Abstract
This study employed Environmental DNA (eDNA) barcoding technology to delve into the influence of the tributaries and mainstem on fish diversity and spatiotemporal distribution in a hotspot fish conservation area in the upper Yangtze River. A total of 123 fish species were detected, belonging to 7 orders, 19 families, and 77 genera. The composition of fish species in tributaries is similar to that in mainstem, with higher fish community diversity in tributaries during the spring and summer. Exploration of fish ecotypes revealed significant differences between mainstem and tributaries. The fish community is mainly influenced by key environmental factors such as water temperature, dissolved oxygen, electrical conductivity, and ammonia nitrogen, with a higher impact of these factors on tributaries than on mainstem. In conclusion, while tributaries and mainstem in the Jiangjin section exhibit similarities in fish community composition, there are notable differences in community structure and diversity. Therefore, the protection of not only mainstem but also tributaries and their associated fish habitats is crucial for promoting the overall health and sustainability.
Similar content being viewed by others
Introduction
The diversity of fish species in aquatic ecosystems is crucial for maintaining overall ecological balance and functionality1,2, especially in some aquatic ecosystems crisscrossed by the mainstem and tributaries3,4. The interactions between the mainstem and tributaries are complex5, with the mainstem habitat systems often having higher water depths and providing effective refuge and overwintering habitats for fish6, while tributary habitat systems (e.g., variable channel topography, abundant bait organisms, etc.) often provide more diverse habitats for fish and contribute to fish migrations and reproductions7,8. Therefore, studying the spatial and temporal dynamics of fish in the networked habitat systems of river mainstem and tributaries can more effectively reveal the changing patterns of fish diversity in such complex ecological networks, and is also crucial for the healthy and sustainable management of aquatic ecosystems9,10.
As a hotspot fish conservation area, the Jiangjin section of the upper Yangtze River, located downstream of the national nature reserve for rare and endemic fish species in the upper Yangtze River (referred to simply as “the Reserve”) and in close proximity to the tail of the Three Gorges Reservoir, occupies a hilly region with both flowing and slow-flowing aquatic habitats. The hydrological conditions in the area are diverse, and the water networks of the mainstem and tributaries are intertwined, harboring rich fishery resources, including 197 species of fish11. Therefore, this region plays a crucial role in maintaining China’s freshwater fish genetic resources and fish diversity12,13,14. However, the region has long faced challenges such as habitat destruction, water pollution and alien fish invasion due to human activities along the river11,15,16, and as a result, fishery resources have declined drastically, resulting in significant changes in the structure of fish communities17,18,19. Therefore, strengthening the water ecology monitoring and scientific research in this region and gaining a deep insight into the current status and spatial and temporal patterns of change of fish communities are necessary to maintain the lasting development of regional fisheries resources and to ensure the healthy and stable development of the water ecosystem.
Environmental DNA (eDNA) technology refers to the molecular biology techniques of obtaining, preserving, extracting, amplifying, sequencing, and classifying DNA from environmental samples to clarify the distribution and presence of target organisms in a study area20,21. The principle behind eDNA is that organisms release DNA into the environment through processes such as feces, skin fragments, urine, etc. This DNA can then be extracted from environmental samples. By analyzing eDNA from environmental samples like water, soil, and air, it is possible to non-invasively detect and monitor the presence and distribution of target organisms without the need for direct observation or capture22,23,24,25. Moreover, when applied at temporal and spatial scales, this method can continuously monitor the diversity dynamics, community composition, and distribution of various riverine species26. Therefore, eDNA technology finds wide applications in ecology, environmental monitoring, and the study of biological invasions27,28,29. Although traditional fishing methods (netting, trapping, electrofishing, etc.) are the main means of fishery resource investigation, providing direct access to the morphological characteristics and abundance of target organisms, their strong invasiveness can cause damage to species. Identification also requires extensive specialized taxonomic knowledge and consumes a significant amount of time and effort21,30. Hence, the efficient, sensitive, and non-invasive advantages of eDNA technology make it a promising tool for biodiversity investigations.
