Abstract
Cadmium (Cd) toxicity significantly threatens agricultural productivity and food safety. Developing effective strategies to enhance plant tolerance to Cd stress is essential. This study investigates the synergistic effects of biochar (BC) and gibberellic acid (GA3) on mitigating Cd toxicity in maize (Zea mays), focusing on their impact on oxidative stress markers and antioxidant enzyme activities. Soil samples were collected from the Cholistan Institute of Desert Studies (CIDS) and analyzed for trace metal ions and other properties. Biochar was produced from fruit and vegetable waste, washed, washed, deashed, and mixed with 10 ppm GA3. FH-1036 hybrid maize seeds were sterilized and planted in pots containing soil with varying Cd levels (0, 8, and 16 mg Cd/kg soil). Twelve treatments were established, including control, GA3, BC, and their combinations under different Cd stress levels. Plants were irrigated to maintain 60% field capacity and harvested at the V10 growth stage. Hydrogen peroxide (H2O2) contents and activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) were measured in roots, stems, and leaves. Statistical analysis was performed using OriginPro 2021, with ANOVA and Fisher’s LSD test used to determine significant differences. GA3 and BC treatments significantly reduced H2O2 levels in maize roots, stems and leaves under Cd stress. The combined treatment of GA3 + BC showed the most significant reduction in H2O2 levels across all plant parts, reducing root H2O2 by 50%, stem H2O2 by 55%, and leaf H2O2 by 53% under severe Cd stress (16 mg Cd/kg). SOD activity increased under non-stress conditions but decreased under Cd stress, with the highest activity observed in the combined treatment. POD activity followed a similar pattern, with GA3 + BC treatment resulting in the most significant increases under non-stress conditions and the least reductions under Cd stress. CAT activity showed substantial increases with GA3 + BC treatment, particularly under severe Cd stress, with a notable rise over the control. APX activity also exhibited enhancements with GA3 and BC treatments, especially in the combined treatment under various Cd stress levels. This study highlights the potential of combined BC and GA3 treatments in improving Cd stress tolerance in maize. Future research should focus on field trials and the long-term impacts of these treatments on crop productivity and soil health.
Similar content being viewed by others
Introduction
Introducing heavy metals (HMs) into the environment through industrial activities, mining operations, and improper waste disposal poses a significant threat to ecosystems and human health. Metals such as lead (Pb), cadmium (Cd), and mercury (Hg) accumulate in soil and water, leading to soil pollution, water contamination, and severe health issues, including neurological disorders and organ impairment1. Persistent heavy metals in agricultural soils are taken up by crops, entering the food chain and presenting risks to human health.
Maize (Zea mays L.), a staple crop, is notably a significant source of dietary Cd for humans. Despite its importance, maize exhibits resilience to Cd stress and is often utilized in the phyto-management of contaminated soils2. However, prolonged Cd exposure profoundly impacts maize growth by compromising its structural integrity and disrupting essential morphological, physiological, and biochemical processes. This includes detrimental effects on photosynthesis, chloroplast ultrastructure, and increased oxidative damage through ROS overproduction. Cd uptake through plant roots adversely affects agricultural production by disrupting photosynthesis, modifying chloroplast ultrastructure, enhancing lipid peroxidation, and increasing oxidative damage due to ROS generation. These effects vary based on plant type, exposure duration, absorption amount, sequestration, and geographical ___location3,4,5.
Cadmium (Cd) accumulation in maize is a complex process influenced by various factors including soil Cd concentration and plant physiology6. Cd is primarily taken up by the maize roots from contaminated soils, where it initially accumulates due to its affinity for root tissues. The extent of Cd uptake and its subsequent distribution within the plant is governed by soil conditions such as pH and organic matter content, which affect the metal’s bioavailability7. Once absorbed, Cd is translocated through the xylem to the above-ground parts of the plant, including the stems and leaves. Although the roots generally accumulate higher concentrations of Cd, significant amounts can also be found in the stems and leaves. The maize grains, or kernels, may also contain Cd, which raises concerns about food safety. The accumulation of Cd in maize tissues leads to various physiological and biochemical disruptions, such as impaired root development, reduced nutrient uptake, and oxidative stress8. These disruptions manifest as stunted growth, chlorosis, and reduced biomass, reflecting the plant’s struggle to manage and detoxify the heavy metal.
Gibberellic acid (GA3) emerges as a crucial plant growth regulator. GA3 initiates various physiological and developmental processes, including root production, flowering, cell division, maturity, and seed germination. It enhances plants’ resilience to environmental challenges such as salt, cold, drought, and heavy metal stress by modulating antioxidant enzyme activity9. GA3 reduces excessive intracellular ROS generated during stress, protecting plants from environmental stressors. Exogenous GA3 effectively alleviates oxidative stress in wheat induced by salt stress, enhancing soil nutrients, and increasing plant output. Gibberellins regulate various plant growth processes, including seed germination, stem elongation, and flowering10,11,12.
Simultaneously, biochar (BC) emerges as a promising solution to mitigate the detrimental effects of heavy metal contamination in soils. Produced by controlled pyrolysis of organic material in an oxygen-limited environment, BC has shown significant positive outcomes in pot and field testing, enhancing growth, biomass, and other factors in Cd-contaminated soils. Incorporating BC into soil is a viable solution to mitigate harmful heavy metal impacts13. BC improves plant growth and soil productivity and is effective in absorbing and reducing HM concentrations in soils, particularly in HM-contaminated areas. As an organic soil amendment, BC aids in soil immobilization, showcasing favorable characteristics such as a fundamental nature, porous texture, energetic functional groups, and greater cation exchange capacity (CEC). Soils treated with BC exhibit reduced Cd transport and accumulation14,15,16. Through compound generation and cation exchange techniques, BC rapidly absorbs Cd, Pb, and Cu. The use of BC to remove Cd from soil has gained considerable interest, improving biological, chemical, and physical soil characteristics. Recycling discarded straws for BC production is ecologically friendly and economically practical. BC rapidly improves soil physicochemical characteristics, promoting plant photosynthetic capacity, enhancing growth, and increasing the retention ability for hydration, nutrients, and nutrition levels17,18.
