Abstract
A highly efficient approach for synthesizing a supramolecular metallogel of Co(II) ions, denoted as CoA-TA, has been established under room temperature and atmospheric pressure conditions. This method employs the metal-coordinating organic ligand benzene-1,3,5-tricarboxylic acid as a low molecular weight gelator (LMWG) in DMF solvent. A comprehensive analysis of the mechanical properties of the resulting supramolecular Co(II)-metallogel was conducted through rheological investigation, considering angular frequency and thixotropic study. The hierarchical rocky network structure of the supramolecular Co(II)-metallogel was unveiled using field emission scanning electron microscopy (FESEM). Transmission electron microscopic (TEM) analysis showed rod-shaped structures via low-magnification high angle annular dark field (HAADF) bright field scanning transmission electron microscopic (STEM) imaging, while energy dispersive X-ray (EDX) elemental mapping confirmed its primary chemical constituents. The formation mechanism of the metallogel was examined via fourier transform infrared spectroscopy (FTIR) spectroscopy. The nature of the synthesized CoA-TA metallogel was affirmed through powder X-ray diffraction (PXRD) analysis. Furthermore, this study involved fabrication of Schottky diode structures in a metal-semiconductor-metal geometry based on cobalt(II) metallogel (CoA-TA), enabling observation of charge transport behavior. Remarkably, a resistive random access memory (RRAM) device utilizing cobalt(II) metallohydrogel (CoA-TA) demonstrated bipolar resistive switching at room temperature and under ambient conditions. The switching mechanism was investigated, revealing the formation and rupture of conductive filaments between metal electrodes that govern the resistive switching behavior. This RRAM device exhibited an impressive ON/OFF ratio (~ 414) and exceptional endurance over 5000 switching cycles. These structures offer great potential for diverse applications such as non-volatile memory design, neuromorphic computing, flexible electronics and optoelectronics. Their advantages lie in their fabrication process, reliable resistive switching behavior and overall performance stability.
Similar content being viewed by others
Introduction
Supramolecular gels formed through the intricate choreography of non-covalent interactions, captivate scientists with their elegance and versatility. Gels, with their jelly-like consistency and unique properties, stand out as a captivating category of materials. They’re not just semi-solid; they adapt to their surroundings with an elastic, interconnected network of particles or molecules suspended in a liquid medium1,2,3. From smoothing out cosmetics to delivering drugs in medicine, gels flex their versatility across a spectrum of industries like cosmetics and skincare products4 etc. But their influence doesn’t stop there; they play pivotal roles in stabilizing, thickening, and emulsifying food products, too. Overall, gels are an essential class of materials that have transformed numerous aspects of modern life through their distinctive characteristics and diverse applications5.
Supramolecular gels are composed of self-assembling molecules that come together in a mesmerizing combination of non-covalent interactions: hydrogen bonding, π-π stacking, and van der Waals forces6. The combination and strength of these interactions determine the stability to mechanical prowess to how quickly they form gel. By tweaking the ingredients, scientists wield absolute control over their mechanical, thermal, and rheological traits, crafting gels that are tailor-made for any task. Supramolecular gels have found applications in various fields, including drug delivery7, tissue engineering8, sensors9, and even as soft electronic devices10,11, showcasing their immense potential to revolutionize diverse industries. The exploration of supramolecular gels continues to be an exciting area of research, offering promising avenues for the development of cutting-edge materials with remarkable functionalities.
Low molecular weight gelators (LMWGs) are small organic molecules with relatively low molecular masses that possess a unique ability to self-assemble and form supramolecular gels. These gelators typically contain both hydrophobic and hydrophilic regions within their chemical structure, enabling interactions with each other and solvent molecules through non-covalent bonds. During self-assembly, LMWGs aggregate in solution, forming a network-like structure that traps the solvent, resulting in a supramolecular gel material. Remarkably, despite their low molecular weight, these gelators can form gels at very low concentrations, often as low as 1–5% or even less, making them highly efficient for gel formation. In recent years, low molecular weight gelators have garnered significant attention due to their potential applications in various fields. Their ability to form gels at low concentrations makes them cost-effective and environmentally friendly. They find utility in drug delivery12, tissue engineering13, sensor technologies14, soft robotics15, and the development of smart materials16. The choice of solvent significantly influences the gelation properties, morphology, and stability of the resulting supramolecular gel. An eclectic mix of solvents, ranging from the commonplace water17,18,19,20to a plethora of organic counterparts like acetone21, alcohol22,23, tetrahydrofuran24, toluene25, carbon tetrachloride26, dimethyl sulfoxide27, dimethylformamide28,29,30, perform the role of dissolving gelators and ushering in gelation. The choice of solvents determine the performance towards optimal design and performance.
Metallogels represent a unique category of supramolecular gels, distinguished by their formation through the self-assembly of metal-containing molecules or coordination complexes31. Unlike traditional supramolecular gels, which primarily utilize organic gelators, metallogels incorporate metal ions or metal complexes as crucial components in the gelation process. This process is typically driven by metal-ligand coordination interactions, where metal ions act as cross-linkers between ligands. These ligands, which can be organic molecules or coordination compounds with specific binding sites, form coordination bonds with metal ions, providing the necessary stability for creating a three-dimensional network. This network effectively entraps the solvent, resulting in a gel-like material. The properties of metallogels can be finely tuned by selecting different metal ions, ligands, and solvents during the gelation process. This tunability, along with their unique mechanical, electrical, magnetic and optical properties makes metallogels promising candidates for a wide range of applications. These include uses in sensors32, controlled drug release systems33, memory devices, semiconducting diodes, catalysis34,35, proton conductivity36, and magnetic behavior37. Researchers have unveiled a fascinating array of metallogels crafted from transition metal ions such as Mn(II), Ni(II)38,39, Cu(II)40, Cd(II), Fe(II/III)41,42,43, Zn(II)44,45, Co(II)46. These metallogels are revolutionizing various scientific fields with their exceptional properties and applications. Notably, Co(II)-based metallogels stand out due to their remarkable versatility. They play pivotal roles in redox switching47, toxic removal48, sensor49, non-linear property50, self-healing property, and functionality in semiconducting devices51and catalysis. These unique materials, with their multifaceted potential, are particularly promising for cutting-edge applications like resistive switching devices52,53,54, making them a hot topic of research and innovation.