In recent years, a decade-long fishing ban in the Yangtze River basin has provided a crucial opportunity for the protection and restoration of fishery resources. Concurrently, the advancement of eDNA technology has presented a powerful tool for the study of fish diversity. Therefore, here, we carried out the present study with the aim of exploring the spatial and temporal dynamics of fish diversity in the water network system of the mainstem and tributaries of the Jiangjin section of the upper Yangtze River by eDNA metabarcoding to provide a scientific basis for better insights into and protection of fishery resources in this area.
Materials and methods
Study area
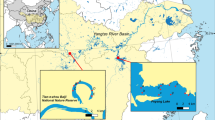
Combined with the characteristics of water network system, the pattern of hydrological change and the fish life cycle in the Jiangjin section of the Yangtze River, a total of 11 sampling sections were set up in the region in December 2021 (winter), April 2022 (spring) and August 2022 (summer) (Fig. 1A), of which six sampling sections were set up in the mainstem, namely, S1, S3, S4, S6, S7, and S10; and 5 in the tributaries, namely, S2 (the Qijiang River), S5 (the Binan River), S8 (the Linjiang River), S9 (the Daluxi River), and S11 (the Tang River).
The ___location of sampling sections in the Jiangjin section of the upper Yangtze River (A) and family level composition map (B). (A: Sampling sections located in tributaries: S2, S5, S8, S9, S11; Sampling sections located in mainstem: S1, S3, S4, S6, S7, S10. B: “N” represents the number of species and “P” represents the percentage of a family in the total number of species).
eDNA sampling and processing
A water sampler was used in each sampling section to collect 9 L of water samples. The water samples collected from the field were immediately placed into a refrigerated environment. Prior to each sampling, all equipment was sterilized with a 10% bleach solution and rinsed with carbonated water, while disposable equipment was used for each sampling to minimize the risk of contamination31. In the laboratory, a composite sample was prepared by mixing water samples from the same sampling section. The composite sample was then divided into three technical replicates, each containing only 2 L of mixed water. The remaining 3 L composite sample was discarded32,33,34. The three technical replicate samples collected were immediately refrigerated and passed through a 0.45 μm mixed cellulose ester filter membrane (Whatman, UK) utilizing a vacuum pump within 24 h. When significant quantities of sediment were found in the water samples, they were prefiltered using sterile medical gauze during collection34. The filtration equipment was disinfected before each sample filtration to ensure no cross-contamination occurred. During the samples processing, a negative control using 2 L of distilled water was also performed to check for any contamination by exogenous DNA. Subsequently, the filter membranes were cryopreserved at -80°C for subsequent DNA extraction.
Total DNA on the filter membrane was extracted using the PowerWater DNA Isolation Kit (Qiagen). The extracted eDNA samples were subjected to 1% agarose gel electrophoresis to assess their quality35. Each sample was independently extracted, with an untreated filter membrane serving as a negative control. The extracted DNA samples were preserved at -20°C for subsequent PCR amplification.
The mitochondrial 12 S rRNA gene fragment was amplified by employing the Tele02 primers36. The PCR was performed utilizing TransStart Fastpfu DNA Polymerase (TransGen AP221-02) within a 20 µL reaction system. The reaction mixture consisted of 4 µL of 5×FastPfu buffer, 2 µL of dNTPs (2.5 mM), 0.4 µL of Fastpfu polymerase, 2 µL of template DNA (10 ng), and 0.8 µL each of the forward and reverse primers (5 µM), which were concentrated to 20 µL with the addition of ddH2O. The PCR amplification program consisted of an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 45 s. A final extension step at 72°C for 10 min was performed. Three independent PCR amplifications were performed for each sample. Subsequently, the PCR products from the same samples were pooled and subjected to 2% agarose gel electrophoresis. In this study, detectable PCR products were successfully amplified from all 36 samples, while no products were observed in the negative controls. Gel extraction and recovery was performed using the AxyPrepDNA Gel Recovery Kit (AXYGEN), followed by Tris HCl elution. Finally, the purified PCR products were sequenced utilizing the Illumina NovaSeq 6000 high-throughput sequencing platform.
