Abstract
Stem nematode (Ditylenchus destructor Thorne) is considered one of the most economically devastating species affecting sweet potato production. Biocontrol offers a sustainable strategy for nematode control. This study conducted a pot experiment to evaluate the biocontrol efficacy of Paecilomyces lilacinus CS-Z and Bacillus pumilus Y-26 against the stem nematode, as well as to examine their influence on the bacterial communities in the sweet potato rhizosphere. The findings indicated that B.pumilus Y-26 and P.lilacinus CS-Z exhibited respective suppression rates of 82.9% and 85.1% against the stem nematode, while also stimulating sweet potato plant growth. Both high-throughput sequencing and Biolog analysis revealed distinct impacts of the treatments on the bacterial communities. At the phylum level, B.pumilus Y-26 enhanced the abundance of Actinobacteria but reduced the abundance of Cyanobacteria, with P.lilacinus CS-Z exhibiting similar effects. Additionally, the treatment with B.pumilus Y-26 resulted in increased abundances of Crossiella, Gaiella, Bacillus, and Streptomyces at the genus level, while the treatment with P.lilacinus CS-Z showed increased abundances of Crossiella and Streptomyces. In contrast, the abundance of Pseudarthrobacter was reduced in the treatment with B.pumilus Y-26. Conversely, the application of the nematicide fosthiazate exhibited minor influence on the bacterial community. The findings indicated that the application of P.lilacinus CS-Z and B.pumilus Y-26 led to an increase in the relative abundances of beneficial microorganisms, including Gaiella, Bacillus, and Streptomyces, in the rhizosphere soil. In conclusion, P.lilacinus CS-Z and B.pumilus Y-26 demonstrated their potential as environmentally friendly biocontrol agents for managing stem nematode disease of sweet potato.
Similar content being viewed by others
Introduction
Sweet potato (Ipomoea batatas (L.) Lam.) is a commercially important crop both for the food market and bio-energy fuel production throughout the world1. Statistically, China is the main sweet potato producer in the world, with a cultivated area of 22,500 km2 and a production of 4.5 × 107 tons ( FAO, 2020, http://faostat.fao.org/). In China, the sweet potato plants are often cropped continuously, which results in the very rapid build-up of soil-borne pathogens. Stem nematode (Ditylenchus destructor Thorne) is one of the most important soil-borne pathogens in sweet potato production, causing yield losses estimated to be around 20–50% usually, and even no yield in the field if seriously infected by stem nematodes in China2,3. Obviously, the current losses may be greater. Since 1937, stem nematode has been documented in China as significant pests. Thus, the management of sweet potato stem nematode is important. In past decades, the application of chemical nematicides appeared to be the important method to this parasite. However, there are many problems associated with the use of nematicides, such as their high toxicity they have adverse effects on non-target organisms, and they can accumulate in the soil damaging the ecological environment4. Therefore, an eco-friendly sweet potato stem nematodes management strategy is urgently needed to replace chemical nematicides.
Plant-parasitic nematodes have many natural enemies, particularly some biocontrol microorganisms (e.g., Paecilomyces spp., Bacillus spp., Fusarium spp., and Syncephalastrum spp.) that can parasitize nematodes and their eggs. Some of these microorganisms have been found to release compounds which have nematicidal effects5,6, promote plant growth7, and survive in the soil for a long time and proliferate quickly in appropriate conditions8. Among these microorganisms, Paecilomyces lilacinus, a nematode egg parasite, is currently used as a biological control agent against various plant-parasitic nematodes8,9. This fungus can reduce soil and root populations of Meloidogyne incognita and increase tomato yield10,11. Furthermore, P.lilacinus has been found to suppress the potato cyst nematode Globodera rostochiensis (Wollenweber) Behrens (Tylenchida: Heteroderidae)12. In addition, few studies have investigated the potential application of Bacillus pumilus against plant-parasitic nematodes. Ramezani et al.13 found that the application of B. pumilus against M. javanica efficiently reduced the number of galls and eggs of in tomato plants. Similarly, inoculation with B. pumilus also resulted in a reduction in the number of egg masses and galls in tomato plants infected by M. arenaria14. Currently, there is few documented research on the application of P. lilacinus or B. pumilus as a biocontrol agent against the stem nematode disease of sweet potato.