Gibberellic acid and biochar contribute significantly to the regulation of antioxidant enzymes, which play a critical role in scavenging reactive oxygen species (ROS) and mitigating oxidative stress in plants exposed to cadmium toxicity19. GA3 enhances the activity of essential antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD)20. SOD catalyzes the dismutation of superoxide radicals into hydrogen peroxide, which is then further broken down by CAT and POD into water and oxygen, thereby reducing ROS levels and protecting cellular components from oxidative damage. GA3 influences the expression and activity of these enzymes, thereby boosting the plant’s ability to manage oxidative stress and maintain cellular redox balance. Concurrently, biochar improves soil health and plant growth by increasing cation exchange capacity (CEC), enhancing soil structure, and supporting beneficial microbial communities21. These improvements contribute to reduced availability and uptake of Cd, decreasing the overall oxidative stress on plants. Additionally, biochar can adsorb heavy metals, including Cd, effectively reducing their bioavailability in the soil and limiting their entry into plant tissues. The combined application of GA3 and BC is hypothesized to offer a synergistic effect by both directly enhancing the antioxidant defense mechanisms and indirectly reducing the oxidative stress imposed by Cd contamination, leading to improved plant growth and resilience.
This study aims to evaluate the potential positive effects of gibberellic acid GA3 and BC on maize growth under Cd stress. Specifically, it seeks to investigate the individual and combined applications of GA3 and BC to determine their effectiveness in mitigating the adverse impacts of Cd on maize. The objectives of this research are to assess the individual effects of GA3 on maize growth, physiological parameters, and biochemical responses under Cd stress, and to evaluate the individual effects of BC on soil properties, Cd bioavailability, and maize growth under Cd stress. Furthermore, the study aims to investigate the combined effects of GA3 and BC on maize growth, physiological parameters, and biochemical responses under Cd stress in maize. By addressing these objectives, this research endeavors to bridge existing knowledge gaps and provide insights into the efficacy of GA3 and BC as potential interventions for mitigating Cd stress in maize. The findings are expected to contribute to the development of practical and sustainable strategies for managing heavy metal contamination in agricultural systems, ultimately supporting food security and environmental sustainability. The novelty of this research lies in its comprehensive approach, integrating GA3 and BC to address Cd stress in maize. This study aims to elucidate the individual and combined effects of these treatments and provide a sustainable, practical solution for improving crop resilience and productivity in Cd-contaminated soils. The findings will contribute to the broader understanding of phyto-management strategies and their potential applications in agricultural practices, particularly in areas affected by heavy metal contamination.
Materials and methods
Soil sampling and analysis
The soil samples for the experiments were sourced from the Cholistan Institute of Desert Studies (CIDS), The Islamia University of Bahawalpur, Pakistan. They consisted of calcareous sandy loams, known for their fertility and low levels of organic matter. Procedures outlined by22 were followed to identify trace metal ions in the soil samples. After collection, the samples underwent a series of steps. Ten milliliters of nitric acid (HNO3) were added to an Erlenmeyer flask containing one gram of dried soil, and the mixture was allowed to stand overnight. Controlled heating up to 200 °C, followed by cooling and treatment with HNO3 and perchloric acid (HClO4), was performed23. Further heating at 280 °C continued until fumes from HClO4 became apparent. After cooling, hydrochloric acid was introduced, and another heating-cooling cycle was conducted. The resulting solution, mixed with 1% hydrochloric acid (HCl), was filtered using Whatman filter paper number 42.
Trace metal ions in the filtered solution were measured using an atomic absorption spectrophotometer (AAS), specifically the Thermo Scientific iCE 3000 Series. Distinct soil attributes were determined using specific methods: pH determination involved analyzing soil-saturated paste with a pH meter (model: Hanna HI2211), soil organic content (SOC) was quantified using the potassium dichromate (K2Cr2O7) method; electrical conductivity (EC) was assessed by mixing soil and deionized water at a 1:10 ratio followed by analysis on an EC meter (model: Hach HQ40d); soil phosphorus (P) analysis followed the Olsen method; for soil potassium (K) analysis, extraction with ammonium acetate (NH₄CH₃CO₂) and flame photometry were employed (model: Jenway PFP7)24,25,26. The properties of the soil are presented in Table 1.
Biochar’s composition and nutritional content
BC was generated using waste materials sourced from local vendors selling fruits and vegetable juices. The gathered waste underwent drying under sunlight and subsequent fragmentation into small pieces. During BC preparation, the maximum temperature reached was 570 ◦C after 20 min of ignition with an average temperature of ~ 325 ± 5 °C throughout the process in a stainless-steel kiln. After the pyrolysis process was completed, the substance was cooled, crushed, and ground into particles smaller than < 2 mm. The properties of BC are presented in (Table 1).
Depletion of Ash from Biochar
It was first washed with tap water to prepare the BC to eliminate any impurities. Once the ash content was eliminated, the BC underwent a thorough rinsing with deionized water to ensure the removal of any remaining ash residues. Subsequently, it was air-dried in a well-ventilated space until it reached complete dryness18. Finally, the deashed BC was properly stored for further use.
Procurement and sterilization of seeds
FH-1036 hybrid seeds were acquired from a local market. The collection of plant material complies with relevant institutional, national, and international guidelines and legislation. The seed sterilization involved three rinses with 95% ethanol followed by the application of 5% sodium hypochlorite (NaOCl). The procedure entailed immersing the seeds in a NaOCl solution for 30 min followed by three washes using 95% ethanol27,28,29.
Experimental setup
Powdered deashed BC was blended with 90% pure GA3. The process commenced with finely grinding the prepared deashed BC into powder. Subsequently, using an analytical balance, 10 ppm GA3 was precisely weighed and mixed with the BC. This resulting powder was promptly employed as a soil amendment after processing. As per the treatment plan, 0.75% BC was manually added to the soil followed by pot filling. Three different Cd levels were applied detailed below in the treatment protocol30. Cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O) (ALDRICH, CAS number − 10022-68-1, MDL number - MFCD00149626, product number − 642045 and batch number - MKCT3996) was employed to induce Cd toxicity22. The spiking technique involved carefully adding cadmium nitrate solution to 35 kg of soil over 21 days to ensure a consistent distribution. The soil, placed in a plastic container for the spiking process, was covered after spiking to prevent contamination and a 21-day incubation period was followed. This incubation duration was selected to facilitate prolonged interaction between Cd and the soil matrix.
