Abstract
Oxidative stress (OS) is believed to be a significant factor in the decline of semen quality, with mitochondrial DNA copy number (mtDNAcn) serving as a sensitive biomarker for both semen quality and mitochondrial dysfunction resulting from oxidative stress. While glutathione S-transferases (GSTs) are commonly known as ‘antioxidant’ enzymes, there is ongoing debate regarding the relationship between GST genotypes and semen quality. In a study involving 568 male volunteers from the outpatient department of Puyang Reproductive Medicine Center, sperm mtDNAcn, semen quality, and GSTM1/GSTT1 genotypes were analyzed to investigate the potential link between GSTM1/GSTT1 gene variations and semen quality, as well as the impact of GSTs gene variations on the connection between sperm mtDNAcn and semen quality. Adjusting for variables such as age, BMI, smoking, and alcohol consumption, it was found that mtDNAcn was significantly correlated with decreased sperm concentration and total sperm count (b = − 0.109, − 0.128, respectively; P = 0.002, 0.001, respectively). GSTM1 was associated with progressive motility (OR 0.390, 95% CI 0.218, 0.697), Straight line velocity (VSL) (OR = 0.606, 95% CI 0.385, 0.953), and Straightness (STR) (OR 0.604, 95% CI 0.367, 0.994), while GSTT1 was linked to progressive motility (OR 0.554, 95% CI 0.324, 0.944) and Beat crossover frequency (OR 0.624, 95% CI 0.397, 0.982). The GSTT1 was found to moderate the relationship between mtDNAcn and sperm motility parameters linearity (LIN), STR, and Wobble (WOB), with additive interaction effects observed between GSTT1 and mtDNAcn on LIN, STR, and WOB (P for interaction = 0.008, 0.034, 0.010, respectively). Overall, this study suggests that GSTT1 and GSTM1 gene variations may play a role in sperm motility, with GSTT1 potentially influencing the impact of oxidative stress on sperm motility.
Similar content being viewed by others
Introduction
The World Health Organization (WHO) recognizes that infertility is a disease that is increasingly becoming a global public health issue. Infertility affects about 8–12% of reproductive-aged couples worldwide1,2,3. Evidence suggests a decline in semen quality among men globally in recent decades4,5, with the male component contributing 50% to infertility cases6. While the precise reasons for this decline are not fully understood, various environmental pollutants have been implicated. Exposure to pollutants like petroleum products, agrochemicals, industrial chemicals, and heavy metals, particularly lead (Pb) and cadmium (Cd), may negatively impact the male reproductive system.
Oxidative stress (OS) refers to the imbalance between pro-oxidation and antioxidant states, which is linked to apoptosis and germ cell apoptosis7. It is known to play a role in the negative impact of various environmental pollutants on semen quality. Mitochondrial DNA, lacking histones and DNA repair capabilities found in nuclear DNA, is particularly vulnerable to oxidative stress8. Mitochondrial DNA copy number (mtDNAcn) serves as a valuable indicator of mitochondrial genome abundance9 and has been recognized as a sensitive biomarker for semen quality and mitochondrial dysfunction caused by oxidative stress10,11,12. Following acute chemical exposure and oxidative stress, mtDNAcn tends to increase as a compensatory mechanism due to reduced energy production. However, prolonged oxidative stress can eventually overwhelm mitochondria, leading to mitochondrial dysfunction and a decline in mtDNAcn levels13. During epididymal transport, sperm undergo a series of biochemical and morphological changes, gradually maturing in the process. As sperm mature, they lose most of their cytoplasmic components and become more vulnerable to oxidative stress, which can damage sperm structure and function. An optimal level of reactive oxygen species (ROS) not only protects against oxidative damage but also aids in sperm capacitation14,15.
Glutathione S-transferases (GSTs) are a group of enzymes that are widely present and have multiple functions. They are crucial for cellular detoxification in phase II reactions and for defending against oxidative stress, often referred to as ‘antioxidant’ enzymes16,17. Some GST enzymes are produced by genes with known functional variations. GSTM1 (encoding GST mu1) and GSTT1 (encoding GST theta1) are important in detoxifying both internal and external toxins. Deletions in GSTM1 and GSTT1 are quite common in human populations18. Variations in genes that encode enzymes involved in drug metabolism and detoxification in phase II can impact their activity, potentially affecting how the male reproductive system processes harmful substances and influencing susceptibility to conditions like infertility19,20,21. In a previous study, we observed that GSTM1/GSTT1 gene variations moderated the relationship between metals and semen quality in male volunteers at the Puyang Reproductive Medicine Center outpatient department. Individuals with GSTT1+ and GSTM1+ genotypes were found to have a protective effect against the harmful impacts of heavy metal exposure18.