In the present study, we investigated the effects of P.lilacinus CS-Z and B.pumilus Y-26 on stem nematode infection and plant growth in sweet potato. Additionally, irrigating roots treated with the nematicide fosthiazate is considered one of the most effective measures to control the stem nematode disease of sweet potato in China, and it acted as the conventional nematicide treatment in this study. Furthermore, the effects of P.lilacinus CS-Z and B.pumilus Y-26 on the rhizosphere bacterial communities of sweet potato were investigated using high-throughput sequencing technology. These results provides a theoretical basis for assessing the microbial ecological risk associated with the application of P.lilacinus CS-Z and B.pumilus Y-26, as well as their efficacy as biocontrol agents for controlling stem nematode disease.
Methods
Test materials
P.lilacinus CS-Z was inoculated into a 500-mL Erlenmeyer flask containing 200mL of potato dextrose broth and incubated at 28 °C with shaking at 180 rpm for 7 days. The liquid culture was filtered through two layers of sterile gauze and adjusted to 1 × 109 CFU/mL with sterile distilled water. B.pumilus Y-26 was inoculated into a 500-mL Erlenmeyer flask containing 200mL of liquid medium supplemented with beef extract and incubated at 37 °C with shaking at 180 rpm for 2 days. Then B.pumilus Y-26 culture was centrifuged at 5000r/min for 10 min to collect the cells. The harvested cells were resuspended in sterile distilled water to generated 1 × 109 CFU/mL. The concentration of P.lilacinus CS-Z and B.pumilus Y-26 suspension was determined by microscope and blood cell counting plate. P. lilacinus CS-Z was isolated from crab shells samples collected in the coast of Qinhuangdao city15. In addition, B.pumilus Y-26 was isolated from the rhizosphere soil of a cucumber field16. Both microbes exhibited strong activity against plant nematode and were preserved in our laboratory. Xiankuang water emulsion, containing 20% (w/w) total effective constituent with 20% fosthiazate, was purchased from Qingdao Grees Agriculture Ltd. (Qingdao, China).
Design of pot experiments
The pot experiments were performed at the greenhouse of the Hebei Normal University of Science & Technology located in Changli County, Qinhuangdao City. A stem nematode susceptible sweet variety Tengfei used in this study, is one of the most important commercial cultivars widely planted in China, it was purchased from Zhongshu Agricultural Technology Co., Ltd. (Lulong, China). Sweet potato seedlings up to 15 cm in size were selected and transplanted into 35 × 30 cm plastic pot with 10 kg soil. The soils used for the pot experiments were collected from a field site in Lu’long, Hebei province, China, with a 5-year history of continuous sweet potato cultivation that suffered from severe stem nematode disease. The soil had the following properties: organic carbon 9.4 g kg− 1, total nitrogen 117.6 mg kg− 1, available P 70.4 mg kg− 1, available K 12.9 mg kg− 1, and a pH of 6.8. The initial population of stem nematodes was 110 individuals per 100 cm3 of soil.
Three days after transplanting, forty potted plants were divided into four groups, ① CK: a negative control or untreated control, treated with 200 ml of sterile distilled water; ② PL, a biocontrol agent control, treated with 200 ml of P.lilacinus CS-Z suspension;③BP: a biocontrol agent control, treated with 200 ml of B.pumilus Y-26 suspension;④FO, a conventional nematicide control, treated with 200 ml of 20% fosthiazate diluted 1500 times. Altogether 240 pots of sweet potato were used in this study, treated at the greenhouse and performed the routine management practices of sweet potato.
Plant growth and disease index
At the harvesting stage of sweet potato, ten plants per treatment from each replicate were dug up, and measurements were taken for vine length, number of branches, number of storage roots, weight of storage roots and fresh weight of vines and leaves per plant. The soil adhering to the roots after gentle shaking was referred to as the rhizosphere soil. The remaining particles were brushed off and combined to form a rhizosphere soil sample17. To eliminate plant material, 24 soil samples were sieved through a 2.0 mm mesh and then stored at -80 °C for microbiomics analysis. Storage roots were examined for lesions and assigned a disease severity rating on a scale of 0–4(0 = healthy; 1 ≤ 25% the lesions areas of storage root; 2 = 25–50% of the lesions areas of storage root; 3 = 50–75% of the lesions areas of storage root; 4 = 75–100% of the lesions areas of storage root) following the reference by Qiao et al.18. The disease incidence, disease index, and biocontrol efficacy were calculated using the formula provided below.
