related complications in the intervention group, with excellent postoperative pain control and a notable decrease in short-term opioid requirements. Consequently, the application of the ERAS protocol in Da Vinci robot-assisted pancreaticoduodenectomy is deemed safe. Given the substantial impact of gastrointestinal surgery on patients’ anatomical structures and physiological functions, traditional surgical recovery plans often involve prolonged drain placement. Additionally, the extended drainage time in pancreaticoduodenectomy is primarily attributed to surgical disruption of normal anatomical and physiological structures and intraoperative field bleeding27,28. Da Vinci robot-assisted surgery significantly enhances surgical precision, enabling precise lesion excision and accurate anastomosis29. Consequently, it significantly reduces surgical trauma, allowing for early drain removal and facilitating gastrointestinal function recovery30. Early postoperative mobilization significantly promotes gastrointestinal function recovery, preventing bedridden complications such as aspiration pneumonia and deep vein thrombosis13,31. Additionally, there was no significant difference in the readmission rate within 30 days after discharge (P > 0.05), partly due to the limited sample numbers and the short period of postoperative days.
This study has several limitations. First, it was a retrospective case-control study, lacking prospective design and randomization, which limits the persuasiveness of the conclusions. Second, the sample size was relatively small, as pancreaticoduodenectomy indications are diverse. Both groups contained patients with various disease types, leading to significant heterogeneity despite no statistical differences in baseline data. Third, excluding postoperative mortality may introduce bias when assessing the benefits of the ERAS protocol. Mortality is a key factor in evaluating both the surgery and postoperative management. While its implementation significantly enhances postoperative recovery, it cannot entirely prevent severe complications associated with pancreaticoduodenectomy, particularly intraoperative complications. Finally, the observed outcomes primarily focused on short-term postoperative results, and the follow-up period was relatively short. While the study evaluated the two-year postoperative reoperation and survival rates, periampullary cancers have a high degree of malignancy, resulting in short patient survival times, making it challenging to effectively assess the long-term survival. Future research should involve prospective randomized controlled trials to further evaluate the value of the ERAS protocol in Da Vinci robot-assisted pancreaticoduodenectomy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ljungqvist, O., Scott, M. & Fearon, K. C. Enhanced recovery after surgery: A review. JAMA Surg. 152, 292–298 (2017).
Su, S. et al. The Global States and hotspots of ERAS Research from 2000 to 2020: A bibliometric and visualized study. Front. Surg. 9, 811023 (2022).
Li, C. et al. The pertinent literature of enhanced recovery after surgery programs: A bibliometric approach. Med. (Kaunas) 57, 172 (2021).
Jiang, X. et al. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: A meta-analysis. Int. J. Surg. 73, 14–24 (2020).
Chen, Q. et al. Prognostic and recurrent significance of SII in patients with pancreatic head cancer undergoing pancreaticoduodenectomy. Front. Oncol. 13, 1122811 (2023).
Xu, X., Zheng, C., Zhao, Y., Chen, W. & Huang, Y. Enhanced recovery after surgery for pancreaticoduodenectomy: Review of current evidence and trends. Int. J. Surg. 50, 79–86 (2018).
Wang, M. et al. Minimally invasive pancreaticoduodenectomy: A comprehensive review. Int. J. Surg. 35, 139–146 (2016).
Rosemurgy, A. et al. Cost analysis of pancreaticoduodenectomy at a high-volume robotic hepatopancreaticobiliary surgery program. J. Am. Coll. Surg. 232, 461–469 (2021).
Rosemurgy, A. et al. Robotic pancreaticoduodenectomy is the future: Here and now. J. Am. Coll. Surg. 228, 613–624 (2019).
Li, B. B. et al. Da Vinci robot-assisted pancreato-duodenectomy in a patient with situs inversus totalis: A case report and review of literature. World J. Gastrointest. Oncol. 14, 1363–1371 (2022).
Wang, R., Li, J., Tan, C. L., Liu, X. B. & Chen, Y. H. Prospects and applications of enucleation in solid pseudopapillary neoplasms of the pancreas. World J. Gastrointest. Oncol. 14, 1227–1238 (2022).
Melloul, E. et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J. Surg. 44, 2056–2084 (2020).
Kuemmerli, C. et al. Impact of enhanced recovery protocols after pancreatoduodenectomy: Meta-analysis. Br. J. Surg. 109, 256–266 (2022).