While some studies have linked mtDNAcn and GSTs gene polymorphisms to decreased semen quality, there are inconsistencies in the results. Previous research has often focused on the impacts of mtDNAcn or GSTs genotypes individually, without considering potential interactions. Given that GSTs possess antioxidant properties that protect against oxidative stress, it is hypothesized that GSTs gene polymorphisms may influence the association between sperm mtDNAcn and impaired semen quality. This study aims to investigate the relationship between sperm mtDNAcn and gene polymorphisms of GSTT1 and GSTM1 in adult male volunteers recruited from the outpatient department of Puyang Reproductive Medicine Center. Additionally, the study will explore the role of gene polymorphisms in the relationship between mtDNAcn and semen quality.
Materials and methods
Study population and design
In our cross-sectional study, we recruited male partners in couples who attended the outpatient department of Puyang Reproductive Medicine Center in Henan, China for semen examination, which has been reported in our previous report18. The sample size calculation formula used is as follows:
In Eq. (1), the variables have the following meanings.
Zα/2 = 1.96 (significance level with α = 0.05, Two-tailed test); Zβ = 1.28 (power of test 1 − β = 0.9, One-tailed test); σ = The estimate of the standard deviation of the two populations is based on findings from published literature22 and the sample collection experience, which is 15 in this case. δ = The mean difference of 4.5 between the two groups is based on findings from published literature and sample collection experience. n1 = n2 = 234. In order to ensure sufficient sample size, the sample size was expanded by 20% on this basis, and N was chosen as 568.
Following the exclusion criteria, 568 participants were ultimately included in the final analytic sample. Upon signing the informed consent form, volunteers completed a comprehensive social-body-behavioral questionnaire covering basic demographics, disease history, lifestyle, and other relevant factors. Height, weight, and various indicators were measured, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Semen samples were collected for semen parameter and genetic polymorphism detection, with storage at − 80 °C designated for research purposes only, not for conception.
Including and excluding criteria
The same inclusion and exclusion criteria were applied as described in our previous study. The inclusion criteria required participants to abstain from sexual activity for 2–7 days and be at least 18 years old, as well as accept the questionnaire and provide informed consent. Additionally, subjects were excluded if they had a history of testicular injury, epididymitis, or genitourinary inflammation diagnosed by a urologist, as well as if they had received treatment for varicocele, had a history of incomplete testicular dislocation, or exhibited certain physical abnormalities identified by the urologist during the physical examination stage, such as absence of pubic hair, breast abnormalities, or penile abnormalities. Individuals with azoospermia, mitochondrial loss, or mtDNAcn deletion were also excluded.
Semen collection and semen parameters measurement
Prior to semen collection, volunteers were instructed to abstain from sexual activity for a period of two to seven days. Each subject received individual guidance before entering the designated semen collection room. Semen samples were obtained through masturbation without the use of a condom in a secluded room adjacent to the semen analysis area. All procedures adhered to the guidelines outlined in the WHO laboratory manual for the examination and processing of human semen (Fifth Edition)23. The collected semen samples were placed in containers and incubated at 37 °C for liquefaction. Computer-assisted sperm analysis (CFT-9201, Jiangsu Ruiqi Life Science Co., LTD, Jiangsu, China) was utilized for the evaluation of semen parameters, including volume, sperm concentration, total sperm count, progressive motility, as well as various sperm motility parameters such as curve line velocity (VCL, µm/s), straight line velocity (VSL, µm/s), average path velocity (VAP, µm/s), wobble (WOB), the head of lateral movement (ALH, µm), linearity (LIN, %), the average degree of movement (MAD, °), straightness (STR, %), beat crossover frequency (BCF, Hz). Sperm morphology assessment was conducted using a microscope, which included evaluation of normal morphology rate, teratozoospermia index, sperm deformity index, and normal acrosome.
mtDNAcn analysis
Genomic DNA was isolated from the semen sample for the mtDNAcn assay. For each semen sample, centrifuged at 3500 r/min for 5 min, and the upper spermatoplasm was discarded. DNA was isolated from the remaining sperm cells using a DNA isolation kit (Life Feng Biotechnology Co, Shanghai) following the manufacturer’s instructions. The method for measuring the relative mtDNAcn was consistent with that described by Gong et al.24. Ct values of the mitochondrial gene mtND1 and the reference gene human β-globulin (HBG) were measured using the QuantiNAVA STBR Green PCR Kit. The relative mtDNAcn was calculated through quantitative real-time polymerase chain reaction (PCR) based on the copy number of mitochondrial gene mtND1 and the copy number of the nuclear gene HBG. The primer sequences used were as follows:
-
mtND1:forward5′-CACCCAAGAACAGGGTTTGT-3′, reverse5′-TGGCCATGGGTATGTTGTTA-3′;
-
HBG:forward5′-GCTTCTGACACAGTGTTCACTAGC-3′,
-
reverse5′-CACCAACTTCATCCACGTTCACC-3′.