Biolog analysis
BIOLOGTM ECO plates (Biolog Inc., Hayward, CA, USA), which contained three replicates of 31 carbon sources and a water blank containing no carbon source19, were used to generate community-level physiological profiles for the four kinds of rhizosphere soil samples. Biolog analysis was conducted following the methodology described previously20.
Illumina MiSeq sequencing and analysis
The total soil DNA was extracted using the Fast Soil DNA kit (Omega Biotek, Inc., Norcross, GA, USA) according to the manufacturer’s instruction. The concentration and purity of the extracted DNA were determined using a spectrophotometer, and the DNA samples were stored at − 20 °C for subsequent experiments. PCR amplification of the bacterial 16 S rRNA gene at the V3-V4 hypervariable region was performed using primers 338 F (5′- ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) as described by Lu et al.21. Illumina MiSeq sequencing was performed at Majorbio Biopharm Technology Co., Ltd. (Shanghai, China). All the raw sequencing data were deposited at the National Center for Biotechnology Information (NCBI) under the accession number PRJNA1075339.
The original sequences were processed using USEARCH software(Version 11.0; http://www.drive5.com/usearch/), and clustering was performed with the UPARSE-OUT algorithm at 97% similarity to group sequences into the same OUT. The Alpha-diversity indices were calculated using Mothur (Version 1.30.2; https://www.mothur.org/). Community structure was analyzed using principal coordinate analysis (PCoA), which was performed with QIIME (Version 1.9.1; http://qiime.org/) and the results were displayed using the vegan package in R (Version 2.15.3). The heatmap figure was generated using custom R scripts. All the bioinformatics analyses were performed by using the online tools of Majorbio Cloud Platform (https://cloud.majorbio.com/page/tools.html).
Statistical analysis
Statistical analyses were conducted using analysis of variance (ANOVA) with SAS statistical software(Version 9.21; SAS Institute Inc., USA). The results in the table are presented as the mean ± standard deviation. Significant differences between treatments were determined according to Duncan’s multiple range test at p < 0.05.
Results
Biocontrol effects of P.lilacinus CS-Z and B.pumilus Y-26 on stem nematode
The biocontrol effects of P.lilacinus CS-Z and B.pumilus Y-26 on stem nematodes were evaluated based on a pot experiment, as shown in Fig. 1; Table 1. At the harvesting stage, the CK treatment resulted in severe stem nematode disease in sweet potato, while the biocontrol agent treatment significantly reduced the disease severity and exhibited excellent biocontrol effects against stem nematodes in the pot experiment. Based on the data in Table 1, the treatments with the biocontrol agents B.pumilus Y-26 and P.lilacinus CS-Z reduced the disease index from 65.28 to 11.11% and 9.72%, respectively. They also inhibited the disease incidence from 83.33 to 44.44% and 38.89%, and achieved a biocontrol efficacy of 82.98% and 85.11% against stem nematode disease, respectively. However, there were no significant differences in both disease incidence and disease index between the different biocontrol agent treatments.
Effects of P.lilacinus CS-Z and B.pumilus Y-26 on the plant growth parameters of sweet potato
Comparing the CK treatment with the biocontrol agents P.lilacinus CS-Z and B.pumilus Y-26 in Table 2, the vine length, number of branches, weight of storage roots per plant, and fresh weight of vines and leaves per plant showed significant differences at the p < 0.05 level during the harvesting stages. These significant differences indicate that the biocontrol agent treatment reduced the losses caused by stem nematodes and increased the plant biomass of sweet potatoes to a considerable extent. However, the plant biomass of sweet potatoes in the B.pumilus Y-26 and P.lilacinus CS-Z treatments was smaller than that in the nematicide fosthiazate treatment. In fact, the nematicide fosthiazate, widely used in the field in China, might significantly increase sweet potato yield and decrease disease incidence, as indicated by the data in Tables 1 and 2.