Bioinformatic analyses and taxonomic assignment
Low-quality bases at the 3’ end of the reads with Phred scores lower than 20 were filtered out during the sequencing data processing flow using Trimmomatic v.0.3637. A sliding window of 10 bp was used to calculate the average quality within the window. If the average quality was below a score of 20, bases from the beginning of the window were trimmed and reads shorter than 100 bp were discarded. Subsequently, paired reads were merged into a single sequence utilizing the FLASH (v.1.2.11) tool, which relies on the overlap between paired-end (PE) reads, with the minimum overlap length set to 10 bp38. During the merging process, the maximum allowable mismatch rate was set to 0.2 to eliminate substandard sequence assembly. Barcodes and primer information on both ends of the sequences were then utilized to distinguish high-quality sequences for each sample and to adjust sequence orientation. Afterwards, chimeras were removed using the Usearch software (version 10, http://drive5.com/uparse/) in conjunction with reference sequences from the GOLD database and denovo sequences. Finally, high-quality sequences with a similarity threshold of ≥ 97% were subjected to OTU cluster analysis using Usearch software. The MitoFish database (http://mitofish.aori.u-tokyo.ac.jp/) and the NCBI database (https://www.ncbi.nlm.nih.gov/) were compared and categorized for annotation using the Blastn tool and the uclust algorithm Sequences with an OTU count below 10 were automatically ignored and OTUs with identity values ≥ 99% and E-values ≤ 10−5 were selected. In addition, OTUs belonging to the same taxonomic unit were merged and non-fish sequences were removed.
Data analysis
The reads from each sample were randomly subsampled using the QIIME v.1.9.0 software and data from all eDNA sample were normalized according to the minimum sequencing depth observed in all samples39. This normalization ensured that all samples had the same sequencing depth, reducing bias due to differences in sequencing depth. OTU cluster analysis and taxonomic information were followed by a series of in-depth statistical and visual analyses of community structure. For fish species classification, the Fishbase database (https://www.fishbase.de/) and the book “Species and Distribution of Inland Fishes in China”40 were referenced. Information on the feeding habits, spawning types, habitat depths and hydrological environments of fish species were mainly referred to the book “The Fishes of the Yangtze River”41 and “The Fishes of Sichuan”42, as well as references to relevant journal articles43,44,45. The spatial distribution of fish ecotypes in the Jiangjin section was visualized using ArcGIS 10.7.
Similarity and diversity indices
The Sorensen similarity index was used to analyze the similarity between traditionally catches fish communities over the past decade and those identified by eDNA in this study, as well as the similarity in species composition between the mainstem and tributaries. The Sorensen index46 was calculated using the formula:
Sorensen = 2a/(b + c).
Here, “a” represents the number of shared fish species between the two groups, and “b” and “c” are the respective total numbers of fish species in each group. The calculated values range from 0 to 1, indicating extremely low similarity and extremely high similarity between the two groups, respectively.
In this study, the Shannon index47, Simpson48 index, and Pielou index49 were used to analyze the α-diversity of the fish community structure. The formulas for these indices are as follows:
Shannon-Wiener index: H’ = ∑PilogPi, Pi = ni/N.
Simpson index: D = [∑ni(ni-1)] /N(N-1).
Pielou index: J = H’/Hmax
Here, “N” represents the total number of detected fish sequences, “ni”and represents the number of sequences for the i-th fish species. “Hmax” is the maximum Shannon index achievable under equal species richness (i.e., all species in the community have the same richness).