The reaction procedure consisted of an initial activation of DNA polymerase at 95 °C for 3 min, followed by 35 cycles. Each cycle included denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 60 s. A standard curve with 5 data points (1.25, 2.50, 5.00, 10.00, and 20.00 ng/µL) was generated for each plate, and the amplification efficiency was calculated, which ranged from 90 to 110% for each plate. Three replicates were measured in each sample. The calculation of relative mtDNAcn was as follows:
GSTT1/GSTM1 polymorphism detection
Genomic DNA was utilized for analyzing GSTT1/GSTM1 polymorphisms following a previously reported method25. DNA was extracted from peripheral blood using a DNA isolation kit (Life Feng Biotechnology Co, Shanghai) as per the manufacturer’s instructions, and stored at − 80℃. The polymorphisms of GSTM1 and GSTT1 were examined through restriction fragment length polymorphism (RFLP) analysis. For each genotype RFLP method, several positive and negative samples were selected randomly to do DNA sequencing to verify the accuracy of the method, and about 20% of samples were repeated secondly to prove the repeatability. Multiplex polymerase chain reaction was employed to determine GSTT1, GSTM1, and ALB (albumin) levels, with the following primer sequences:
Gene | Primer sequence (5′–3′) |
|---|---|
GSTT1 | Forward: TTCCTTACTGGTCCTCACATCTC |
Reverse: TCACCGGATCATGGCCAGCA | |
GSTM1 | Forward: GAACTCCCTGAAAAGCTAAAGC |
Reverse: GTTGGGCTCAAATATACGGTGG | |
ALB | Forward: GCCCTCTGCTAACAAGTCCTAC |
Reverse: GCCCTAAAAAGAAAATCGCCAATC |
Statistical analysis
All analyses were conducted using SPSS 22.0 and R 4.3.0 (R Development Core Team, Vienna, Austria). The total sperm count, sperm concentration, normal morphology rate, and mitochondrial DNA copy number were transformed using the natural-log(measure) (ln) method to achieve a normal distribution approximation. Semen quality parameters and mtDNAcn for the study population were described using data prior to transformation and were presented as mean ± SD or M (P25, P75). Descriptive analysis was utilized to summarize characteristics with counts or proportions for variables such as smoking status (smokers and ever smokers, non-smokers), alcohol usage (users, non-users), and GST genotypes (−, +).
In the association analysis, the multivariate linear regression was utilized initially to investigate the association of semen parameters as continuous variables with mtDNAcn, GSTM1/GSTT1 genotypes. Additionally, Independent sample t-tests and ANOVA tests were employed to compare semen quality parameter levels among different genotype populations. To ensure the reliability of our results, sensitivity analyses were conducted. Firstly, mtDNAcn levels were categorized into quartiles based on the overall study population’s distribution to examine the relationships. Secondly, associations were explored when each semen quality parameter was dichotomously classified as ‘normal’ or ‘abnormal’ according to WHO guidelines, which define normal as semen volume ≥ 1.5 ml, sperm concentration ≥ 15 × 106/ml, total sperm number ≥ 39 × 106, normal morphology rate ≥ 4%, and progressive motility ≥ 32%, respectively. For semen parameters without established standards, the P25 was used as a classification criterion in this study. A multivariate logistic model was employed to assess the association between semen parameters as dichotomous variables and mtDNAcn, GST genotypes adjusting for age (continuous), body mass index (BMI; continuous), smoking (categorical), and drinking status (categorical). The regression results were presented as effect estimates and 95% confidence intervals (CI).
SPSS PROCESS26 was utilized to investigate the moderation effect of GSTs gene polymorphisms on the relationships between mtDNAcn and semen quality. The variables were centered to ensure that the coefficients for the product of the two variables were interpretable within the data range. To further explore the impact of gene polymorphisms on the connection between sperm mtDNAcn and semen quality, we conducted a stratified analysis based on GSTM1 and GSTT1 genotypes. The correlation between semen parameters and mtDNAcn in each stratum was examined. When assessing biological interaction, emphasis was placed on additive interaction rather than multiplicative interaction. In linear regression analysis, the regression coefficient of the product term indicated whether there was additive interaction or departure from additivity. Therefore, interaction terms of the stratified variables and the independent variable were included in a linear regression model, and the coefficient of the interaction term was directly assessed to determine if there was an interaction effect between GSTM1/GSTT1 gene polymorphisms and mtDNAcn on semen parameters using R. The statistical significance level was set at P < 0.05 (two-tailed).