Carbon utilization capacity of the bacterial community
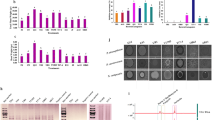
The Biolog EcoPlates comprised a total of 31 carbon sources, including nine carboxylic acids, six amino acids, four polymers, two amines, eight carbohydrates, and two aromatic compounds. Figure 2 illustrates the relative utilization efficiency of the six types of carbon sources by the rhizosphere bacterial community. Carbohydrates, amino acids, carboxylic acids, and polymers exhibited a high relative utilization efficiency. Conversely, aromatic compounds and amines demonstrated a low relative utilization efficiency. Compared to the control group (CK) and nematicide fosthiazate treatments, the rhizosphere bacterial community of sweet potato plants treated with the two biocontrol agents exhibited the most efficient utilization of amines. Slight differences were observed among the six types of carbon sources. These findings suggest that the application of biocontrol agents and the nematicide fosthiazate can influence carbon utilization and metabolism by bacteria in the rhizosphere soil. Principal component analysis (PCA) was performed for in-depth understanding of the variations in carbon utilization by rhizosphere bacteria. Figure 3 shows the PCA results of the carbon substrate utilization profiles of the rhizosphere bacterial community, with PC1 accounting for 53.1% and PC2 accounting for 39.4% of the data variability. The catabolic profiles of the rhizosphere bacterial community displayed distinct differences between the CK and B.pumilus Y-26 treatments, indicating a marked divergence in the rhizosphere bacterial community structure. However, the communities from the P.lilacinus CS-Z and nematicide fosthiazate treatments exhibited closer proximity to each other in quadrant IV. Additionally, significant differences (p < 0.05) were observed between the B. pumilus Y-26 and CK treatments in terms of average well color development (AWCD), Shannon’s diversity index, and substrate richness (Table 3).
Rhizosphere bacterial diversity
The richness and diversity of rhizosphere bacterial communities were assessed through alpha diversity analysis (Table 4). The Ace and Chao1 indices represent measurement of community richness, and the Shannon and Simpson indices represent indicators of community diversity and distribution evenness, respectively. In comparison to the CK treatment, the Chao1 and ACE indices exhibited a significant decrease (P < 0.05) following B.pumilus Y-26 treatment, whereas the nematicide fosthiazate and P.lilacinus CS-Z treatments did not show significant differences. This suggests that B.pumilus Y-26 treatment had a substantial influence on the richness of rhizosphere bacterial communities. Furthermore, there were significant differences in the Shannon and Simpson indices between the B.pumilus Y-26 and CK treatment, indicating that B.pumilus Y-26 treatment had a significant effect on the diversity of the rhizosphere bacterial communities.
Rhizosphere bacterial composition
The differences and similarities of bacterial community structures across all rhizosphere soil samples were illustrated using PCoA analysis (Fig. 4). This plot clearly separates the four treatment samples and indicates their unique clustering. Furthermore, the B.pumilus Y-26 treatment exhibited significant differences compared to the CK treatment, with the greatest distance observed. The PCoA analysis revealed that the first principal component (PC1) and the second principal component (PC2) accounted for 32.5% and 22.22% of the variation respectively, with a cumulative contribution rate of 54.72%.
Eleven bacterial phyla were identified and found to be presented in all rhizosphere soil samples (Fig. 5). The dominant bacterial phyla in rhizosphere soil were Actinobacteriota (28.79–33.94%), Proteobacteria (20.52–21.46%), Chloroflexi (14.78–17.56%), Acidobacteria (9.56–11.98%), Cyanobacteria (2.68–8.36%), follow by the minor phyla Bacteroidota (2.32–6.52%), Firmicutes (2.67–3.81%), Gemmatimonadota (2.53–2.98%), Myxococcota (2.18–2.71%), Verrucomicrobiota (0.95–1.26%), and Planctomycetota (0.81–1.05%). Compared to the CK treatment, application of the biocontrol agent B.pumilus Y-26 increased the relative abundances of Actinobacteriota, while the relative abundances of Cyanobacteria were reduced in the B.pumilus Y-26, P.lilacinus CS-Z, and nematicide fosthiazate treatments (p < 0.05).