On the other hand, resistive switching is a fascinating phenomenon that has garnered significant attention in the field of electronic materials and device research. It involves the reversible change in resistance of a material or device when subjected to an external electrical stimulus. This effect has been harnessed for various applications, including non-volatile memory devices, synaptic emulation in neuromorphic computing55,56, and analog computing. The fundamental mechanism behind resistive switching is the modulation of the conductivity of certain materials, often referred to as resistive switching materials (RSMs), between high-resistance and low-resistance states. This transition can be controlled by applying a voltage or current pulse, which induces the migration of ions or defects within the material, leading to the formation or dissolution of conductive paths. The resulting change in resistance can be precisely controlled and utilized for storing information or performing computational tasks. Resistive switching phenomena have been observed in a wide range of materials, including transition metal oxides, organic compounds, and polymers. The combination of metallogels’ distinctive characteristics and the ability of resistive switching devices to toggle between resistance states which make them promising candidates for advanced electronic applications.
In this study, we successfully synthesized a metallogel called CoA-TA using cobalt(II) acetate tetrahydrate salt (CoA) and benzene-1,3,5-tricarboxylic acid i.e. trimesic acid (TA) as the low molecular weight gelator ligand. The synthesis was carried out under ambient reaction conditions in a N, N-Dimethylformamide (DMF) medium. Resistive random access memory (RRAM) devices possess exceptional memory capabilities, making them valuable in various fields such as switching, non-volatile memory design, and neuromorphic computing57,58. These devices operate based on physical phenomena like vacancy migration and charge carrier trapping, which facilitate transitions between high- and low-resistance states. Metallogels offer promising opportunities as alternatives to oxide materials in RRAM design which enables the development of flexible electronics and metal-semiconductor (MS) junctions59,60,61. Our approach of creating a flexible and functional soft gel scaffold using Co(II)-metallogel contributes to the advancement of memory devices, especially in data-driven applications like Internet of Things (IoT) and 5G connectivity. Due to their non-volatility and nonlinearity, memristors have a wide range of applications in memory, logic gate, and brain synaptic networks62. Our research has focussed on the function of memristor-based logic circuits in memory applications and provides a succinct overview of the technology. The potential use of memristors for implementing logic in memristor arrays is also explored in this work. Memristive devices with in-memory computing technology differ from conventional computing systems in that they offer more possibilities for artificial intelligence applications. However, research on memristor-based logic circuits opens up new avenues and techniques for creating novel logic architectures63.
Experimental methods
Reagents
Cobalt(II) acetate tetrahydrate (98%), benzene-1,3,5-tricarboxylic acid (95%) were purchased from Sigma-Aldrich chemical company and used as received. Dry solvent i.e. N, N-dimethyl formamide (DMF) was used for entire work.
Apparatus and measurements
A SHIMADZU made UV-3101PC spectrophotometer to collect UV-vis absorption spectral data.
Rheology experiment of the gel was done by an Anton Paar 100 rheometer with a cone and plate geometry (CP 25 − 2). All the mechanical measurements were done fixing the gap distance between the cone and the plate at 0.05 mm. The gels were scooped on the plate of the rheometer. An oscillatory strain amplitude sweep experiment was performed at a constant oscillation frequency of 1 Hz for the applied strain range 0.001-10% at 20°C.
Microstructural feature was analysed using a JEOL JSM 7900 F instrument.
Transmission electron microscopy (TEM) was conducted in an aberration corrected FEI Titan Themis operating at 300 kV. The energy-dispersive X-ray spectroscopy (EDX) analyses in scanning mode were carried out with a Bruker Super detector. The CoA-TA metallogel sample was sonicated in ethanol and drop-casted on holey carbon grids.
A Perkin Elmer made FTIR Spectrum 400 spectrometer was used for IR study.
The Powder X-Ray diffraction (PXRD) data of CoA-TA metallogel was collected at room temperature by of Phillips PANalytical X’PERT PRO instrument.
X-ray photoelectron spectroscopy (XPS) of metallogel was collected by Nexsa-Thermofisher made instrument.
A digital gel melting point measurement apparatus (Aplab MPA-01) was used to the Tgel of the CoA-TA metallogel.
The current-voltage (I-V) measurements of our synthesized metallohydrogel material-based devices were performed at room temperature using a Keithley 2400 sourcemeter interfaced through Labview.
Synthetic Procedure of Co(II)-metallogel (CoA-TA)
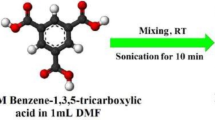
A stable pink-colored Co(II)-metallogel (CoA-TA) was synthesized using a one-shot mixing approach. Specifically, 1 ml of a DMF solution containing 0.249 g (1 mmol) of cobalt(II) acetate tetrahydrate and 1 ml of a DMF solution containing 0.210 g (2 mmol) of benzene-1,3,5-tricarboxylic acid were mixed, followed by continuous sonication of the mixture for 10 min at room temperature (Fig. 1a). The resulting CoA-TA metallogel was found to resist gravitational force, as demonstrated by the inversion of the vial without gel collapse (Fig. 1a). To determine the minimum critical gelation concentration (MGC) of CoA-TA metallogel, different concentrations of Co(CH3COO)2·4H2O and benzene-1,3,5-tricarboxylic acid in the range of 30–459 mg mL−1 were used. It is important to note that the ratio of the CoA-TA metallogel components was maintained at a constant 1:2 (w/w) for Co(CH3COO)2·4H2O and benzene-1,3,5-tricarboxylic acid. The optimal condition for obtaining the stable pinkish-colored CoA-TA metallogel was found to be at a concentration of 459 mg mL−1 for both Co(II)-acetate salt and benzene-1,3,5-tricarboxylic acid in DMF solvent (Fig. 1b). Moreover, the gel melting temperature (Tgel) of the CoA-TA metallogel was determined to be 120ºC ± 2ºC using a digital melting-point measuring apparatus.