Ethical approval
This study was approved by the Ethics Committee of Xinxiang Medical University (December 15, 2017) (Ethical review approval document No: XYLL-20170311). The research content and process of this project adhere to the ethical requirements for biomedical research set forth by international and national governments. All methods were conducted in alignment with the applicable guidelines and regulations, and informed consent was obtained from all participants and/or their legal guardians.
Consent for publication
Informed consent was obtained from all the participants who answered the questionnaire and provided samples.
Results
Participant characteristics and semen parameters
A total of 568 male volunteers were recruited for the study. Table 1 displays the characteristics, semen parameters, GSTT1 and GSTM1 genotype distribution, as well as other basic information of the participants. The average age of the participants was 29.61 years, with an average BMI of 24.74 kg/m2. Among the participants, 50.30% were GSTT1+, 45.70% were GSTM1+, 49.90% were GSTT1+/GSTM1+, and 23.00% were both GSTT1+ and GSTM1+. Furthermore, 49.80% of the participants were smokers and 39.50% were drinkers. The median values for sperm concentration, total sperm number, and mtDNAcn were 58.20 million/mL, 202.90 million, and 91.77 copies, respectively. The mean levels for various semen parameters were as follows: VCL 57.78 μm/s, LIN 65.71%, VSL 37.48 μm/s, STR 68.56%, VAP 49.34 μm/s, WOB 80.04%, ALH 2.67 μm, BCF 5.65 Hz, and MAD 38.68°.
The association of semen quality with mtDNAcn, GSTM1 and GSTT1 gene polymorphisms
Relationship between mtDNAcn and semen parameters
Sperm mitochondrial DNA copy number (mtDNAcn) was found to be inversely correlated with semen quality even after adjusting for age, BMI, smoking, and alcohol consumption. Specifically, the study revealed a significant negative association between mtDNAcn and sperm concentration (b = − 0.109, P = 0.002) as well as total sperm count (b = − 0.128, P = 0.001) (Fig. 1). Supplementary Table S1 further supported these findings by showing a negative correlation between mtDNAcn levels and sperm concentration (P = 0.004) as well as total sperm count (P = 0.004). Additionally, an increase in mtDNAcn was associated with a 0.076 (95% CI 0.000, 0.153) reduction in BCF.
The association between mtDNAcn and semen parameters in multiple linear model while adjusting for age (continuous), BMI (continuous), smoking (categorical) and drinking status (categorical). Sperm concentration, total sperm count, and normal morphological rate and mtDNAcn were ln-transformed. *P < 0.05 and **P < 0.01. TZI teratozoospermia index, SDI sperm deformity index, VCL curve line velocity, VSL straight line velocity, VAP velocity of average path, ALH lateral head movement, MAD average motion degree, LIN linearity, STR straightness, WOB wobble, BCF beat cross frequency.
Relationship between GSTM1 and GSTT1 gene polymorphisms and semen parameters
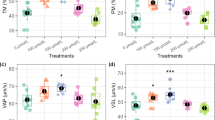
The study found that the levels of STR (P = 0.034) and ALH (P = 0.042) were significantly higher in the GSTM1+ genotype group compared to the GSTM1− genotype group. Additionally, sperm concentration (P = 0.047), total sperm count (P = 0.025), progressive motility (P = 0.020), VCL (P = 0.055), VSL (P = 0.049), and BCF (P = 0.008) levels were all notably higher in the GSTT1+ genotype group than in the GSTT1− genotype group, as illustrated in Fig. 2 and Supplementary Table S2.
(a) Semen quality parameters levels of the population with different genotypes (GSTM1, GSTT1) Independent sample t test was applied. *P < 0.05 and **P < 0.01. (b) Semen quality parameters levels of the population with different genotypes (GSTT1TM1). ANOVA test was applied. “*”indicates P < 0.05 and “**”indicates P < 0.01 gotten from LSD or Dunnett’s T3 method.