The effect of different treatments on the dominant genera of bacteria (top 20) in rhizosphere soil is also shown in Fig. 6. The genera Crossiella, Gaiella, Bacillus, and Streptomyces exhibited significantly higher abundance in the rhizosphere soil samples treated with B.pumilus Y-26 compared to the CK treatment(p < 0.05). Additional, the relative abundances of Crossiella and Streptomyces were also increased in the P.lilacinus CS-Z treatment (p < 0.05). However, the relative abundance of Pseudarthrobacter deceased in the B.pumilus Y-26 treatment (p < 0.05). Interestingly, the nematicide fosthiazate treatment had minimal impact on the bacterial community compared to the CK treatment.
Discussion
Although the stem nematode can attack sweet potatoes and adversely affect both their yields and quality, information on the biocontrol of stem nematode disease in sweet potatoes is limited. This study evaluated the effectiveness of two biocontrol agents and the nematicide fosthiazate in controlling sweet potato stem nematode in a pot experiment. The biocontrol efficacy of B.pumilus Y-26 and P.lilacinus CS-Z, the two biocontrol agents, against stem nematode reached 82.98% and 85.11%, respectively. Furthermore, there were no differences in disease incidence and disease index among the various biocontrol agent treatments. Additionally, the treatments with biocontrol agents significantly reduced the losses caused by stem nematode and substantially increased the plant biomass of sweet potatoes. These results demonstrate that the application of the two biocontrol agents is an effective method for controlling sweet potato stem nematode.
Soil microorganisms act as vital components of the soil ecosystem, play a crucial role in nutrient cycling, organic matter formation and decomposition, soil structure, and plant systemic resistance22,23. The introduction of various beneficial microorganisms, such as symbiotic and free-living N-fixing bacteria, plant growth promoting rhizobacteria (PGPR), biocontrol agents, and arbuscular mycorrhizal fungi (AM), can modify soil microbial communities, thereby impacting the entire ecosystem24,25,26,27. Therefore, it is crucial to comprehend the impact of biocontrol agents on soil microbial communities before their application in agriculture. The high-throughput sequencing approach provides a powerful tool for obtaining information about microbial diversity, structure, and composition from environmental soil samples. In this study, a high-throughput sequencing approach was employed to assess the effects of B.pumilus Y-26 and P.lilacinus CS-Z on the bacterial community in the rhizosphere of sweet potatoes. The diversity of bacterial communities serves as an indicator of the toxic effects resulting from pesticide use in agricultural practices28. Sang and Kim indicated that the application of metalaxyl could decrease the Shannon–Wiener indices of both bacterial and fungal communities29. Furthermore, Liu et al. reported that the inoculation of B.cereus L90 in the rhizosphere soil of Juglans regia significantly increased the Shannon–Wiener index of microbial communities30. In this study, our data indicated that the biocontrol agent B.pumilus Y-26 significantly affected the diversity of the sweet potato rhizosphere bacterial community, as evidenced by the analysis of Chao1, ACE, Shannon, and Simpson indices (p < 0.05). PCA analysis revealed that the biocontrol agent B.pumilus Y-26 had a distinct and unique effect on the structure and diversity of the rhizosphere bacterial community, which was corroborated by similar results obtained from Biolog analysis. In contrast, the biocontrol agent P.lilacinus CS-Z and the nematicide fosthiazate exerted minimal influence on the rhizosphere bacterial communities.