Results and discussion
Rheological analysis
The mechanical properties of the CoA-TA metallogel were characterized through a rheometer instrument of angular frequency and strain-sweep measurement. Gels are unique materials with viscoelastic properties, exhibiting the ability to both store and dissipate energy. Their rheological behavior is characterized by oscillatory stress experiments, which allow for the measurement of key properties. The storage modulus (\(\:G'\)) represents the energy stored in the system when shear is applied within the viscoelastic region. On the other hand, the loss modulus (\(\:G''\)) indicates the amount of energy dissipated as the gel behaves more like a liquid under the application of oscillatory stress. Together, these moduli provide valuable insights into the energy dynamics of gels. The storage modulus (\(\:G'\)) and loss modulus (\(\:G''\)) of the samples are defined as
Where, σ0 represents the shear stress amplitude, γ0 is the strain amplitude, and δ is the phase angle. The amplitude ratio (σ0/γ0) is also important in these calculations. Notably, gels always satisfy the conditions \(\:G'\)(ω) > \(\:G''\)(ω) and \(\:G'\)(ω) ≈ ω°, with ω being the angular frequency. From the rheological measurements, various mechanical properties of the CoA-TA metallogel can be determined. These properties include the mechanical strength (\(\:G'\)), fragility (σ*, which represents the minimum stress required to break the gel), elasticity (\(\:G'\)−\(\:G''\)), stiffness (\(\:G'\)/\(\:G''\)), and the complex modulus (G*), which is calculated as [G* (G* = (\(\:G'\)2+\(\:G''\)2)1/2)].
These measurements provide valuable insights into the behaviour and characteristics of the gels. The rheological investigation of CoA-TA metallogel at a specific concentration of Co(CH3COO)2·4H2O and benzene-1,3,5-tricarboxylic acid (MGC = 459 mg/mL) reveals interesting findings. The storage modulus (\(\:G'\)) of CoA-TA metallogel is significantly higher than the loss modulus (\(\:G''\)) as shown in Fig. 2a. This notable difference between \(\:G'\) and \(\:G''\) confirms the gel-like nature of the material with a semi-solid performance. The average storage modulus of CoA-TA metallogel (\(\:G'\) > 105 Pa) was found to be considerably higher than the loss modulus (\(\:G''\)), indicating a substantial endurance limit for the CoA-TA metallogel (Fig. 2a). These results provide strong evidence for the gel-like behavior of the CoA-TA metallogel with excellent mechanical strength.
(a-c) Rheological analysis of CoA-TA metallogel: (a) Graph depicting the storage modulus (\(\:G'\)) and loss modulus (\(\:G''\)) as functions of angular frequency, (b) Strain-sweep assessments conducted on Co(II)-metallogel at a consistent frequency of 6.283 rad/sec, (c) Cycling of \(\:G'\) and \(\:G''\) values of the metallogel under significant strain (γ = 50%) and varying loading periods ranging from 10 to 100 s.
In Fig. 2b, a strain-sweep experiment was conducted on the CoA-TA metallogel material at a constant frequency of 6.283 rad/sec. The experiment revealed important findings about the gel’s behavior. Specifically, the critical strain, which represents the lowest strain leading to the breakdown of the CoA-TA metallogel, was observed at 0.45%. At this strain value, the storage modulus (\(\:G'\)) becomes comparable to the loss modulus (\(\:G''\)) as shown in Fig. 2b. These results provide valuable insights into the mechanical properties and stability of the CoA-TA metallogel under varying strains, and indicate the point at which the gel transitions to a different state.
The strain-sweep experiment (Fig. 2c) revealed intriguing dynamics in the CoA-TA metallogel. Initially, the storage modulus (\(\:G'\)) exceeded the loss modulus (\(\:G''\) ) until the strain reached approximately 0.1%, indicating a gel-to-sol transition. To further investigate the self-healing behavior, a thixotropy test was conducted (Fig. 2c). Surprisingly, at a strain of about 0.01%, the soft material remained in the gel state, but when the strain was abruptly increased to around 100%, it immediately transformed into a sol. However, upon relieving the high strain, the soft material quickly reverted back to its viscoelastic gel state. This cyclic process of low/high strain demonstrated the reversible transition of the soft material from a gel to a sol state, providing strong evidence of its self-healing behavior. The CoA-TA metallogel showed remarkable resilience and the ability to recover its gel state after experiencing high strains, making it a promising material for various applications.