Treatment protocol
A completely randomized design served as the foundation for the experimental setup. All treatments were replicated three times for robustness. The investigations included twelve different treatments. These are; T1: Control (No Cd stress), T2: Mild Cd stress (8 mg Cd/kg soil), T3: Severe Cd stress (16 mg Cd/kg soil), T4: 10 ppm GA3 (No Cd stress), T5: 10 ppm GA3 + Mild Cd stress, T6: 10 ppm GA3 + Severe Cd stress, T7: 0.75% Biochar (No Cd stress), T8: 0.75% Biochar + Mild Cd stress, T9: 0.75% Biochar + Severe Cd stress, T10: 10ppm GA3 + 0.75% Biochar (No Cd stress), T11: 10ppm GA3 + 0.75% Biochar + Mild Cd stress and T12: 10ppm GA3 + 0.75% Biochar + Severe Cd stress. The optimization of different doses in the experiment was based on a combination of previous research, pilot studies, and a desire to cover a range of Cd stress levels. The selection of specific concentrations such as 8 mg Cd/kg soil for mild stress, 16 mg Cd/kg soil for severe stress and 10 ppm GA3, as well as the combination of BC and GA3, have been influenced by established literature on Cd toxicity thresholds, known effective concentrations of GA3 and the aim to observe a spectrum of responses in maize plants under varying stress conditions. The goal was to create a comprehensive experimental design that allows for a thorough evaluation of the effects of different treatments on plant growth under diverse Cd stress scenarios.
Fertilizer application
The application of recommended fertilizer rates for macronutrients involved 200 kg N, 150 kg P, and 100 kg K per hectare31,32,33. Urea, single superphosphate and sulfate of potash were used as sources for nitrogen (N), phosphorus (P) and potassium (K) respectively to supply these nutrients. The experimental design considered the application of normal fertilization practices to ensure proper plant nutrition across all treatment groups. This approach aimed to isolate the specific impact of BC and GA3 on Cd stress alleviation without confounding factors related to nutrient deficiencies. By providing a consistent nutritional baseline, the study aimed to assess the unique contributions of BC and GA3 in mitigating Cd stress, ensuring that any observed effects could be attributed to these amendments rather than variations in nutrient availability.
Irrigation practices
Water was provided to the pots three to four days a week until the maize plants reached full physiological development. According to Danish and Zafar-ul-Hye, maize plants were irrigated to maintain 60% field capacity (FC) during the growth season34,35.
Crop Harvest
Maize plants were harvested at the V10 Zadoks growth stage. Samples of roots, shoots, and leaves were obtained to collect data.
Determination of relative water content (RWC) of leaf
RWC of fresh leaves was determined using Weatherley’s method36. First, when the leaf discs had been detached from the young leaves, their fresh weight was measured. After being positioned in petri plates filled with distilled water and given time to saturate the identical discs’ turgid weights were calculated. The leaf discs’ dry weight was computed following oven drying. The formula below was used to determine the RWC:
where: FW = fresh weight, DW = dry weight, TW = turgid weight.
Enzyme extraction
Enzyme extraction was carried out from 200 mg of fresh root and shoot samples. These samples were homogenized in 5 ml of 50 mM potassium phosphate buffer (pH 7.8) containing 1% polyvinylpyrrolidone (PVP), 1 mM ascorbic acid and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenate was centrifuged at 22,000 g for 10 min at 4 °C. The resulting supernatant was collected and used for enzyme activity assays.
Protein content determination
The protein content of the samples was determined using the Bradford method. Briefly, a standard curve was prepared using bovine serum albumin (BSA). The supernatant obtained from the enzyme extraction was mixed with Bradford reagent, and the absorbance was measured at 595 nm. The protein concentration was then calculated using the standard curve.
Determination of catalase (CAT) activity
Catalase activity was measured by following the decrease in absorbance at 240 nm due to the decomposition of H2O2. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 10 mM H2O2, and the enzyme extract. The change in absorbance was recorded for 2 min. Catalase activity was calculated using an extinction coefficient (ε) of 0.036 mM− 1 cm− 1 and expressed as EU mg− 1 protein37.
Determination of peroxidase (POD) activity
Peroxidase activity was measured using guaiacol as the substrate. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 1 mM guaiacol, 1 mM H2O2, and the enzyme extract. The increase in absorbance at 470 nm was recorded for 3 min. POD activity was calculated using an extinction coefficient (ε) of 26.6 mM− 1 cm− 1 and expressed as EU mg− 1 protein38.
Determination of Superoxide dismutase (SOD) activity
Superoxide dismutase activity was determined by measuring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 13 mM methionine, 75 µM NBT, 0.1 mM EDTA, 2 µM riboflavin, and the enzyme extract. The reaction was initiated by exposing the mixture to light, and the reduction of NBT was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of NBT reduction and expressed as EU mg− 1 protein39.
Determination of ascorbate peroxidase (APX) activity
Ascorbate peroxidase activity was measured by monitoring the decrease in absorbance at 290 nm due to the oxidation of ascorbate. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.2 mM ascorbic acid, 0.2 mM EDTA, 1 mM H2O2, and the enzyme extract. The change in absorbance was recorded for 7 min at 30-second intervals. APX activity was calculated using an extinction coefficient (ε) of 2.8 mM− 1 cm− 1 and expressed as EU mg− 1 protein40.
Statistical analysis
Statistical analysis was performed using OriginPro 2021 software. Analysis of variance (ANOVA) followed by Fisher’s LSD test was used to determine significant differences between treatments.
Results
Effect of gibberellic acid (GA3) and Biochar (BC) on maize hydrogen peroxide (H2O2) contents in root, stem, and leaves under Cd stress
The application of GA3 and BC significantly influenced hydrogen peroxide (H2O2) contents in maize roots, stems, and leaves under Cd stress (CdS). According to the two-way ANOVA results, treatments (GA3, BC, GA3 + BC, and Control) and Cd stress levels (0, 8, 16 mg Cd/kg soil) had substantial effects on H2O2 contents. In the control plants, H2O2 content increased with higher Cd levels.