The bacteria abundances in each treatment were analyzed. The results revealed that Actinobacteriota, Proteobacteria, Chloroflexi, Acidobacteria, and Cyanobacteria were the predominant populations in the sweet potato rhizosphere soil. The abundance of Actinobacteriota was significantly higher in the B.pumilus Y-26 treatment compared to the CK treatment, whereas the abundance of Cyanobacteria was significantly lower in the B.pumilus Y-26 treatment compared to the CK treatment (p < 0.05). At the genus level, Crossiella, Gaiella, Bacillus, and Streptomyces exhibited significantly higher abundances in the B.pumilus Y-26 treated rhizosphere soil samples compared to the CK treatment, while the relative abundance of Pseudarthrobacter was decreased in the B.pumilus Y-26 treatment. And the relative abundances of Crossiella and Streptomyces were also increased in the P.lilacinus CS-Z treatment. However, the nematicide fosthiazate treatment had a lesser impact on the bacterial community compared to the CK treatment. Among them, certain species within the genus Bacillus have the ability to produce a wide range of bioactive peptides that potentially exhibit inhibitory effects on phytopathogens31. Additionally, as spore-forming bacteria, these organisms are highly suitable candidates for the development of effective biopesticide products32. Streptomyces is known as a prolific producer of a broad range of antibiotics. Some species have been shown to suppress diverse plant pathogens via antibiosis or resource competition33. Moreover, Streptomyces spp. are also recognized as promising taxa for plant growth promotion (PGP) and mycorrhizal helper bacteria due to their capabilities in phosphate solubilization, chitinase and growth regulator production34,35, as well as the stimulation of arbuscular mycorrhizal fungi (AMF) spore germination and hyphal growth36,37,38. The genus Gaiella demonstrates catalase- and oxidase-positive characteristics, indicating its ability to utilize various organic compounds as a source of energy. Currently, research on Gaiella primarily focuses on the degradation of herbicides. Chen et al. reported Gaiella as the primary degrader of sulfadiazine39. These results indicate that the application of biocontrol agents B.pumilus Y-26 and P.lilacinus CS-Z led to the enrichment of beneficial microbes in the rhizosphere soil, thereby enhancing the efficacy of the biocontrol agents against stem nematode.
Conclusion
This study successfully evaluated the effectiveness of biocontrol agents B.pumilus Y-26 and P.lilacinus CS-Z in controlling sweet potato stem nematode through a pot experiment. Additionally, it investigated the potential influence of B.pumilus Y-26 and P.lilacinus CS-Z on the bacterial community structure in the sweet potato rhizosphere. The results demonstrated that the application of B.pumilus Y-26 and P.lilacinus CS-Z was an effective method for controlling sweet potato stem nematode. Furthermore, treatments with B.pumilus Y-26 and P.lilacinus CS-Z resulted in alterations in the diversity of the bacterial community in the rhizosphere soil. The application of B.pumilus Y-26 and P.lilacinus CS-Z had a minor beneficial impact on the native bacterial community in the rhizosphere soil of sweet potato. Consequently, employing B.pumilus Y-26 and P.lilacinus CS-Z as a means of controlling sweet potato stem nematodes acts as a promising ecological approach and can be an alternative to chemical treatments, thereby facilitating the development of a sustainable agricultural management strategy to mitigate agrochemical misuse in sweet potato crops. Future research will concentrate on investigating the roles of B.pumilus Y-26 and P.lilacinus CS-Z in sweet potato stem nematode disease specifically in Hebei Province.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Zang, N. et al. Efficient production of transgenic plants using the bar gene for herbicide resistance in sweet potato. Sci. Hortic. 122, 649–6539. https://doi.org/10.1016/j.scienta.2009.06.023 (2009).
Zhou, Z. W. & Ma, D. F. Research status and prospect of sweet potato stem nematode disease. Rain Fed. Crops 23, 288–290. https://doi.org/10.3724/sp.j.1011.2008.00921 (2003).
Xie, Y. Z. et al. Inheritance and breeding for resistance to sweetpotato plants transformed with the bar gene. Biotechnol Lett. 29, 669–675. https://doi.org/10.1007/s10529-006-9278-1 (2004).
Caboni, P. et al. Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. J. Agr Food Chem. 61, 9784–9788. https://doi.org/10.1021/jf403684h (2013).
Zeng, L. et al. Isolation and identiffcation of chemical constituents from the bacterium Bacillus sp. and their nematicidal activities. J. Basic Microbiol. 55, 1239–1244. https://doi.org/10.1002/jobm.201400798 (2015).
Luo, T. et al. Nematodes avoid and are killed by Bacillus mycoides-produced styrene. J. Invertebr Pathol. 159, 129–136. https://doi.org/10.1016/j.jip.2018.09.006 (2018).
El-Nagdi, W. M. A. & Youssef, M. M. A. Soaking faba bean seed in some bio-agent as prophylactic treatment for controlling Meloidogyne incognita root-knot nematode infection. J. Pestic Sci. 77, 75–78. https://doi.org/10.1007/s10340-003-0029-y (2004).