Microstructural study
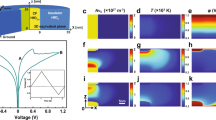
The FESEM images of the CoA-TA metallogel revealed a distinct hierarchical network resembling a rocky structure (Fig. 3a-d). This intricate arrangement observed through FESEM analysis was achieved by synthesizing the metallogel using a combination of Co(OAc)2·4H2O and benzene-1,3,5-tricarboxylic acid in a DMF medium while employing consistent sonication. The well-defined microstructural network of the CoA-TA metallogel is likely attributed to the prevailing supramolecular interactions that contribute to its structural stability. In Fig. 3e, the rod-shaped structural morphology of the CoA-TA metallogel is evident through low-magnification high angle annular dark field (HAADF) bright field STEM imaging. Within the gel, as indicated by the white arrow in Fig. 3f, high-resolution TEM images reveal the distribution of Co nanoparticles inside CoA-TA metalogel. Selected area diffraction pattern reveals the cubic phase of Co nanoparticles formed inside the CoA-TA metallogel in Fig. 3g. High resolution TEM in Fig. 3h shows lattice fringes of Co atoms. TEM analysis enables the precise measurement of interplanar distances (d-spacing) in different position which is around 0.40 nm (Fig. 3h), providing a deeper understanding of the crystallographic arrangement and the material’s overall stability. It is concluded that this CoA-TA mettalogel is a polycrystalline material. From SAED pattern of corresponding TEM image of this metallogel, we have observed three circular rings with different d-spacing values such as 3.36 nm, 1.6 nm, 1.05 nm which are comparable with the (hkl) values obtained from the XRD pattern. From the high-resolution TEM image, we have characterized the size distribution of the Cobalt nanoparticles which offers crucial information about their morphology and uniformity. The average size of Co nanoparticles is 9.593 nm and the average area of this nanoparticle is 1.94 nm2 which is determined by the histogram plot (Fig. 3i, j). The presence of essential elements, including C, O, Co, and N, derived from Co(OAc)2·4H2O, benzene-1,3,5-tricarboxylic acid, and DMF molecules, crucial for the formation of the CoA-TA metallogel networks, is confirmed through elemental composition mapping by STEM EDX (Fig. 3k-p).
(a-d) The FESEM microstructural pattern of CoA-TA metallogel; (e) Bright field TEM image of CoA-TA metallogel; (f) shows Co nano particle distribution marked by white arrows; (g) SEAD pattern of Co nanoparticles; (h) HRTEM of Co nanoparticle inside; (i, j) Size of Co nanoparticles and the average area of this nanoparticle is determined by the histogram plot; (k, l) The corresponding region for elemental mapping is shown in STEM; (m-p) Elemental composition of C, O, Co and N are mapped by STEM EDX.
FT-IR, PXRD and XPS analysis of CoA-TA metallogel
The FTIR spectrum of the xerogel form of CoA-TA metallogel reveals prominent peaks at specific wavenumbers: 3420 –3200 cm−1 for OH stretching, 2940 cm−1 for –CH stretching, 1650 cm−1 for C = O (carboxylic) stretching, 1370 cm−1 for –CH3, and 1100 cm−1 for (-COO). Additionally, there are distinctive peaks observed at 680 cm−1, attributed to Co-O stretching vibrations (Fig. 4a). These FTIR spectral findings provide insights into the supramolecular interactions within the CoA-TA metallogel, shedding light on the interplay among its constituent chemical components.
The CoA-TA metallogel’s nature was analysed using PXRD (Fig. 4b). The diffraction pattern exhibited seven sharp peaks at specific 2θ values: 12.36º, 18.80º, 25.75º, 27.05º, 28.65º, 32.48º, 35.60º respectively. Other peaks were narrow peaks. The sharp and narrow peaks indicate a high degree of crystallinity in the synthesized compound. Depending on the XRD profiles at room temperature, the presence of Co acetate monohydrate, tartaric acid and DMF was identified. Specifically, the 2θ values such that 12.36º corresponded to (Co(OAc)2·H2O), 18.80º corresponded to (Co(OAc)2), 25.75º corresponded to (both Co(OAc)2·H2O and Co(OAc)2) and 27.05º, 28.65º and 32.48º corresponded to tartaric acid and 35.60º corresponded to DMF which was present in the metallogel.
XPS is a surface sensitive, non-destructive spectroscopic tool for elemental analysis. The kinetic energy of the photoelectrons emitted contains the information about the chemical composition and electronic states of elements present in the compound. As the binding energy of photoelectrons gets affected by the chemical environment, it also gives insight into the chemical structure64. The deconvolution of individual elemental peaks reveals the possible chemical bonds. Figure 4(c-f) shows the core level XPS spectra of C 1s, N 1s, Co 2p and O 1s. All the spectrum was analysed using XPSPEAK4.1 software. Figure 4c shows the XPS spectra of C 1s. The deconvolution of peaks gives four subpeaks. While the peaks at 284.8 eV and 285.0 eV corresponds to C-C bond. Peaks at 1.5 eV and 3.8 eV above the main C 1s (284.8 eV) is ascribed to functionality group. Thus 286.3 eV and 288.6 eV peaks indicate the presence of C-O-C and O-C = O respectively65. The Co 2p spectra, shown in Fig. 4d consists of two spin orbit split doublets with associated shake-up satellite peaks. The peaks at 780.9 eV and 782.4 eV correspond to 2p3/2state and its satellite structure appears at 785.59 eV and 789.80 eV66. These peaks are ascribed to the Co2+ species. The Co 2p1/2 peaks appear at higher binding energy. Different peak position of Co and its satellite indicates the presence of CoO chemical state, also found from FTIR. Figure 4e shows the XPS spectrum of N 1s. The deconvoluted spectrum shows three peaks at 399.4 eV, 400.61 eV and 401.51 eV, which are assigned to N-C = O and N-O bonds respectively. The O 1s spectrum is shown in Fig. 4f. The peaks positioned at 529.8 eV, 531.8 eV and 533.4 eV are attributed to C = O and O-C, O-H bonds respectively.
Optical characterization
Figure 5 illustrates the analysis of the UV-vis absorption spectrum using the Tuac’s Plot to identify the optical properties of the synthesised CoA-TA based metallogel. A wavelength range of 300 nm to 800 nm was used for the optical measurement (shown in the inset of Fig. 5). Using Tauc’s equation (given by Eq. 1), we determined the optical bandgap of the CoA-TA metallogel:
Where α, h, Eg and ν are absorption coefficient, Planck’s constant, band gap, and frequency of the light respectively. In the processes of electron transitions, the exponent “n” is a constant. The value of the constant “A” is 1. With n = 2, the direct optical band gap was computed. We determined the direct optical band gap (Eg), which is assumed to be 3.60 eV, by extending the linear region of the plot (αhν)2 vs. hν (Fig. 5) to the region where absorption vanishes.