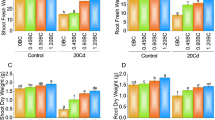
Specifically, root H2O2 content increased by 100% under 8 mg Cd/kg and 16 mg Cd/kg, respectively, compared to control. Similar trends were observed in stems and leaves. GA3 and BC treatments individually reduced H2O2 levels compared to control plants. Under 8 mg Cd/kg, GA3 reduced root H2O2 by 20%, stem H2O2 by 22%, and leaf H2O2 by 18%, while BC reduced root, stem, and leaf H2O2 by 25%, 30%, and 28%, respectively. Under 16 mg Cd/kg, GA3 reduced root, stem and leaf H2O2 by 30%, 35%, and 33%, while BC reduced these levels by 35%, 40%, and 38%. The combined GA3 + BC treatment showed the most significant reduction in H2O2 levels across all plant parts. Under 8 mg Cd/kg, GA3 + BC reduced root H2O2 by 40%, stem H2O2 by 45%, and leaf H2O2 by 42%. Under 16 mg Cd/kg, this treatment reduced root, stem, and leaf H2O2 by 50%, 55% and 53%, respectively. This indicates a synergistic effect of GA3 and BC in mitigating oxidative stress induced by Cd, highlighting their potential to enhance Cd stress tolerance in maize by significantly reducing H2O2 accumulation (Fig. 1).
Effect of gibberellic acid (GA3) and Biochar (BC) on maize Superoxide Dismutase (SOD) contents on root, stem and leaves under Cd stress
The application of GA3 and BC significantly influenced SOD activity in the roots, stems, and leaves of maize under Cd stress. Under control conditions (0 Cd), root SOD activity increased by approximately 30% with GA3, 25% with BC and 35% with combined GA3 + BC treatment. However, under moderate Cd stress (8 Cd), root SOD activity decreased by 10% for GA3, 15% for BC, and 20% for the combined treatment (Fig. 2).
At high Cd stress (16 Cd), the reductions were more pronounced, with decreases of 40%, 35%, and 50% for GA3, BC and the combined treatment, respectively. Stem SOD activity under control conditions increased by 20% with GA3, 15% with BC, and 25% with GA3 + BC, but decreased under moderate Cd stress by 10%, 5%, and 15%, and under high Cd stress by 30%, 25%, and 35% for GA3, BC and the combined treatment, respectively. Similarly, leaf SOD activity under control conditions rose by 25% for GA3, 20% for BC, and 30% for GA3 + BC. Under moderate Cd stress, it decreased by 15% for GA3, 10% for BC, and 20% for the combined treatment, while under high Cd stress, the reductions were 35%, 30%, and 40% for GA3, BC, and combined treatment, respectively. The combined GA3 and BC treatment demonstrated the highest SOD activity under non-stress conditions but also the most significant decline under severe Cd stress, with statistical analysis confirming highly significant differences in treatment effects, Cd stress levels, and their interactions on SOD activity across root, stem and leaf tissues.
Effect of gibberellic acid (GA3) and Biochar (BC) on maize peroxidase (POD) activity of root, stem and leaves under Cd stress
In the control group, a substantial 94.40% increase in root POD was noticed, which decreased to 56.88% under 8Cd stress and further to 21.56% in high Cd stress (16Cd). GA3 treatment without stress increased root POD by 102.1%, while GA3 with 8Cd stress raised it to 71.93%, and with 16Cd stress, it measured by 33.84%. BC treatment without stress significantly enhanced root POD to 113.02% and under 8Cd and 16Cd stress, it reached 82.15% and 42.55%, respectively. Combining GA3 + BC treatment during 0Cd stress led to a remarkable 132.83% increase in root POD, while under 8Cd and 16Cd stress, it measured 86.80% and 49.18%, respectively (Fig. 3).
Similarly, in the stem, 0Cd stress resulted in a 25.20% increase, dropping to 18.36% under 8Cd and further to 8.93% in 16Cd stress. GA3 treatment without stress raised stem POD by 28.57%, and with 8Cd stress, it reached 19.96%, while with 16Cd stress, it measured by 11.91%. BC treatment without stress significantly enhanced stem POD to 34.27%, and under 8Cd and 16Cd stress, it reached 20.61% and 13.84%, respectively. Combining GA3 + BC treatment during 0Cd stress resulted in a substantial 35.61% increase in stem POD, while under 8Cd and 16Cd stress, it measured 23.51% and 15.96%, respectively. In the leaves, 0Cd stress increased POD by 56.58%, decreasing to 34.88% under 8Cd and further to 19.67% under 16Cd stress. GA3 treatment without stress raised leaf POD by 64.54%, and with 8Cd stress, it reached 40.83%, while with 16Cd stress, it increased by 27.11%. BC treatment without stress significantly enhanced leaf POD to 70.33%, and under 8Cd and 16Cd stress, it reached 45.41% and 30.75%, respectively. Combining GA3 + BC treatment during 0Cd stress resulted in a substantial 79.83% increase in leaf POD, while under 8Cd and 16Cd stress, it measured 49.60% and 32.69%, respectively.
Effect of gibberellic acid (GA3) and Biochar (BC) on maize catalase (CAT) activity of root, stem and leaves under Cd stress
The results indicate distinct responses in CAT activity based on different stress and treatment conditions. In the root, exposure to 0Cd stress led to a substantial 51.96% increase in CAT activity compared to the control, whereas under 8Cd stress, a decrease to 35.38% was observed, further dropping to 12.91% under intense 16Cd stress (Fig. 4).
Treatment with GA3 alone, particularly under 0Cd stress, significantly elevated root CAT activity by 10.64%, while the combined GA3 and 8Cd stress increased it by 18.71%.The addition of BC without stress (0Cd) remarkably enhanced root CAT activity by 20.07%, and under 8Cd stress, it marked a substantial 32.33% increase. The GA3 + BC treatment during 0Cd stress resulted in a considerable 31.23% rise, and under 8Cd stress, it showcased a notable 40.89% increase. High CdS (16Cd) with GA3 + BC treatment displayed a significant 192.25% rise over the control. Similar patterns of response were observed in the stem and leaves, highlighting the varied impact of GA3 and BC on maize CAT activity under different Cd stress conditions.