The results presented in Fig. 3 indicate that GSTM1 was significantly associated with progressive motility (OR 0.390, P = 0.001), sperm motility parameters VSL (OR 0.606, P = 0.030) and STR (OR 0.604, P = 0.047), while GSTT1 showed a correlation with progressive motility (OR 0.554, P = 0.030) and BCF (OR 0.624, P = 0.042). Individuals with ‘GSTM1+/GSTT1+’ had a reduced risk of abnormal progressive motility and VSL (P = 0.022, 0.005, respectively), as did those with ‘GSTM1+ and GSTT1+’ (P = 0.001, 0.009, respectively) compared to individuals with ‘GSTM1− and GSTT1−’ (Fig. 3 and Supplementary Fig. S1).
The associations of GSTT1, GSTM1 and GSTT1TM1 gene polymorphisms with sperm parameters (binary categorical variable). The logistic regression model was applied adjusting for age (continuous), BMI (continuous), smoking (categorical) and drinking status (categorical). GSTT1TM1 was a multicategory variable and the reference group was GSTM1− and GSTT1−.
Moderation effect of the GSTM1 and GSTT1 gene polymorphisms on association between mtDNAcn and semen quality
Supplementary Table S3 illustrates the moderating impact of GSTT1 gene polymorphisms on the correlation between mtDNAcn and semen quality. Specifically, for the sperm motility parameter LIN, the regression coefficient of the product term of mtDNAcn and GSTT1 (mtDNAcn*GSTT1) was1.7738 (P = 0.0077), while the regression coefficient of mtDNAcn was − 0.5755, showing opposite signs. GSTT1 appears to have a negative regulatory effect. The change in R-square was 0.0167, indicating that the moderating effect contributes approximately 1.67%. Furthermore, GSTT1 may also modulate the negative relationships between mtDNAcn and STR (b = 4.0424, t = 2.0450, P = 0.0415) and WOB (b = 4.0494, t = 2.6683, P = 0.0079). Additionally, individuals with GSTM1+/GSTT1+ genotypes may mitigate the adverse impact of mtDNAcn on LIN, STR, and WOB (P = 0.016, 0.026, 0.003, respectively) (Supplementary Table S4).
Interaction effect of mtDNAcn and GSTM1 and GSTT1 gene polymorphisms on sperm mobility parameters
The results presented in Table 2 indicate a positive correlation between mtDNAcn and sperm mobility parameters LIN (β = 1.186, 95% CI 0.265, 2.107) in individuals with the GSTT1+ genotype. Additionally, an association was observed between mtDNAcn and an increase in LIN (β = 1.266, 95% CI 0.240, 2.293) and WOB (β = 1.013, 95% CI 0.001, 2.025) in subjects with the GSTM1+/GSTT1+ genotype. And a negative correlation between mtDNAcn and ALH (β = − 0.111, P = 0.042) and MAD (β = − 1.068, P = 0.036) in individuals with the GSTT1− and GSTM1− genotype was found. Further analysis showed that the association between mtDNAcn and LIN, when examined by quartiles of mtDNAcn level, was evident in men with GSTT1+ genotype (β = 1.230, 95% CI 0.384, 2.076), as well as with GSTM1+ and GSTT1+ (β = 1.146, 95% CI 0.269, 2.023) (Supplementary Table S5).
Moreover, significant interaction effects were observed between GSTT1 gene polymorphisms and mtDNAcn on sperm motility parameters LIN (P for interaction = 0.008), STR (P for interaction = 0.034), and WOB (P for interaction = 0.010). However, there was limited evidence for interaction effects on other semen quality parameters as detailed in Table 3. The interaction effect results by mtDNAcn quartile grouping, as presented in Supplementary Table S6, further support the significant interaction between GSTT1 and mtDNAcn on LIN (P for interaction = 0.014) and WOB (P for interaction = 0.040), consistent with the aforementioned findings.
Discussion
In this study, it was discovered that mtDNAcn was significantly inversely correlated with sperm concentration and total sperm count, even after adjusting for age, BMI, smoking, and alcohol consumption. Additionally, a positive correlation was observed between GSTM1 and GSTT1 gene polymorphisms and sperm mobility. Further analysis suggested that GSTT1 gene polymorphisms may moderate the relationship between mtDNAcn and sperm LIN, STR, and WOB.