Bossi, P., De Luca, R., Ciani, O., D’angelo, E. & Caccialanza, R. Malnutrition management in oncology: An expert view on controversial issues and future perspectives. Front. Oncol. 12, 910770 (2022).
Mantzavinou, A., Uppara, M., Chan, J. & Patel, B. Robotic versus open pancreaticoduodenectomy, comparing therapeutic indexes; a systematic review. Int. J. Surg. 101, 106633 (2022).
Marino, M. V. et al. Robotic-assisted pancreaticoduodenectomy with vascular resection. Description of the surgical technique and analysis of early outcomes. Surg. Oncol. 35, 344–350 (2020).
Cao, Y. et al. Impact of enhanced recovery after surgery on postoperative recovery for pancreaticoduodenectomy: Pooled analysis of observational study. Front. Oncol. 9, 687 (2019).
Aarts, M. A. et al. Postoperative ERAS interventions have the greatest impact on optimal recovery: Experience with implementation of ERAS across multiple hospitals. Ann. Surg. 267, 992–997 (2018).
Lassen, K. et al. Pancreaticoduodenectomy: ERAS recommendations. Clin. Nutr. 32, 870–871 (2013).
Sánchez-Iglesias, J. L. et al. Importance of enhanced recovery after surgery (ERAS) protocol compliance for length of stay in ovarian cancer surgery. Ann. Surg. Oncol. 28, 8979–8986 (2021).
Hosoda, K., Niihara, M., Harada, H., Yamashita, K. & Hiki, N. Robot-assisted minimally invasive esophagectomy for esophageal cancer: Meticulous surgery minimizing postoperative complications. Ann. Gastroenterol. Surg. 4, 608–617 (2020).
Shyr, Y. M., Wang, S. E., Chen, S. C., Shyr, B. U. & Shyr, B. S. Robotic pancreaticoduodenectomy for pancreatic head cancer and periampullary lesions. Ann. Gastroenterol. Surg. 5, 589–596 (2021).
Kornaropoulos, M. et al. Total robotic pancreaticoduodenectomy: A systematic review of the literature. Surg. Endosc. 31, 4382–4392 (2017).
Di Franco, G. et al. Robot-assisted pancreatoduodenectomy with the Da Vinci Xi: Can the costs of advanced technology be offset by clinical advantages? A case-matched cost analysis versus open approach. Surg. Endosc. 36, 4417–4428 (2022).
Liu, R. et al. Single-port (SP) robotic pancreatic surgery using the Da Vinci SP system: A retrospective study on prospectively collected data in a consecutive patient cohort. Int. J. Surg. 104, 106782 (2022).
Klotz, R. et al. Gastrointestinal complications after pancreatoduodenectomy with epidural vs patient-controlled intravenous analgesia: A randomized clinical trial. JAMA Surg. 155, e200794 (2020).
Wang, Q. et al. Is routine drainage necessary after pancreaticoduodenectomy?. World J. Gastroenterol. 20, 8110–8118 (2014).
Kawaida, H. et al. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J. Gastroenterol. 25, 3722–3737 (2019).
Shi, Y. et al. Short-term outcomes after robot-assisted vs open pancreaticoduodenectomy after the learning curve. JAMA Surg. 155, 389–394 (2020).
Strobel, O. & Büchler, M. W. Drainage after pancreaticoduodenectomy: Controversy revitalized. Ann. Surg. 259, 613–615 (2014).
Fukushima, T. et al. Role of early mobilization on the clinical course of patients who underwent pancreaticoduodenectomy: A retrospective cohort study. Tohoku J. Exp. Med. 254, 287–294 (2021).
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for the language editing service.
Funding
This work was funded by the Science and Technology Joint Foundation of Zunyi [HZ (2023) 241].
Author information
Authors and Affiliations
Contributions
Zhenxing Liu: Methodology, Writing–original draft, performing the experiments. Honghong Chen: Methodology, Writing–review & editing. Zhengbiao Li: Methodology, performing the experiments, Writing–review & editing. Jinlong Liang: Formal analysis. Tao zhang: performing the experiments, and data curation.Weiwei Ning: data curation and Formal analysis. Jiwei Wang: Supervision, Conceptualization, Project administration. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Z., Chen, H., Li, Z. et al. Clinical efficacy of enhanced recovery surgery in Da Vinci robot-assisted pancreatoduodenectomy. Sci Rep 14, 21539 (2024). https://doi.org/10.1038/s41598-024-72835-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72835-9