Effect of gibberellic acid (GA3) and Biochar (BC) on maize Ascorbate peroxidase (APX) activity of root, stem, and leaves under Cd stress
The results reveal distinct patterns in APX activity based on various treatments. Notably, under 0Cd stress, root APX exhibited a significant increase while GA3 treatment without stress further augmented this activity by 11.43%. In contrast, BC treatment without stress remarkably enhanced root APX by 23.44%. Interestingly, combining GA3 + BC treatment during 0Cd stress resulted in a substantial 38.93% increase in root APX. Similar trends were observed in stem and leaf APX activities, highlighting the nuanced responses to different treatments and Cd stress levels (Fig. 5).
Discussion
Under stress, H2O2 has a significant signaling role, but it can also negatively affect plant cells. According to recent research, the treatment of jasmonic acid and gibberelline in chickpea plants under Cd stress lowered H2O2 levels41. Cu and Ni stress increased H2O2 levels in all cultivars, with maize showing the most increase in H2O2 with Ni stress, no matter the cultivars, using Trichoderma asperellum and BC together reduced the oxidative stress brought on by Cu poisoning42. The study found that treating the ML-2056 variety with Cd increased H2O2 levels, while exogenous GA3 reduced ROS generation, thus mitigating Cd-induced oxidative damage. Similar effects were observed in fenugreek43. Recent research showed decreased H2O2 in GA-treated Solanum lycopersicum (tomato)44. Under Ni stress, maize had the highest H2O2 increase. Application of T. asperellum and BC combination reduced Cu-induced oxidative stress, regardless of cultivars45. Thiourea and BC together effectively decreased H2O2 and EL levels by 26%, 20%, and 21% in comparison to the control group46. High concentrations of heavy metals enhance the generation of ROS, such as hydrogen peroxide, hydroxyl radicals, and superoxide radicals, which disrupt metabolic activities in plants47. Despite H2O2’s role in signaling during stress, CdS induced higher H2O2 production in chickpea plants48. However, a previous study found that Chickpea plants under Cd stress were given JA and GA3 treatments exhibited lower H2O2 and MDA levels, indicating defense against Cd stress-induced lipid peroxidation; jasmonic acid has a reputation for protecting cell membrane components49.
Previous research revealed that the application of Bio + Asa’s combo application had a significant positive influence. Further studies found that different kinds of BC significantly increased wheat grain yield while also reducing Cd bioavailability50. P and GA3 administration resulted in a reduction in MDA, H2O2, EL, and MDA was found in the roots and leaves of C. capsularis, and plants grown under different levels of Cu stress showed an increase in the enzyme activity of SOD, POD, CAT, and APX. SOD, CAT, GR, and APX enzymatic antioxidant activity increased in plants subjected to growth regulators (GA3 and Db-H)51. Current findings suggest that BC has a more pronounced effect in enhancing stem SOD activity, the antioxidant defense system in maize plants under CdS is considerably strengthened by the combination with GA3. The mechanisms behind the synergistic action of GA3 and BC in improving plant tolerance to heavy metal toxicity require further study. Without regard to cultivars, the combination application of T. asperellum and BC increased the concentration of SOD and APX under Cu toxicity. In seedlings subjected to Cd stress, SOD, CAT, and POX activities were higher than in controls, and they grew in proportion to the amount of metal present in the medium. The presence of BC considerably lowered the Cd levels52. Low GA3 concentrations (3–5 mg L− 1) significantly increased the activities of antioxidant enzymes (SOD, POD, CAT, and APX) and biomass. These elements may be the root of this phenomenon: According to Kohli, (i) low GA3 concentration can minimize the oxidative damage to plants brought on by high Cu concentration, and hence lessen the harm brought on by Cu53.
The presence of HMs in the water interfered with the important enzyme activities for plant absorption; this interference was prevented by the administration of GA3. Our findings align with a previous study, that investigated mung beans54. Increased antioxidant enzyme activities (APX, GPX, GST), better Gly enzyme (Gly I and II) activities, improved nutritional uptake, and efficient ROS detoxification were all produced when JA and GA3 were applied to chickpea plants under Cd stress. Furthermore, the favorable characteristics of BC, including its porous structure, high surface charge density, and extensive surface area, enhance its ability to adsorb inorganic contaminants55. Previous studies have demonstrated that GA increases antioxidant enzyme activity, which may promote plant growth, and reduces the negative impacts of a variety of abiotic stimuli, as was seen in our work. The APX activities significantly changed because bamboo charcoal was added to the soil. There was no discernible difference between the impacts of the ZnO NPs alone and the bamboo BC on the APX activities in maize, according to a recent study56. Spring corn’s leaf APX concentration was substantially lower when exposed to Ni toxicity than the control. However, regardless of the cultivars, Trichoderma asperellum and BC applied together improved the APX concentration under Cu toxicity. According to earlier research, the administration of Fe-BC under Cd stress inhibited the activities of CAT and APX. P and GA3 administration decreased MDA, H2O2, and EL in C. capsularis roots and leaves while increasing SOD, POD, CAT, and APX activity57.