Additionally, the four ecotypes of fish were statistically categorized: water flow velocity, habitat layer, diet, and spawning types. Pie charts were created based on the number of fish species and their relative sequence abundance. At the same time, the one-way analysis of variance (ANOVA) was conducted on each ecological type to test for significant differences. Based on the Bray-Curtis distance, Analysis of Similarities (ANOSIM) was conducted to determine if the groupings are meaningful. Then, Principal Coordinate Analysis (PCoA) used the Bray-Curtis distance matrix to analyze the similarity among fish communities in various seasons. Permutational Multivariate Analysis of Variance (PerMANOVA) was performed using R version 4.0.3 to assess multivariate variance. Conduct the Detrended Correspondence Analysis (DCA) using the relative sequence abundance of fish as the variable. If the length of the axis is between 2 and 4, proceed with the Redundancy Analysis (RDA) to explore the relationship between environmental factors and fish communities. Data visualization analysis was carried out on the BioMicroClass platform. Variance analysis was conducted using SPSS statistical software.
Result
Comparison with traditional survey
Over the past decade, 103 fish species belonging to 7 orders, 19 families, and 69 genera have been collected in the Jiangjin section by traditional survey methods (e.g., trawling and netting)12,13,50 (Table S1), including 21 endemic fish species in the upper Yangtze River, seven national protected fish, and seven alien fish (Cirrhinus mrigala, Oreochromis mossambicus, Gambusia affinis, Hypseleotris punctatus, Micropterus salmoides, Neosalanx taihuensis, and Pseudorasbora parva). Comparing the traditional survey data with the results of the eDNA survey conducted in this study (123 species), 69 species were co-identified, 54 species were uniquely identified by eDNA, and 34 species were uniquely identified by the traditional survey method. The coverage of the eDNA survey method was 66.99% compared to the traditional method, indicating its good sensitivity and detection ability in surveying fish diversity. In addition, the Sorensen similarity index of 0.6106 (Table 1) also indicated that the fish communities identified by the two methods were moderately similar.
Fish species diversity and spatial-temporal variations
Overall species diversity and spatial-temporal variations.
123 fish species from 7 orders, 19 families, and 77 genera were detected (Fig. 1B), of which 99, 88, and 84 fish species were identified in winter, summer, and spring, respectively (Table 2; Table S2). Of the 123 fish species, 27 were endemic fish species in the upper Yangtze River, and seven were national protected fish species, including Rhinogobio ventralis, Procypris rabaudi, Schizothorax davidi, Onychostoma macrolepis, Leptobotia elongata, L. rubrilaris, and Liobagrus kingi. In addition, there were 12 alien fish species originating from abroad or other regions of China, namely G. affinis, I. punctatus, Megalobrama amblycephala, M. terminalis, M. salmoides, N. taihuensis, O. niloticus, Coptodon zillii, P. parva, Tinca tinca, Danio rerio, and Clarias gariepinus (Table S3). The histogram of relative sequence abundance of the top 30 genera showed that Cyprinus, Ctenopharyngodon, Carassius, Pelteobagrus, and Hemiculter had higher sequence abundance (Fig. 2). The histogram of relative sequence abundance at the species level revealed that C. carpio, C. idella, C. auratus, P. fulvidraco, and H. tchangi were at the top of the list (Fig. 3). In particular, alien fish species accounted for 5.34% of all fish species sequences, with O. niloticus accounting for 1.99% and P. parva accounting for 1.02%.
Comparison of fish species composition and similarities between the mainstem and tributaries.
A total of 117 fish species belonging to 7 orders, 19 families, and 76 genera were detected during the three seasons in the mainstem, of which 94, 76, and 75 fish species were identified in winter, summer, and spring, respectively (Table 2). Among these 117 species, 26 endemic fish species in the upper Yangtze River (with S. prenanti not detected), seven national protected fish, and 11 alien fish (T. tinca not detected) were found (Table S3). A total of 118 fish species belonging to 7 orders, 18 families, and 74 genera were detected in the tributaries, of which 94, 79, and 79 fish species were identified in winter, summer, and spring, respectively (Table 2). Among these 118 species, 27 endemic fish species in the upper Yangtze River, six national protected fish (O. macrolepis not detected), and 12 alien fish were found (Table S3). The shared species identified both the mainstem and tributaries were 112, of which five fish species were detected only in the mainstem and six species only in the tributaries. The Sorensen similarity index between the entire mainstem and the tributaries at the species level was 0.9532, indicating extremely high similarity (Table 1). The Sorensen similarity indices between the tributaries (S2, S5, S8, S9, S11) and the entire mainstem were 0.8796, 0.9014, 0.9321, 0.8879, and 0.8654, respectively, which also indicated that there was a very high degree of similarity in the species composition of the fish between the tributaries and the entire mainstem (Table 1).