Device fabrication
We prepared a device based on a metal-semiconductor junction in horizontal configuration with the structure of ITO (Indium Tin Oxide)/CoA-TA/ITO (Device 1) to investigate the electrical properties of the synthesised material. We used the doctor blade method to deposit a thin layer of synthesised CoA-TA metallogel on an ITO coated glass substrate to create the junction device. The film had already been annealed to remove the solvent. ITO is an appropriate material for research involving photo-excitations since it is an optically transparent and electrically conducting material commonly used in various electronic devices and displays.
In this work, we developed two sandwich-like structures such as ITO/CoA-TA/Cu (referred to as Device 2) and Cu/CoA-TA/Cu (referred to as Device 3) in vertical configuration to create RRAM devices based on the CoA-TA metallogel. Here, ITO acts as a bottom electrode in both the configurations which had been cleaned using ultrasonicator. Then synthesized CoA-TA metallogel had been deposited on ITO substrate. After that, the top electrode was placed on the sample to create these RRAM devices.
The device structures are given by the following Table S1 in Supporting Information.
Electrical characterization of device
To investigate the semiconducting nature of the material, the charge transport properties of CoA-TA metallogel in the thin film geometry were observed. In Fig. 6, I-V curve for device 1 is displayed on a linear scale within the voltage range (-4 V to + 4 V). Within this voltage range, it was noticed that the current increased quickly as the voltage increased for both positive and negative polarity. After measuring I-V characteristics, the primary diode characteristics were calculated using the thermionic emission theory (TE Theory) from the I-V curve of a non-linear Schottky diode. Cheung67 proposed using this approach to learn how these systems transmit electricity. Using the following Eqs. (2)-(3), the measured I-V curve was evaluated.
Here, I0 = Saturation Current, q = Electronic Charge, kB = Boltzmann’s Constant, T = Temperature, V = Applied voltage, A = Effective diode area, η = Ideality factor, ΦB = Barrier potential height, RS = Series resistance, A* = Effective Richardson constant which is considered as 32 AK−2cm−2 for this device.
We have also plotted the log I vs. log V graph, which is shown in Fig. 6 (right: inset), to better understand the conduction mechanism. In the lower voltage region, the current follows an ohmic conduction behaviour with a slope of m = 1, whereas in the higher voltage region, it is seen to follow a space charge limited conduction mechanism with a slope of m = 5.37.
The series resistance, ideality factor, and barrier potential height were determined using Eqs. (4–6). Cheung suggested these equations. We plotted the graphs of dV/d(ln I) vs. I and H vs. I in order to estimate the diode parameters for device 1, as depicted in Fig. S1(shown in Supporting Information). The ideality factor (\(\:\eta\:\)) was determined using the intercept of the dV/d(ln I) vs. I graph, and the barrier height was calculated using the intercept of the H vs. I graph. In our experiment, the ideality factor (\(\:\eta\:\)) is 8.5167. This value is higher than the ideal value of 1.1. The discrepancy of these results could be attributed to the presence of interface states, series resistance at the interface and inhomogeneities inside the Schottky barrier itself.
A barrier height (\(\:{\varphi\:}_{B}\)) of 0.0258 eV was measured for device 1. These two characteristics such as a lower barrier potential height and a higher ideality factor in the prepared diode structure, must be present in a Schottky diode. From the slopes of the dV/d(ln I) vs. I and the H vs. I graph, we determined the value of series resistance which is 830.85 Ω. By taking into account all the measured characteristics, it can be concluded that such diode structures can be useful for the application of electronic device.
Figure S2 shows the differences between two I-V features for the CoA-TA metallogel-based device under dark and light environments (shown in Supporting Information). For light environment, the current values increase rapidly with an increase in voltage compared to the dark medium. This CoA-TA based device may exhibit photoconductive behavior, that means they become more conductive under light exposure. When the material becomes more conductive, the resistance of the device decreases which leads to an increase in current for a given voltage. This is the reason behind the change in IV characteristics. The resistive switching behaviour is also measured for device 1, which is shown in Fig S3 (shown in Supporting Information). Here, the voltage starts from 0 V → 10 V → -10 V→ 0 V. It exhibited a proper hysteresis behaviour of I-V characteristics.
We have also investigated the resistive switching behaviour of ITO/CoA-TA/Cu heterostructure based on CoA-TA metallogel, where ITO acts as the bottom electrode and Cu acts as the top electrode (see Fig. 7(right: inset)). The compliance current (CC) is set at 100 mA before starting any measurements for our investigations in order to eliminate any leakage contribution to the I-V measurements. In Fig. 7, the I-V characteristic of device 2 is shown on a linear scale. This voltage sequence used for I-V measurement is 0 V→ 10 V→-10 V→10 V. Here, we have seen a complete hysteresis, which is a characteristic of memristor behaviour. When the voltage is low, the current increases linearly from point 1 to point 2 and then it increases, as the voltage increases. Next, it reaches its highest value at 5 V at point 2, and then it slightly decreases with an increase in voltage from point 2 to 3. Then the device is converted from a higher resistance state (HRS) to a lower resistance state (LRS) at a voltage of 9.95 V, also known as the SET voltage (VSET), and this is the process known as the SET process. The device is still turned on at that point. When the device transitions from the ON state to the OFF state from point 3 to point 4, the current starts to rapidly deteriorate as the voltage drops. After that, the current will increase in reverse process until it reaches to LRS at point 5. When the RESET value (VRESET) reaches at -9.95 V, then the device returns to the HRS mode. The arrow between points 5 and 6 defines the RESET process which indicates that the device switches from LRS to HRS. Since a negative voltage is required to return the device to its initial resistance state, this is an important sign of bipolar resistive switching behaviour of this device. The I-V characteristic of the device 2 is displayed on a semi-logarithmic scale in Fig. 7 inset (left). Current fluctuations are primarily caused by the formation and rapture of the conductive filaments, which are used to explain the switching of the device between the ON and OFF states. This mechanism is described in the next section.