By applying exogenous foliar Thiourea and BC incorporation to the soil, CAT activities in maize were markedly improved. In seedlings subjected to the concurrent treatment of GA3 and Cd, a significant rise in the enzymatic activity was also observed58. Previous studies have demonstrated that GA increases the activity of antioxidant enzymes, reducing the negative effects of a variety of abiotic stressors, which, as demonstrated by our research, may accelerate plant growth. According to previous research, several forms of BC wheat grain showed a significant increase and Cd bioavailability was reduced59. The agricultural area that has high levels of Cd contamination has an impact on plant growth. To reduce the toxicity of Cd in plants, Cd uptake and accumulation must be restricted. Cd poisoning harmed the development of maize plants. P and GA3 administration resulted in a reduction in MDA, H2O2, EL, and MDA C. Capsular roots and leaves, while plants grown under varied levels of Cu stress showed an increase in the activities of SOD, POD, CAT, and APX60. However, regardless of the cultivars, the combination application of Trichoderma asperellum and BC increased the CAT concentration under Cu toxicity. CAT activities were increased in maize leaves after GA3 treatment, which may help to lower the H2O2 level. In comparison to controls, soybean leaves with higher Cd toxicity produced less SOD and CAT and more POD61. In comparison to controls, soybean leaves with higher Cd toxicity produced less SOD and CAT and more POD. BC treatment boosted SOD and CAT production while decreasing POD formation in soil with low and high Cd spiking levels62. By reducing oxidative stress and enhancing antioxidant defenses, gibberellins (GA) can protect plants from the damaging effects of trace metals. POD activity rises as a result of BC enhancement of the stem’s antioxidant defense mechanism. According to earlier studies, treatment with BC and thiourea decreased the negative effects of Cd on lipid peroxidation and cell membrane permeability. Neelam and desi makai, however, displayed the maximum development at 200 m Cadmium chloride and 0.25 mg GA63. The POD concentration under Cu toxicity was increased by the combination treatment of T. asperellum and BC, regardless of the cultivars, these are consistent with our findings. Recent studies have shown that plants under Cd stress treated with uniconazole and GA3 have higher POD activity than the plants in the control group. POD activities were increased in maize leaves after GA3 treatment, which may help to lower the H2O2 level, similar to the current study64.
For future investigations, it is recommended to delve deeper into the combined application of BC with other soil amendments to understand their synergistic effects on plant growth and stress tolerance. Long-term field trials are essential to assess the persistent impacts of these amendments on soil properties and crop productivity over multiple seasons. Exploring the influence of BC and GA3 on soil microbial communities can provide insights into their role in enhancing soil fertility and ecosystem functioning. Additionally, practical field application strategies need to be optimized to ensure the effective delivery of these amendments to plant roots while minimizing environmental risks. Crop-specific responses to BC and GA3 under heavy metal stress conditions should be investigated to tailor management practices for different agricultural systems. Furthermore, assessing the ecological impacts and scaling up studies to larger agricultural landscapes can validate the feasibility and sustainability of these interventions for mitigating heavy metal stress in agriculture.
Conclusion
This study focused on addressing the environmental challenge posed by cadmium (Cd), a prevalent contaminant due to widespread industrial use. The stress induced by Cd significantly disrupts essential physiological and metabolic functions in plants, emphasizing the need for effective mitigation strategies. Through a comprehensive investigation, this research explored the combined impacts of BC and GA3 in alleviating CdS, specifically in maize. The results revealed that the application of GA3 + BC significantly enhanced growth parameters, including root, stem, leaf H2O2, SOD, APX, CAT, and POD compared to the control under different Cd levels. The findings suggest the potential for the sustainable mitigation of heavy metal-induced stress in crop cultivation, offering valuable insights into environmental and agricultural practices. Detailed molecular studies can unravel the specific pathways influenced by GA3 + BC treatment, shedding light on how these components interact at the cellular and genetic levels.
Data availability
The author confirms that all data generated or analyzed during this study are included in this published article.
References
Anjum, S. A. et al. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. CLEAN-Soil Air Water 44, 29–36. https://doi.org/10.1002/clen.201400905 (2016).
Cao, J., Feng, Y., He, S. & Lin, X. Silver nanoparticles deteriorate the mutual interaction between maize (Zea mays L.) and arbuscular mycorrhizal fungi: A soil microcosm study. Appl. Soil. Ecol. 119, 307–316. https://doi.org/10.1016/j.apsoil.2017.04.011 (2017).
Rizwan, M. et al. Use of maize (Zea mays L.) for photo management of Cd-contaminated soils: A critical review. Environ. Geochem. Health 39, 259–277. https://doi.org/10.1007/s10653-016-9826-0 (2017).
Ghous, M. et al. Halophyte quinoa: A potential hyperaccumulator of heavy metals for phytoremediation. Asian J. Agric. Biol. 2022(4): 2021444. https://doi.org/10.35495/ajab.2021.444
Jiang, W., Liu, D. & Hou, W. Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic (Allium sativum L). Bioresour. Technol. 76, 9–13. https://doi.org/10.1016/S0960-8524(00)00086-9 (2001).
Suleman, M. et al. Determining the Cadmium accumulation in Maize (Zea mays L.) and soil influenced by phosphoric fertilizers in two different textured soils. Land 11, 1313. https://doi.org/10.3390/land11081313 (2022).
Xu, W. et al. Uptake and distribution of cd in sweet maize grown on contaminated soils: A field-scale study. Bioinorg. Chem. Appl. 2013, 959764. https://doi.org/10.1155/2013/959764 (2013).
Soni, S., Jha, A. B., Dubey, R. S. & Sharma, P. Mitigating cadmium accumulation and toxicity in plants: The promising role of nanoparticles. Sci. Total Environ. 912, 168826. https://doi.org/10.1016/j.scitotenv.2023.168826 (2024).
Zulfiqar, U. et al. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 250, 109557 (2019).
Anwar, Z. et al. Biofortification of maize with zinc and iron not only enhances crop growth but also improves grain quality. Asian J. Agric. Biol. 2022(2): 202102079. https://doi.org/10.35495/ajab.2021.02.079
Sandalio, L. M., Dalurzo, H. C., Gomez, M., Romero-Puertas, M. C. & Del Rio, L. A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 52, 2115–2126. https://doi.org/10.1093/jexbot/52.364.2115 (2001).
Sharma, P. & Dubey, R. S. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentration of aluminum. Plant. Cell. Rep. 26, 2027–2038. https://doi.org/10.1007/s00299-007-0416-6 (2007).
Feng, J. et al. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (cd) toxicity in Cucumis sativus L. Sci. Hortic. 123, 521–530. https://doi.org/10.1016/j.scienta.2009.10.013 (2010).
Tuna, A. L., Kaya, C., Dikilitas, M. & Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 62, 1–9. https://doi.org/10.1016/j.envexpbot.2007.06.007 (2008).