Fish community structure and spatial-temporal changes
Ecotypes of fish in the mainstem and tributaries.
Based on the fish ecotypes composition and classification status (Table S4), the spatial distribution was mapped using ArcGIS 10.7 (Fig. 4). According to the species-level results of fish ecotypes, the fish community in the Jiangjin section was dominated by species preferring quiet and slow-flowing water (N), omnivorous (O), inhabiting the bottom layer (B), and spawning demersal eggs (D) (Fig. 4). The percentage of fish species preferring quiet and slow-flowing water (N) was 59.06%, compared to those preferring flowing water (R) was 40.94% (Fig. 4A). The percentage of omnivorous (O), herbivorous (H), and carnivorous (C) fish species was 66.27%, 4.07%, and 29.67%, respectively (Fig. 4B). The percentage of fish species inhabiting the upper layer (U), the bottom layer (B), and the lower middle layer (L) was 21.16%, 28.65%, and 50.18%, respectively (Fig. 4C). The percentage of fish species with floating eggs (P), drifting eggs (F), sticky eggs (V), demersal eggs spawning (D), and those favoring bivalve spawning (S) was 2.71%, 31.37%, 16.99%, 42.48%, and 6.44%, respectively (Fig. 4D).
Ecotype spatial distribution map at the species level. (A: water flow velocity; B: habitat layer; C: diet; D: spawning types; “100 species” indicates that the size of the pie chart corresponds to the presence of 100 species of fish at that sampling section. The magnitude of the pie chart is proportional to the number of fish species at the sampling site.).
The results of one-way analysis of variance for various ecotypes of fish communities showed that the number of fish species with spawning demersal eggs (D), floating eggs (P) and drifting eggs (F) ecotypes between the mainstem and tributaries did not differ significantly (P > 0.05) (Table S5). Compared to the mainstem, tributaries had a significantly higher proportion of fish preferring bivalve spawning (S) (P < 0.01), while the mainstem had a significantly higher proportion of fish preferring sticky eggs (V) (P < 0.05). There was no significant difference in the number of omnivorous (O), herbivorous (H), and carnivorous (C) fish species between the mainstem and tributaries (P > 0.05). The number of fish species inhabiting the lower middle layer (L) was not significantly different between the mainstem and tributaries (P > 0.05), whereas the number of fish species inhabiting the bottom layer (B) was significantly higher in the mainstem than in tributaries (P < 0.01), and the number of the upper layer (U) species was significantly lower in the mainstem than in tributaries (P < 0.05). There was no significant difference between the number of fish species preferring quiet and slow-flowing water (N) in the mainstem and tributaries (P > 0.05), while the number of fish species preferring flowing water (R) was significantly higher in the mainstem than in tributaries (P < 0.05).
Based on the sequence abundance level, the fish ecotypes analysis showed that the fish community was dominated by species that preferred quiet and slow-flowing water (N), omnivorous (O) and inhabited the bottom layer (B), and spawning demersal eggs (D) (Fig. 5). At different flow rates, 65.32% of fish species preferred quiet and slow-flowing water (N)and 34.68% preferred flowing water (R) (Fig. 5A). In terms of feeding habits, 67.17% were omnivorous (O)fish species, 12.67% were herbivorous (H) fish species and 20.16% were carnivorous (C) fish species (Fig. 5B). Among the different water layers, the upper layer (U)fish species accounted for 15.08%, the lower middle layer (L) fish species accounted for 30.64% and the bottom layer (B) fish species accounted for 54.28% (Fig. 5C). Among the different spawning types, 0.17% were floating eggs (P) fish species, 27.81% were drifting eggs (F) ecotype, 31.42% were demersal eggs spawning (D) ecotype, 37.87% were sticky eggs (V) ecotype and 2.72% were bivalve spawning (S) ecotype (Fig. 5D).