Schematic representation of a glass/ITO/CoA-TA/Cu-based device (right: inset), along with I-V characteristics plotted on a linear scale for the same device (Point 1 to 2 indicates a linear increase in current, Point 2 to 3 indicates slightly decrease in current, Point 3 to 4 indicates abrupt decrease in current, Point 4 to 5 indicates change in the direction of current and then Point 5 to 6 indicates an increase in current continuously up to 0 V) and I-V Curve of glass/ITO/CoA-TA/Cu based device in semi-logarithemic scale (left: inset).
In order to explain the conduction process and the charge transport mechanism of device 2, we have also plotted the I-V curve throughout the SET procedure using a logarithmic scale as seen in Fig. S4 (shown in the Supporting Information). With a slope of m = 1, the current exhibits Ohmic conduction behaviour in the lower voltage region, while in the higher voltage region, it also displays same behaviour with a slope of m = 1.5.
In device 3 (glass/Cu/CoA-TA/Cu), where Cu acts as the top and bottom electrodes, we have also looked into the resistive switching behaviour. In Fig. 8, the behaviour of the memristor is depicted during full voltage cycling with discrete LRS and HRS states during the SET and RESET operations (VSET = 3.978 V and VRESET = -3.978 V). Device 3 exhibits similar switching behaviour which is comparable with device 2. Figure 8 (inset: left) displays I-V characteristic of device 3 on a semi-logarithmic scale.
In order to explain the conduction mechanism and the charge transport procedure for device 3, we have also fitted the I-V curve in a logarithmic scale during the SET procedure (shown in the Supporting Information in Fig. S5). Here, it is seen that the current varies linearly with a slope of m = 1 in the lower voltage area which indicates the behaviour of Ohmic conduction. Similar to this, it exhibits Ohmic conduction behaviour with a slope of m = 1.35 at the higher voltage area.
We have measured the complete IV curves for device 3 (Cu/Co-A-TA/Cu) for consecuitive cycles and observed minor variation in the multiple cycles (~ 3000) which gets stabilised later (shown in the Supporting Information in Fig. S7). The initial variation can arise due to ion migration. Cu ion migration plays an important role in the formation of conductive filament for switching process. RRAM devices often depend on the movement of oxygen vacancies within the material to achieve resistive switching. However, with repeated cycling, the distribution and concentration of oxygen vacancies can change. This redistribution can alter the resistive states and affect the hysteresis behaviour observed in the I-V curve.
To understand the reliability of the switching process for device 3, an endurance test is conducted at room temperature, as shown in Fig. 9. The results of this endurance test have revealed that the switching process of device 3 remained stable up to 5000 multiple cycles. Additionally, the endurance test has provided valuable information about the average ON/OFF ratio, which is determined to be approximately ~ 414. This high ratio indicates a strong and reliable switching behaviour. It suggests that the memory device can work without any degradation, which is advantageous for cost-effective memory circuit designs. Based on the observed stability and the high ON/OFF ratio, it can be concluded that the switching process of device 3 is reliable and can be trusted for practical applications.
We have also performed retention test upto 1000 sec at room temperature for device 3, as shown in Fig. S6 (shown in the Supporting Information). The switching procedure for device 3 is found to be reliable up to 103 sec. We have also observed that for device 3, ON/OFF ratio is around 100. From the retention test, we can conclude that this device can safely perform storage functionality upto 103 sec without any degradation.
Redox reaction, the formation of the Schottky barrier with electrochemical migration and valence change memory are some phenomena that can be used to explain the physical reasons of the resistive switching process. The valence change memory (VCM) and electrochemical metallization (ECM) processes are affected by the mobility of oxygen defects and metal cations in our research. Here, it is shown that both metal ions and oxygen vacancies considerably affect the change in resistance of the device (Fig. 10). The formation and rupturing of the Cu filaments are used to explain how the device switches from HRS to LRS in the semiconducting layers. This work indicates that the migration of Cu ions, Co ions and oxygen vacancies plays a crucial role in the resistive switching behaviour of device 2. As we already know, Cu→ Cu2+ + 2e−, so, Cu ions move in the direction of an applied electric field and ionise in the presence of an electric field. While applying a positive voltage, Cu ions, Co ions and oxygen vacancies move towards the intermediate layer, where they transform into metallic Cu. After completing the SET process, the device changes from HRS to LRS and then Cu ions, Co ions and oxygen vacancies go towards the bottom electrode. At this moment, the conductivity of this layer increases. The device remains in the LRS state until a sufficient voltage of the opposite polarity is applied to dissolve the Cu filaments and oxygen vacancies through an electrochemical RESET operation. In the RESET process, the device enters into the HRS and its conductivity decreases when a negative voltage is applied. At the end of the procedure, Cu2+, Co ions, and oxygen vacancies return to the top electrode. Furthermore, this method can be utilised to explain the resistive switching behaviour of device 3 through the migration of Cu2+ ions, Co ions and other ions.
Schematic representation of the mechanism of the resistive switching process of a glass/ITO/CoA-TA/Cu-based device using the conductive filament model along with the I-V curve: (a) The ions move towards the intermediate layer after applying a positive voltage of 0.45 V; (b) At 9.926 V, the Cu ions, Co ions, and oxygen vacancies produced a conducting filament-type structure; (c) At − 9.893 V, the Cu ions, Co ions, and oxygen vacancies return to the top electrode; and (d) At -0.4 V, all of the ions are gathered at the top electrode and switched into HRS.
Further, we have shown how metallogel based RRAM devices in a crossbar array works in memory computing using logic gate operation. For making crossbar device, the gel sample is placed at the crossing point of two perpendicular metal lines, as shown in Fig. 11. Here, in this work we have prepared 2 × 2 cross bar devices based on Cu/CoA-TA/Cu structure where Cu acts as both top and bottom electrode (Fig. 11) and four RRAM devices are designated by A, B, C, D.