Muddassir, M. & Alotaibi, B. A. Farmers’ awareness of Improved Maize Cropping practices in Punjab, Pakistan. Int. J. Agric. Biosci. 12(1), 8–17 (2023).
Pangaribuan, D. H., Widagdo, S., Hariri, A. M., Siregar, S. & Sardio, M. I. The effect of rice straw mulch and cow urine on growth, yield, quality on sweet corn and pest population density. Asian J. Agric. Biol. 2023(1): 202103123. https://doi.org/10.35495/ajab.2021.03.123
Noman, M. U. & Azhar, S. Metabolomics, a potential way to Improve Abiotic stresses tolerance in cereal crops. Int. J. Agric. Biosci. 12(1), 47–55 (2023).
Zulfiqar, U. et al. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil. Sci. Plant. Nutr. 1–58 (2022).
Anwar, T. et al. Synergistic effect of biochar-based compounds from vegetable wastes and gibberellic acid on wheat growth under salinity stress. Sci. Rep. 13, 19024. https://doi.org/10.1038/s41598-023-46487-0 (2023).
Gilani, S. A. Q. et al. Gibberellic Acid and Boron enhance antioxidant activity, phenolic content, and yield quality in Pyrus communis L. Gesunde Pflanzen 73, 395–406. https://doi.org/10.1007/s10343-021-00555-5 (2021).
Wu, Y. et al. The critical role of biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front. Plant. Sci. 14, 1163451. https://doi.org/10.3389/fpls.2023.1163451 (2023).
Abedinzadeh, M., Etesami, H., Alikhani, H. A. & Shafiei, S. Combined use of municipal solid waste biochar and bacterial biosorbent synergistically decreases cd (II) and pb (II) concentration in edible tissue of forage maize irrigated with heavy metal–spiked water. Heliyon 6, e04688. https://doi.org/10.1016/j.heliyon.2020.e04688 (2020).
Shahniza, S. S., Firdaus, M. I. & Roslan, I. Effect of time of application and concentrations of plant growth regulators on growth and yield of sweet corn (Zea mays L). Res. Crops 21, 46–53. https://doi.org/10.31830/2348-7542.2020.007 (2020).
Vwioko, E. D. Performance of soybean (Glycine max L.) variety in salt-treated soil environment following salicylic acid mitigation. J. Niger Soc. Exp. Biol. 13, 44–49 (2021).
Liu, Y. et al. Biochar application as a soil amendment for decreasing cadmium availability in soil and accumulation in Brassica chinensis. J. Soils Sediments 18, 2511–2519. https://doi.org/10.1007/s11368-018-1927-1 (2018).
Ahmad, S., Aziz, A. R., Ullah, A. & Muhammad Ali Raza, M. A. Effect of vermicompost and organic matter in enhancing wheat tolerance against Drought stress. Int. J. Agri Biosci. 11(3), 165–167 (2022).
Qian, T. T. et al. Screening of wheat straw biochars for the remediation of soils polluted with Zn (II) and Cd (II). J. Hazard. Mater. 362, 311–317. https://doi.org/10.1016/j.jhazmat.2018.09.034 (2019).
Wu, C. et al. Adsorption of cadmium on degraded soils amended with maize-stalk-derived biochar. Int. J. Environl Res. Public. Health 15, 2331. https://doi.org/10.3390/ijerph15112331 (2018).
Paz-Ferreiro, J., Lu, H., Fu, S., Mendez, A. & Gasco, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 5, 65–75. https://doi.org/10.5194/se-5-65-2014 (2014).
Liu, X. et al. Effects of PASP/NTA and TS on the phytoremediation of pyrene-nickel contaminated soil by Bidens pilosa L. Chemosphere 237, 124502. https://doi.org/10.1016/j.chemosphere.2019.124502 (2019).
Hussan, M. U. et al. Impact of soil applied humic acid, zinc and boron supplementation on the growth, yield and zinc translocation in wheat. Asian J. Agric. Biol. 2022(1): 202102080. https://doi.org/10.35495/ajab.2021.02.080
Zulfiqar, U. et al. Chromium toxicity, speciation, and remediation strategies in soil-plant interface: A critical review. Front. Plant. Sci. 13, 1081624 (2023).
Haroon, M. et al. Autoimmunity in plants; a powerful Weapon in Kingdom Plantae to Combat stresses. Int. J. Agri Biosci. 12(3), 159–164 (2023).
Sparks, D. L., Page, A. L., Helmke, P. A. & Loeppert, R. H. (eds) Methods of soil Analysis, part 3: Chemical Methods Vol. 14 (Wiley, 2020).
Pratt, P. F. Potassium. Potassium. Methods of soil analysis: Part 2. Chem. Microbiol. Prop. 9, 1022–1030. https://doi.org/10.2134/agronmonogr9.2.c20 (1965).
Ahmad, I. et al. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut Res. 21, 11054–11065. https://doi.org/10.1007/s11356-014-3010-9 (2014).
Sultan, H., Ahmed, N., Mubashir, M. & Danish, S. Chemical production of acidifed activated carbon and its infuences on soil fertility comparative to thermo-pyrolyzed biochar. Sci. Rep. 10, 595. https://doi.org/10.1038/s41598-020-57535-4 (2020).
Ćwieląg-Piasecka, I. et al. Deashed wheat-straw biochar as a potential superabsorbent for pesticides. Materials 16, 2185. https://doi.org/10.3390/ma16062185 (2023).
Naz, I. et al. Effectiveness of ACC-deaminase containing Pseudomonas strains to induce salinity tolerance in maize under fertilized and unfertilized field conditions. Soil. Environ. 32, 167–172 (2013).
Abd El-Fattah, D. A., Hashem, F. A. & Abd-Elrahman, S. H. Impact of applying organic fertilizers on nutrient content of soil and lettuce plants, yield quality and benefit-cost ratio under water stress conditions. Asian J. Agric. Biol. 2022(2): 202102086. https://doi.org/10.35495/ajab.2021.02.086
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant. Physiol. 59, 309–314. https://doi.org/10.1104/pp.59.2.309 (1977).