During sperm differentiation and maturation in the epididymis, plasma droplets gradually fall off, cytoplasmic content decreases, and the sperm head becomes predominantly occupied by DNA. This leads to an increase in polyunsaturated fatty acids (PUFA) in the sperm plasma membrane, making mature sperm more sensitive to ROS27. Exposure to oxidative stress impairs the functionality and vitality of human sperm, affecting the fertilization process. The mtDNAcn serves as an indicator of mitochondrial DNA quantity. In semen of poor quality, the increase in sperm mtDNAcn can be attributed to two main factors. Mild oxidative stress promotes mitochondrial biosynthesis and mtDNAcn28. However, prolonged exposure to excessive ROS impairs mitochondrial respiratory chain function, hindering ATP synthesis. As a compensatory mechanism, mtDNA copy number increases to address functional defects caused by damage, although this compensation may not fully restore mitochondrial function and normal spermatogenesis. Additionally, during normal spermatogenesis, sperm mtDNAcn decreases significantly to ensure low mtDNAcn levels at fertilization29,30, as mitochondrial DNA is maternally inherited. Abnormal spermatogenesis or disrupted apoptosis processes may result in an elevated mtDNA copy number in human sperm31. Our findings support the notion that mtDNAcn negatively impacts sperm concentration and total sperm count.
GSTs, a supergene family of related isozymes, are known for detoxifying electrophiles by facilitating the binding of electrophilic groups from harmful substances to the sulfhydryl groups of reduced glutathione. This process results in the formation of a more soluble, non-toxic derivative that is easily excreted from the body32. GSTs are typically composed of two subunits of 25–27 kDa that polymerize in a homologous or heterologous manner. Each subunit contains two ligand binding sites, including a glutathione (GSH)-specific binding site (G-site) located at the N-terminal end. A conserved serine/tyrosine (Ser/Tyr) residue at this site forms a hydrogen bond with the sulfhydryl group of GSH, leading to the ionization of GSH and the formation of a stable, highly reactive thiolate anion. Genetic variations in human GST can influence gene activity, with GSTM1 and GSTT1 potentially impacting an individual’s susceptibility to OS damage33. Studies have shown that the GSTT1- genotype is associated with lower levels of GSTT1 expression, which may increase vulnerability to oxidative DNA damage and excessive ROS34. Our results suggest a link between the GSTT1 gene polymorphisms and improved sperm motility parameters, indicating that individuals with the null genotype may have reduced detoxification capacity and a higher risk of poorer semen quality.
The impact of GSTM1 and GSTT1 gene polymorphisms on the association between mtDNAcn and semen quality was investigated. It was observed that GSTT1 acts as a moderator in the relationship between mtDNAcn and semen parameters LIN, STR, and WOB, mitigating the inverse correlation. Previous research indicated that individuals with the GSTT1- genotype exhibited higher levels of oxidative stress markers such as malondialdehyde (MDA) and nitric oxide (NO), suggesting a potential mechanism35. Males with the GSTT1- genotype may have increased mtDNAcn, with the GSTT1 gene helping to maintain a balance between pro-oxidation and anti-oxidation states. Certain oxidative stress biomarkers could be influenced by a GST null genotype, leading to elevated ROS levels and reduced antioxidant capacity36. The absence of histones and DNA repair mechanisms in mitochondrial DNA makes it more vulnerable to oxidative stress, potentially resulting in mitochondrial dysfunction and decreased mtDNAcn. Significant DNA damage in sperm cells could accelerate germ cell death, lower sperm count, and impact semen quality. Our study highlighted the significant moderating effect of GSTT1, rather than GSTM1, on the relationship between mtDNAcn and sperm motility parameters, suggesting that GSTT1 may play a more substantial role in mitigating the adverse impact of mtDNAcn on semen quality.
Sperm motility parameters were a key focus of this research, with nine parameters considered. This is in contrast to most studies which typically only report conventional semen parameters that indicate semen quality. In our analysis of moderating effects, we utilized the PROCESS method and ensured that variables were centered. Centering is essential in estimating a moderation model as it helps reduce multicollinearity between the product and its constituent terms, ensuring that coefficients remain interpretable within the range of the data. Furthermore, this study incorporated interaction terms of GSTM1/GSTT1 gene polymorphism and mtDNAcn in a linear regression model to assess their combined effect on semen quality, providing valuable insights into biological interaction. However, it is important to acknowledge several limitations of this study. Firstly, the male volunteers were recruited from the Center for Reproductive Medicine, potentially introducing admission bias. Secondly, despite adjusting for some potential confounders, residual confounding from unmeasured covariates or unknown factors cannot be entirely ruled out. Lastly, although the primary focus was on oxidative stress, direct observation of parameters related to oxidative stress was not conducted.