Now, for logic gate operations we have used two RRAM devices (A and B). For OR logic gate, if memristors A and B are both in logical “0” that means no voltage is applied, then output voltage is 0.03 V which is considered as “0” state. When we applied 5 V at Input A and 0 V at Input B, then output voltage is 4.81 V which is considered as logical “1” state. Similarly, when we applied 0 V at Input A and 5 V at Input B, then output voltage is 4.82 V which is also considered as logical “1” state. When we applied 5 V at both the terminals, then the output voltage is also 4.81 V, considered as logical “1” state. The truth table of OR logic gate is shown in Table S2 (in the Supporting Information). Similarly, we have designed NOT gate logic circuit (shown in Fig. 11) using device C and it also satisfied NOT gate truth table which is shown in Table S3 (in the Supporting Information). The current structure can be extended further with a larger size of cross-point arrays to perform advanced logic and computing operations, which can act as a central part for in-memory computing where the computation and information storage are done at the same circuit level, as demonstrated here. In this way, memristor based logic gate circuits using crossbar arrays will help us to explore different engineering methodologies that depend on in-memory computing principles.
Conclusions
A novel supramolecular Co(II)-metallogel was synthesized using a benzene-1,3,5-tricarboxylic acid gelator. The gel was formed by quickly mixing cobalt acetate and benzene-1,3,5-tricarboxylic acid in DMF, followed by sonication at room temperature. The gel’s stability arises from various non-covalent interactions. The CoA-TA metallogel’s microstructure was examined using FESEM, revealing a distinctive rocky hierarchical architecture. TEM analysis showcased rod-shaped structures via low-magnification HAADF bright field STEM imaging, while EDX elemental mapping confirmed its primary chemical constituents. Rheological tests confirmed the material’s mechanical stability and thixotropic behavior. FTIR analysis elucidated potential non-covalent interactions between cobalt salt and benzene-1,3,5-tricarboxylic acid ligands in DMF solvent. The nature of the CoA-TA metallogel was further understood through PXRD patterns. Our synthesized CoA-TA metallogel exhibited a semiconducting nature which is confirmed by its optical band gap. We extended our study to include the fabrication of RRAM devices, namely ITO/CoA-TA/Cu and Cu/CoA-TA/Cu configurations, revealing bipolar resistive switching behavior. The resistive switching is attributed to conduction filament formation and rupture between the vertical electrodes. Impressively, these heterostructures demonstrated non-volatile and non-destructive switching with a consistent ON/OFF ratio of ~ 414 maintained over 5000 cycles. Our recent findings underscore the Co(II)-metallogel’s suitability for non-volatile memory applications due to its robust resistive switching, remarkable endurance, and high ON/OFF ratio. Here, Cu/CoA-TA/Cu based 2 × 2 crossbar array was also successfully developed to show how this crossbar device acts as in-memory computing. Therefore, our current work on the CoA-TA metallogel, derived from benzene-1,3,5-tricarboxylic acid and cobalt(II) source, introduces an innovative method and material for efficient RRAM device applications. Besides this, specific properties can be optimized for desired memristor behaviors, such as low power consumption, fast switching, and high scalability for integration into neuromorphic computing and data storage systems by fine-tuning synthesis conditions and comprehensively characterizing the metallogels. The current methodology guides the design and synthesis of metallogels that meet the specific electrical and mechanical requirements for reliable and high-performance memristive devices.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kuosmanen, R., Rissanen, K. & Sievänen, E. Chem. Soc. Rev. 49 1977–1998. (2020).
Whitesides, G. M., Mathias, J. P. & Seto, C. T. Science 254 1312–1319. (1991).
Steed, J. W. Chem. Commun. 47 1379–1383. (2011).
Sangeetha, N. M. & Maitra, U. Chem. Soc. Rev. 34 821–836. (2005).
Dastidar, P. Chem. Soc. Rev. 37 2699–2715. (2008).
Dhibar, S. et al. ACS Appl. Electron. Mater. 1 1899–1908. (2019).
Hamley, I. W. Angew Chem. Int. Ed. 42 1692–1712. (2003).
Kato, T., Mizoshita, N. & Kishimoto, K. Angew Chem. Int. Ed. 45 38–68. (2006).
Panja, S., Panja, A. & Ghosh, K. Mater. Chem. Front. 5 584–602. (2021).
Dhibar, S. et al. Dalton Trans. 47 17412–17420. (2018).
Karmakar, K. et al. ACS Appl. Electron. Mater. 5 3340–3349. (2023).
Draper, E. R. & Adams, D. J. Chem 3 390–410. (2017).
Skilling, K. J. et al. Soft Matter 10 237–256. (2014).
Mandegani, F., Zali-Boeini, H., Khayat, Z. & Scopelliti, R. Talanta 219 121237. (2020).
de Loos, M., Feringa, B. L., & van Esch J. H. Eur. J. Org. Chem. 3615–3631. (2005).
Toronyi, A. Á., Giuri, D., Martiniakova, S. & Tomasini, C. Cosmetics 10 38. (2023).
Dhibar, S., Jana, R., Ray, P. P. & Dey, B. J. Mol. Liq 289 111–126. (2019).
Dhibar, S., Dey, A., Ghosh, D., Mandal, A. & Dey, B. J. Mol. Liq 276 184–193. (2019).
Dhibar, S. et al. New. J. Chem. 43 15691–15699. (2019).
Ghosh, D. et al. J. Mol. Liq 280 1–12. (2019).
Jiang, B. et al. Chem. Commun. 53 172–175. (2017).
Karan, C. K. & Bhattacharjee, M. ACS Appl. Mater. Interfaces 8 5526–5535 (2016).
Lin, Q. et al. Soft Matter 10 8427–8432. (2014).
Yao, Z., Wang, Z., Yu, Y., Zeng, C. & Cao, K. Polymer 119 119, 98–106. (2017).