Moran, J. F. et al. Drought induced oxidative stress in pea plants. Planta 194, 346–352. https://doi.org/10.1007/BF00197534 (1994).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880. https://doi.org/10.1016/0032-3861(81)90258-5 (1981).
Saeed, M. Z., Hayat, H., Shafiq, F. & Tareen, W. H. Assessing the prospects and challenges of Organic Agriculture in the Pothwar Region of Punjab, Pakistan. Int. J. Agri Biosci. 12(1), 1–7 (2023).
OriginLab & Corporation OriginPro (OriginLab, 2021).
Ahmad, M. et al. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 194, 327–339. https://doi.org/10.1016/j.chemosphere.2017.11.156 (2018).
Hasan, S., Sehar, Z. & Khan, N. A. Gibberellic acid and sulfur-mediated reversal of cadmium-inhibited photosynthetic performance in mungbean (Vigna radiata L.) involves nitric oxide. J. Plant. Growth Regul. 39, 1605–1615. https://doi.org/10.1007/s00344-020-10175-4 (2020).
Amanullah, F. & Khan, W. U. D. Trichoderma asperellum L. coupled the effects of biochar to enhance the growth and physiology of contrasting maize cultivars under copper and nickel stresses. Plants 12, 958 (2023). https://doi.org/10.3390/plants12040958
Mukarram, M., Mohammad, F., Naeem, M. & Khan, M. M. A. Exogenous gibberellic acid supplementation renders growth and yield protection against salinity-induced oxidative damage through upregulating antioxidant metabolism in fenugreek (Trigonella Foenum-Graceum L.) Fenugreek. Biol. Appl. 99–117. https://doi.org/10.1007/978-981-16-1197-1_6 (2021).
Kaya, C., Sarioglu, A., Ashraf, M., Alyemeni, M. N. & Ahmad, P. Gibberellic acid-induced generation of hydrogen sulfide alleviates boron toxicity in tomato (Solanum lycopersicum L.) plants. Plant. Physiol. Biochem. 153, 53–63. https://doi.org/10.1016/j.plaphy.2020.04.038 (2020).
Haider, F. U. et al. Integrated application of thiourea and biochar improves maize growth, antioxidant activity and reduces cadmium bioavailability in cadmium-contaminated soil. Front. Plant. Sci. 12, 809322. https://doi.org/10.3389/fpls.2021.809322 (2022).
Souguir, D., Ferjani, E., Ledoigt, G. & Goupil, P. Sequential effects of cadmium on genotoxicity and lipo-peroxidation in Vicia faba roots. Ecotoxicol. Environ. Saf. 20, 329–336. https://doi.org/10.1007/s10646-010-0582-0 (2011).
Nazir, F., Fariduddin, Q. & Khan, T. A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252, 126486. https://doi.org/10.1016/j.chemosphere.2020.126486 (2020).
Ali, E. et al. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to cd toxicity. J. Zhejiang Univ. Sci. B 19, 130–146. https://doi.org/10.1631/jzus.B1700191 (2018).
Yaseen, S. et al. Supplemental effects of biochar and foliar application of ascorbic acid on physio-biochemical attributes of barley (Hordeum vulgare L.) under cadmium-contaminated soil. Sustainability 13, 9128. https://doi.org/10.3390/su13169128 (2021).
Rehman, M. Z. et al. Residual effects of frequently available organic amendments on cadmium bioavailability and accumulation in wheat. Chemosphere 244, 125548. https://doi.org/10.1016/j.chemosphere.2019.125548 (2020).
Dad, F. P. et al. Influence of iron-enriched biochar on cd sorption, its ionic concentration and redox regulation of radish under cadmium toxicity. Agriculture 11, 1. https://doi.org/10.3390/agriculture11010001 (2020).
Rady, M. M. et al. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 10, 748. https://doi.org/10.3390/plants10040748 (2021).
Zheng, R. et al. Mitigating heavy metal accumulation into rice (Oryza sativa L.) using biochar amendment-a field experiment in Hunan, China. Environ. Sci. Pollut Res. 22, 11097–11108. https://doi.org/10.1007/s11356-015-4268-2 (2015).
Gong, Q. et al. Gibberellic acid application on biomass, oxidative stress response, and photosynthesis in spinach (Spinacia oleracea L.) seedlings under copper stress. Environ. Sci. Pollut Res. 28, 53594–53604. https://doi.org/10.1007/s11356-021-13745-5 (2021).
Kohli, S. K. et al. Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol. Environ. Saf. 147, 382–393. https://doi.org/10.1016/j.ecoenv.2017.08.051 (2018).
Chauhan, R. et al. Heavy metal tolerance in crop plants: Physiological and biochemical aspects. Plant Adapt. Strateg. Changing Environ. 253–267 (2017). https://doi.org/10.1007/978-981-10-6744-0_10
Hossain, M. A., Hasanuzzaman, M. & Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 16, 259–272. https://doi.org/10.1007/s12298-010-0028-4 (2010).
Xu, X., Cao, X. & Zhao, L. Comparison of rice husk- and dairy manure derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 92, 955–961. https://doi.org/10.1016/j.chemosphere.2013.03.009 (2013).
Acknowledgements
This project was supported by Researchers Supporting Project Number (RSP2024R98) King Saud University, Riyadh, Saudi Arabia.
Funding
This project was supported by Researchers Supporting Project Number (RSP2024R98) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
TA: Methodology, supervision, Writing and drafting, and research design; HQ: Experimentation, validation, Software, and data Curation; MJ: writing, Investigation, drafting, statistical analysis; and validation; EHS: writing, Software, Resource, research design, validation; WZ: validation, data collection, drafting, statistical analysis; SAA, MJA: writing, statistical analysis, Resource, software, validation. All authors have read and approved the final manuscript and declare that they have no competitive interest.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Consent for publication
Not applicable.
Study protocol must comply with relevant institutional, national, and international guidelines and legislation
Our experiment follows the relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Anwar, T., Qureshi, H., Jabeen, M. et al. Exploring the synergistic benefits of biochar and gibberellic acid in alleviating cadmium toxicity. Sci Rep 14, 24196 (2024). https://doi.org/10.1038/s41598-024-73678-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73678-0