Conclusions
Our results indicated a significant negative correlation between sperm mtDNAcn and semen parameters, suggesting that oxidative stress may have a detrimental impact on semen quality. Furthermore, GST gene polymorphisms were found to play a protective role against oxidative stress, potentially reducing the decline in semen quality. Specifically, GSTT1 gene polymorphisms may moderate the relationship between oxidative stress and sperm motility parameters. Furthermore, studies on glutathione S-transferases gene polymorphisms and levels of oxidative stress should be done in a multicenter, multi-ethnic population in the future. And its biological significance still needs to be verified in future longitudinal studies.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ombelet, W., Cooke, I., Dyer, S., Serour, G. & Devroey, P. Infertility and the provision of infertility medical services in developing countries. Hum. Reprod. Update 14, 605–621. https://doi.org/10.1093/humupd/dmn042 (2008).
Inhorn, M. C. & Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 21, 411–426. https://doi.org/10.1093/humupd/dmv016 (2015).
Fainberg, J. & Kashanian, J. A. Recent advances in understanding and managing male infertility. F1000Research 8, 1–8. https://doi.org/10.12688/f1000research.17076.1 (2019).
Auger, J., Eustache, F., Chevrier, C. & Jégou, B. Spatiotemporal trends in human semen quality. Nat. Rev. Urol. 19, 597–626. https://doi.org/10.1038/s41585-022-00626-w (2022).
Huang, C. et al. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 107, 83-88.e82. https://doi.org/10.1016/j.fertnstert.2016.09.035 (2017).
Concepcion-Zavaleta, M. et al. Assessment of hormonal status in male infertility. An update. Diabetes Metab. Syndr. 16, 102447. https://doi.org/10.1016/j.dsx.2022.102447 (2022).
Ko, E. Y., Sabanegh, E. S. & Agarwal, A. Male infertility testing: Reactive oxygen species and antioxidant capacity. Fertil. Steril. 102, 1518–1527. https://doi.org/10.1016/j.fertnstert.2014.10.020 (2014).
Yakes, F. M. & Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 94, 514–519. https://doi.org/10.1073/pnas.94.2.514 (1997).
Smith, A. R. et al. Prospective associations of early pregnancy metal mixtures with mitochondria DNA copy number and telomere length in maternal and cord blood. Environ. Health Perspect. 129, 117007. https://doi.org/10.1289/ehp9294 (2021).
Chen, Y. et al. Seminal plasma cell-free mitochondrial DNA copy number is associated with human semen quality. Eur. J. Obst. Gynecol. Reprod. Biol. 231, 164–168. https://doi.org/10.1016/j.ejogrb.2018.10.048 (2018).
Malik, A. N. & Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction?. Mitochondrion 13, 481–492. https://doi.org/10.1016/j.mito.2012.10.011 (2013).
Rosati, A. J. et al. Sperm mitochondrial DNA biomarkers and couple fecundity. Hum. Reprod.35, 2619–2625. https://doi.org/10.1093/humrep/deaa191 (2020).
Kupsco, A. et al. Prenatal manganese and cord blood mitochondrial DNA copy number: Effect modification by maternal anemic status. Environ. Int. 126, 484–493. https://doi.org/10.1016/j.envint.2019.02.029 (2019).
Aitken, R. J. & Drevet, J. R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants (Basel) 9, 111. https://doi.org/10.3390/antiox9020111 (2020).
Barati, E., Nikzad, H. & Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 77, 93–113. https://doi.org/10.1007/s00018-019-03253-8 (2020).
Schuppe, H. C. et al. Xenobiotic metabolism, genetic polymorphisms and male infertility. Andrologia 32, 255–262. https://doi.org/10.1046/j.1439-0272.2000.00393.x (2000).
Hemachand, T. & Shaha, C. Functional role of sperm surface glutathione S-transferases and extracellular glutathione in the haploid spermatozoa under oxidative stress. FEBS Lett. 538, 14–18. https://doi.org/10.1016/s0014-5793(03)00103-0 (2003).
Ren, J. et al. Mixed exposure effect of seminal metals on semen quality, mediation of total antioxidant capacity, and moderation of GSTM1/GSTT1 gene deletion in Chinese reproductive-aged men. Environ. Res. 229, 115888. https://doi.org/10.1016/j.envres.2023.115888 (2023).
Hekim, N. et al. SNP’s in xenobiotic metabolism and male infertility. Xenobiotica 50, 363–370. https://doi.org/10.1080/00498254.2019.1616850 (2020).
Yu, B. & Huang, Z. Variations in antioxidant genes and male infertility. Biomed. Res. Int. 2015, 513196. https://doi.org/10.1155/2015/513196 (2015).
Nebert, D. W. & Vasiliou, V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 1, 460–464. https://doi.org/10.1186/1479-7364-1-6-460 (2004).