Offiler, C. A., Jones, C. D. & Steed, J. W. Chem. Commun. 53 2024–2027. (2017).
Bunzen, H., Nonappa, E., Kalenius, S., Hietala, E. & Kolehmainen Chem. -Eur J. 19 12978–12981. (2013).
Ganta, S. & Chand, D. K. Dalton Trans. 44 15181–15188. (2015).
Dhibar, S. et al. ChemistrySelect 8 e202204214. (2023).
Dhibar, S. et al. New. J. Chem. 46 17189–17200. (2022).
Dhibar, S. et al. Ind. Eng. Chem. Res. 59 5466–5473. (2020).
Tam, A. Y. Y. & Yam, V. W. W. Chem. Soc. Rev. 42 1540–1567. (2013).
Sarkar, S., Dutta, S., Chakrabarti, S., Bairi, P. & Pal, T. ACS Appl. Mater. Interfaces 6 6308–6316. (2014).
Choudhary, P., Gaur, R., Rambabu, D., Dhir, A. & Gupta, A. Pooja ChemistrySelect 6, 9139–9143. (2021).
Dhibar, S., Ghosh, D., Majumdar, S. & Dey, B. ACS Omega 5 2680–2689. (2020).
Dhibar, S. et al. Dalton Trans. 48 17388–17394. (2019).
Saha, S., Schon, E. M., Cativiela, C., Diaz, D. D. & Banerjee, R. Chem. Eur. J. 19 9562–9568. (2013).
Saha, S. et al. ChemistrySelect. 7 e202203307. (2022).
Pal, B. et al. Mater. Adv. 4 3628–3635. (2023).
Dhibar, S. et al. Chemistry Africa. DOI: (2023). https://doi.org/10.1007/s42250-023-00680-w
Dhibar, S. et al. J. Mol. Liq 370 121021. (2023).
Chen, J., Wang, T. & Liu, M. Inorg. Chem. Front. 3 1559–1565. (2016).
Zhong, J. L. et al. Soft Matter 12 191–199. (2016).
Arnedo-Sánchez, L. et al. Dalton Trans. 46 7309–7316. (2017).
Dhibar, S. et al. J. Mol. Liq 375 121348. (2023).
Karmakar, K. et al. RSC Adv. 13 2561–2569. (2023).
Dhibar, S. et al. J. Mol. Liq 370 121020. (2023).
Zhou, Q. et al. Langmuir 35 15344–15351. (2019).
Alam, N., Majumder, S., Ray, S. J. & Sarma, D. Langmuir 38 10601–10610. (2022).
Malviya, N., Sonkar, C., Ganguly, R. & Mukhopadhyay, S. Inorg. Chem. 58 7324–7334. (2019).
Lepcha, G. et al. Dalton Trans. 51 13435–13443. (2022).
Dey, A. et al. J. Phys. Chem. Solids 160 110300. (2023).
Roy, A. et al. Mater. Adv. 5 3459–3471. (2024).
Roy, A. et al. Sci. Rep. 1 13109. (2024).
Dhibar, S. et al. Langmuir 1 179–192. (2023).
Lv, Z. et al. Adv. Mater., 33 2405145. (2024).
Lv, Z. et al. Matter, 5 1702–1719. (2021).
Arpita Roy, K., Kumari, S., Majumder, Soumya, J. & Ray ACS Appl. Bio Mater. 7 5147–5157. (2024).
Majumder, S., Kumari, K. & Ray, S. J. Appl. Phys. A 129, 357 (2023).
Majumder, S., Kumari, K. & Ray, S. J. Mater. Lett., 302 130339 (2021).
Kumari, K., Thakur, A. D. & Ray, S. J. Mater. Today Commun. 26 102040. (2021).
Kumari, K., Kar, S., Thakur, A. D. & Ray, S. J. Curr. Appl. Phys. 35 16–23. (2022).
Karmakar, K. et al. Sci. Rep. 1 22318. (2023).
Roy, A. & Ray, S. J. J. Mater. Science: Mater. Electron. 9 643. (2024).
Wagner, C. D., Riggs, W. M., Davis, L. E., Moulder, J. F. & Muilenberg, G. E. Handbook of X-Ray Photoelectron Spectroscopy, Volume 38 Vol. 38 (Perkin-Elmer Corp, Eden Prairie, 1979).
Greczynski, G. & Hultman, L. Appl. Surf. Sci. 606 154855. (2022).
Biesinger, M. C., Payne, B. P., Grosvenor, A. P., Lau, L. W. M. & Gerson, A. R. R. St. C. Smart, Appl Surf Sci. 257 2717. (2011).
Cheung, S. K. & Cheung, N. W. Appl. Phys. Lett. 49 85–87. (1986).
Acknowledgements
S.D. is grateful to the UGC, New Delhi, for awarding him Dr. DS Kothari Postdoctoral Fellowship (Award letter number: No.F.4 − 2/2006 (BSR)/CH/19–20/0224). S.B. gratefully acknowledges DST Inspire Faculty Research Grant (Faculty Registration No.: IFA18-CH304; DST/INSPIRE/04/2018/000329).
Author information
Authors and Affiliations
Contributions
Experimental: Material synthesis (SD), Rheological measurements (PR), XRD, FESEM, XPS (PG), TEM, FTIR, Optical and Electrical measurements (AR). Data Analysis: Rheological measurements (PR), XPS (SK), TEM, FTIR, Optical and Electrical measurements (AR). Manuscript writing: AR, SK, SD, PR. Manuscript editing: SJR. Supervision: SB, AB, BS and SJR. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roy, A., Dhibar, S., Kumar, S. et al. A semiconducting supramolecular Co(II)-metallogel based resistive random access memory (RRAM) design with good endurance capabilities. Sci Rep 14, 26848 (2024). https://doi.org/10.1038/s41598-024-74994-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74994-1