Anastasiadis, I. A. et al. The combined effect of the application of a biocontrol agent Paecilomyces Lilacinus, with various practices for the control of root-knot nematodes. Crop Protect. 27, 352–361. https://doi.org/10.1016/j.cropro.2007.06.008 (2008).
Khan, A. et al. Control of plant-parasitic nematodes by Paecilomyces lilacinus and monacrosporium lysipagum in pot trials. Biocontrol. 51, 643–658. https://doi.org/10.1007/s10526-005-4241-y (2006).
Lara, J. et al. Biological control of Meloidogyne Incognita in tomato in Puerto Rico. Nematropica. 26, 143–152. https://doi.org/10.46429/jaupr.v74i3.6658 (1996).
Siddiqui, I. A. et al. Biological control of rot-root knot disease complex of tomato. Plant Soil 227, 163–169. https://doi.org/10.1023/a:1026599532684 (2000).
Cannayane, I. & Sivakumar, C. V. Nematode egg–parasitic fungus 1: Paecilomyces lilacinus-A review. Agric. Rev. 22, 79–86. https://doi.org/10.5455/vetworld.2011.168-170(2001) (2001).
Ramezani, M. M. et al. The first report of Bacillus pumilus influence against Meloidogyne Javanica in Iran. J. Crop Prot. 3, 105–112. https://doi.org/10.18681/pjn.v35.i02.p155-156(2014) (2014).
Lee, Y. S. & Kim, K. Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. J. Phytopathol. 164, 29–39. https://doi.org/10.1111/jph.12421 (2016).
Shi, F. Y. et al. Isolation, characterization and degradation products of a chitosanase from Paecilomyces Sp. CS-Z Mycosystema. 32, 721–728. https://doi.org/10.13346/j.mycosystema.2013.04.016 (2013). (in Chinese).
Mei, F. Z. H. et al. Identification, fermentation optimization and determination of nematicidal activity of biocontrol bacterium Y26. Chin. J. Biol. Control. 40, 424–434. https://doi.org/10.16409/j.cnki.2095-039x.2024.02.005 (2024). (in Chinese).
Zheng, H. et al. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 41, 517–532. https://doi.org/10.1111/pce.12944 (2018).
Qiao, Y. J. et al. Effect of different planting patterns on yield of sweet potato and the number of nematodes in rhizosphere. Res. Agric. Modern. 35, 800–803. https://doi.org/10.13872/j.1000-0275.2014.0052 (2014) (in Chinese).
Garland, J. L. & Mills, A. L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359. https://doi.org/10.1128/aem.57.8.2351-2359 (1991).
Zhu, Y. B. et al. Rhizosphere bacterial communities associated with healthy and Heterodera glycines-infected soybean roots. Eur. J. Soil. Biol. 58, 32–37. https://doi.org/10.1016/j.ejsobi.2013.05.001 (2013).
Lu, R. et al. Bacterial community structure in atmospheric particulate matters of different sizes during the haze days in Xi’an. China Sci. Total Environ. 637–638, 244–252. https://doi.org/10.1016/j.scitotenv.2018.05.006 (2018).
Smith, K. P. & Goodman, R. M. Host variation for interactions with beneficial plant-associated microbes. Annu. Rev. Phytopathol. 37, 473–491. https://doi.org/10.1146/annurev.phyto.37.1.473 (1999).
Cycoń, M. et al. Shortterm effects of the herbicide napropamide on the activity and structure of the soil microbial community assessed by the multi-approach analysis. Appl. Soil. Ecol. 66, 8–18. https://doi.org/10.1016/j.apsoil.2013.01.014 (2013).
Ravnskov, S. et al. Soil inoculation with the biocontrol agent Clonostachys Rosea and the mycorrhizal fungus Glomus intraradices results in mutual inhibition, plant growth promotion and alteration of soil microbial communities. Soil. Biol. Biochem. 38, 3453–3462. https://doi.org/10.1016/j.soilbio.2006.06.003 (2006).
de Salamone, I. E. G. et al. Field response of rice paddy crop to Azospirillum inoculation: Physiology of rhizosphere bacterial communities and the genetic diversity of endophytic bacteria in different parts of the plants. Plant. Soil. 336, 351–362. https://doi.org/10.1007/s11104-010-0487-y (2010).