Amaral, A., Ramalho-Santos, J. & St John, J. C. The expression of polymerase gamma and mitochondrial transcription factor A and the regulation of mitochondrial DNA content in mature human sperm. Hum. Reprod. 22, 1585–1596. https://doi.org/10.1093/humrep/dem030 (2007).
Department of Reproductive Health and Research WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen 5th edn. (WHO Press, 2011).
Gong, S. Y. et al. Association between mitochondrial DNA copy number and lead level in peripheral blood among lead-exposed workers. J. Environ. Occup. Med. 35, 189–195. https://doi.org/10.13213/j.cnki.jeom.2018.17670 (2018).
Zhang, G. H. et al. Effect of polymorphic metabolizing genes on micronucleus frequencies among benzene-exposed shoe workers in China. Int. J. Hyg. Environ. Health 217, 726–732. https://doi.org/10.1016/j.ijheh.2014.03.003 (2014).
PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling.
O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 7, 662–668. https://doi.org/10.1111/andr.12630 (2019).
Gao, J. F. et al. Correlation of sperm mitochondrial DNA copy number and integrity with semen parameters in male adults. J. Third Mil. Med. Univ. 39, 1286–1291. https://doi.org/10.16016/j.1000-5404.201702074 (2017).
Hecht, N. B., Liem, H., Kleene, K. C., Distel, R. J. & Ho, S. M. Maternal inheritance of the mouse mitochondrial genome is not mediated by a loss or gross alteration of the paternal mitochondrial DNA or by methylation of the oocyte mitochondrial DNA. Dev. Biol. 102, 452–461. https://doi.org/10.1016/0012-1606(84)90210-0 (1984).
St. John, J. C., Facucho-Oliveira, J., Jiang, Y., Kelly, R. & Salah, R. Mitochondrial DNA transmission, replication and inheritance: A journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum. Reprod. Update 16, 488–509. https://doi.org/10.1093/humupd/dmq002 (2010).
Rajender, S., Rahul, P. & Mahdi, A. A. Mitochondria, spermatogenesis and male infertility. Mitochondrion 10, 419–428. https://doi.org/10.1016/j.mito.2010.05.015 (2010).
Strange, R. C., Spiteri, M. A., Ramachandran, S. & Fryer, A. A. Glutathione-S-transferase family of enzymes. Mutat. Res. 482, 21–26. https://doi.org/10.1016/s0027-5107(01)00206-8 (2001).
Zhang, H. et al. Genetic polymorphisms of glutathione S-transferase M1 and T1, and evaluation of oxidative stress in patients with non-small cell lung cancer. Eur. J. Med. Res. 19, 67. https://doi.org/10.1186/s40001-014-0067-3 (2014).
Wu, W. et al. GSTM1 and GSTT1 null polymorphisms and male infertility risk: An updated meta-analysis encompassing 6934 subjects. Sci. Rep. 3, 2258. https://doi.org/10.1038/srep02258 (2013).
Wu, Q. F. et al. Genetic polymorphism of glutathione S-transferase T1 gene and susceptibility to idiopathic azoospermia or oligospermia in northwestern China. Asian J. Androl. 10, 266–270. https://doi.org/10.1111/j.1745-7262.2008.00347.x (2008).
He, J. et al. Glutathione S-transferase genetic polymorphisms and fluoride-induced reproductive toxicity in men with idiopathic infertility. Asian J. Androl. 25, 404–409. https://doi.org/10.4103/aja202271 (2023).
Acknowledgements
We would like to express our gratitude to all the subjects who participated in the survey.
Funding
This work was supported by the Youth project of National Natural Science Foundation of China (82003489), and the Key Lab of Medical Protection for Electromagnetic Radiation (2022DCKF001), and the Clinical Research Project of Shanghai Municipal Commission of Health and Family planning (20224Y0085). Shanghai’s three-year Action Plan to strengthen public health system construction (2023–2025) Excellent Youth Project (GWVI-11.2-YQ30).
Author information
Authors and Affiliations
Contributions
T.Z. and S.Z.: Writing—original draft, data curation, methodology, resources. C.Z., H.L., M.L., and G.Z.: Investigation, methodology, project administration, resources. G.D.: Supervision, validation, writing−review and editing. S.C.: Writing—review and editing. J.R.: Conceptualization, formal analysis, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, T., Zhang, S., Zhang, C. et al. The moderation effect of GSTM1/GSTT1 gene polymorphisms on the association of sperm mitochondrial DNA copy number and sperm mobility. Sci Rep 14, 24790 (2024). https://doi.org/10.1038/s41598-024-74968-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74968-3