Ecotype spatial distribution map at the relative sequence abundance level. (A: water flow velocity; B: habitat layer; C: diet; D: spawning types; “120,000” represents that the size of the pie chart signifies a total relative sequence abundance of fish of 120,000 at that sampling section. The size of the pie chart corresponds to the level of relative sequence abundance of fish species at the sampling site.).
The results of one-way analysis of variance on the various ecotypes of fish communities in the mainstem and tributaries indicated that there was no significant difference (P > 0.05) in the sequence abundance of drifting eggs (F) and floating eggs (P) ecotype fish (Table S4). However, the sequence abundance of bivalve spawning (S), demersal eggs spawning (D), and sticky eggs (V) ecotype fish species in the tributaries was significantly higher (P < 0.01) than in the mainstem (Table S4). Sequence abundance of fish species preferring flowing water (R) in the mainstem and tributaries was not significantly different the between (P > 0.05), but the sequence abundance of fish species preferring quiet and slow-flowing water (N) in the tributaries was significantly higher than in the mainstem (P < 0.01). Sequence abundance of herbivorous (H) and carnivorous (C) fish species in the mainstem and tributaries were not significantly different (P > 0.05), but the sequence abundance of omnivorous (O) fish species in the tributaries was significantly higher than that in the mainstem (P < 0.05). Sequence abundance of the upper layer (U) and the lower middle layer (L) fish species were not significantly different between the mainstem and tributaries (P > 0.05), but the sequence abundance of the bottom layer (B) fish species in the tributaries was significantly higher than that in the mainstem (P < 0.01).
Diversity indices of fish in the mainstem and tributaries.
One-way analysis of the mean values of fish diversity indices showed that there was no significant spatial variation in fish diversity indices, but there were significant seasonal variations (Table 3). The Shannon index ranged from 1.9662 to 3.2255, with the lowest in spring, which was significantly lower than in winter and summer (P < 0.05). The Simpson index ranged from 0.7589 to 0.9457 with no significant temporal and spatial differences (P > 0.05). The Pielou index ranged from 0.4783 to 0.7616, with significantly higher values in summer than in winter and spring (P < 0.05). Comparison of fish diversity indices in different seasons showed that in terms of the Shannon index, there was no significant difference between the mainstem and tributaries in spring and winter (P > 0.05), whereas the values in the tributaries were significantly higher than the mainstem in summer (P < 0.01). In terms of the Simpson index, there was no significant difference between the mainstem and tributaries in winter (P > 0.05), but the index values of tributaries in spring were significantly lower than those of mainstem (P < 0.05), and those of tributaries in summer were significantly lower than those of the mainstem (P < 0.01). There was no significant difference between the mainstem and tributaries in all seasons with respect to the Pielou index (P > 0.05).
Similarity of fish communities between the mainstem and tributaries.
The results of ANOSIM similarity analysis based on the Bray-Curtis matrix (R = 0.851, P = 0.001) indicated that the intergroup differences were greater than intragroup differences among the three seasons. The differences in fish community structure among the three seasons were highly significant (P < 0.01). A PCoA analysis based on the fish species sequence data (Table S3) were implemented to examine the fish community structure in the mainstem and tributaries among different seasons (Fig. 6). The cumulative explanatory rate for Axis 1 and Axis 2 was 53% (PCoA1 = 36%, PCoA2 = 17%). The results of PerMANOVA showed an R² value of 0.5928 and a P-value of 0.001, which indicated an extremely significant difference among the groups in spring, summer, and winter (P < 0.01). Furthermore, pairwise comparisons among the three seasons also revealed highly significant differences (spring vs. summer: R²=0.6089, P < 0.01; spring vs. winter: R²=0.2761, P < 0.01; winter vs. summer: R²=0.4089, P < 0.01). Comparisons between the mainstem and tributaries in different seasons showed significant differences between the mainstem and tributaries in spring and winter (spring mainstem vs. tributaries: R²=0.1938, P < 0.05; winter mainstem vs. tributaries: R²=0.2031, P < 0.01), while there is no significant difference in summer (R²=0.0832, P = 0.698).