Yin, Z. et al. Enhancement of maize growth and alteration of the rhizosphere microbial community by phosphate-solubilizing fungus Aspergillus aculeatus P93. J. Agric. Biotechnol. 2, 1–10. https://doi.org/10.1080/01490451.2016.1146373 (2017).
Jin, N. et al. The effect of combined application of Streptomyces rubrogriseus HDZ-9-47 with soil biofumigation on soil microbial and nematode communities. Sci. Rep. 9, e16886. https://doi.org/10.1038/s41598-019-52941-9 (2019).
Wang, Y. S. et al. Effect of carbendazim and pencycuron on soil bacterial community. J. Hazard. Mater. 172, 84–91. https://doi.org/10.1016/j.jhazmat.2009.06.142(2009) (2009).
Sang, M. K. & Kim, K. D. Plant growth-promoting rhizobacteria suppressive to Phytophthora blight affect microbial activities and communities in the rhizosphere of pepper (Capsicum annuum L.) in the field. Appl. Soil Ecol. 62, 88–97 (2012). https://doi.org/10.1016/j.apsoil.2012.08.001(2012).
Liu, F. C. et al. Effects of inoculating plant growth-promoting rhizobacteria on the biological characteristics of walnut (Juglans regia) rhizosphere soil under drought condition. Chin. J. Appl. Ecol. 25,1475–1482. https://doi.org/10.13287/j.1001-9332.2014.0014 (2014) (in Chinese).
Baysal, Ö. et al. A proteomic approach provides new insights into the control of soilborne plant pathogens by Bacillus species. PloS One 8, e53182 (2013).
Ongena, M. & Jacques, P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. https://doi.org/10.1016/j.tim.2007.12.009(2008) (2008).
Schlatter, D. et al. Resource amendments influence density and competitive phenotypes of Streptomyces in soil. Microb. Ecol. 57, 413–420. https://doi.org/10.1007/s00248-008-9433-4 (2009).
Mohandas, S. et al. Guava (Psidium guajava L.) Rhizosphere Glomus mosseae spores harbor actinomycetes with growth promoting and antifungal attributes. Sci. Hortic. Amsterdam 150, 371–376. https://doi.org/10.1016/j.scienta.2012.11.019(2013) (2013).
Hamedi, J. & Mohammadipanah, F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol. 42, 157–171. https://doi.org/10.1007/s10295-014-1537-x(2015) (2015).
Mugnier, J. & Mosse, B. Spore germination and viability of a vesicular arbuscular mycorrhizal fungus. Glomus mosseae. Trans. Br. Mycol. Soc. 88, 411–413. https://doi.org/10.1016/s0007-1536(87)80018-9 (1987).
Tylka, G. L. et al. Axenic germination of vesicular–arbuscular mycorrhizal fungi: Effects of selected Streptomyces species. Phytopathology 81, 754–759. https://doi.org/10.1094/phyto-81-754(1991) (1991).
Carpenter-Boggs, L. et al. Spore germination of Gigaspora margarita stimulated by volatiles of soil-isolated actinomycetes. Soil. Boil Biochem. 27, 1445–1451. https://doi.org/10.1016/0038-0717(95)00075-p(1995) (1995).
Chen, J. et al. Sulfadiazine degradation in soils: dynamics, functional gene, antibiotic resistance genes and microbial community. Sci. Total Environ. 691, 1072–1081. https://doi.org/10.1016/j.scitotenv.2019.07.230 (2019).
Acknowledgements
This work was funded by the Department of Science and Technology of Hebei Province (grant number: 21326502D).
Author information
Authors and Affiliations
Contributions
Fengyu Shi, Dan Yang, Xinpeng Meng, Jiaxin Li performed the experiments, collected data, analyzed data, interpreted data, and maintenance drafted the manuscript. Yingbo Zhu conceived and designed the study, obtained financial support, provided the study material, and helped revise the manuscript. Jianbin Liu helped perform the experiments and participated in the discussion. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
In this study, experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, F., Yang, D., Meng, X. et al. Effects of Paecilomyces lilacinus and Bacillus pumilus on stem nematode and rhizosphere bacterial communities of sweet potato. Sci Rep 14, 23290 (2024). https://doi.org/10.1038/s41598-024-74268-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74268-w