Fish diversity and correlation with environmental factors
Differences in environmental factors between the mainstem and tributaries.
The results of the water quality tests for the Jiangjin section are presented in Table S6. Single-factor analysis of variance was performed for various environmental factors, and the results showed that there were significant differences in pH, water temperature (WT), suspended solids (SD), and dissolved oxygen (DO) in different seasons. Specifically, pH was significantly higher in summer than in winter (P < 0.01), while pH was significantly higher in winter than in spring (P < 0.05). Water temperature was significantly higher in summer than in spring (P < 0.01), and in spring than in winter (P < 0.01). Additionally, SD were significantly higher in summer than in spring (P < 0.05), and DO was significantly higher in winter and spring than in summer (P < 0.05). Electrical conductivity (EC), total phosphorus (TP), and ammonia nitrogen (NH3-N) differed significantly among the sampling sections. EC was significantly higher in S9 than in the other sections (P < 0.01), and the TP at S8 is significantly higher than in other sections (P < 0.05). NH3-N was significantly higher in both S8 and S9 than in the other sections (P < 0.05). Furthermore, SD, EC, and NH3-N in the tributaries were significantly higher than those in the mainstem (P < 0.05).
Analysis of the correlation between fish diversity and environmental factors in the mainstem and tributaries.
DCA analysis using relative sequence abundance of fish as response variable showed that the lengths of the first ordinal axis was 3.5184, 3.2765, and 2.6898 in winter, spring and summer, respectively, which fell in the range of 2–4. Subsequently, the relationship between environmental factors and fish communities was explored using the RDA method. The RDA analyses of fish communities in the three seasons were illustrated in Fig. 7. In winter, the cumulative interpretation of species information Axis I and Axis II reached 72.95% (Fig. 7A). WT (R²=0.707, P < 0.05), DO (R²=0.520, P < 0.05), EC (R²=0.968, P < 0.01), and NH3-N (R²=0.913, P < 0.05) were considered to be the most significant factors affecting the fish community structure during winter. In spring, the cumulative explanation of species information for both axes reached 78.83% (Fig. 7B). DO (R²=0.766, P < 0.05) and EC (R²=0.933, P < 0.01) were considered as the main environmental factors affecting the fish community structure in spring. In summer, the cumulative explanation of species information for both axes reached 59.45% (Fig. 7C). In winter, the S9 was mainly affected by WT, EC and NH3-N, while the S11 was most affected by DO. In spring, the fish community in S9 was mainly influenced by WT and EC. As both S9 and S11 are tributaries, they exhibit a stronger correlation with major environmental factors, indicating a significant association between tributaries and environmental factors. In contrast, the mainstem is less influenced by these key environmental factors.
Discussion
Fish species composition and historical comparison
A total of 123 fish species belonging to 7 orders, 19 families, and 77 genera were detected in the Jiangjin section across the three seasons. Among them, species in the family Cyprinidae dominated in terms of species diversity and sequence abundance, which is consistent with the results of historical surveys in this region29,51. Compared with the catch data of the past decade12,13,50, there has been an increase of 20 fish species, with 54 species not recorded in the past decade. This suggests that the implementation of local ecological conservation policies may herald an increased in fish diversity in this region, implying the continued enrichment and restoration of fish ecosystems within this basin. The limitations of traditional survey methods may have led to the oversight of elusive or rare fish species in the area21,30. eDNA application have detected the presence of such species with its high sensitivity, allowing for differences in fish composition between the two time periods due to different technological approaches. However, it is crucial to recognize the limitations of eDNA technology, such as the potential for false positives, although many measures have been taken to minimize the occurrence of false positives52. Additionally, it is important to recognize the advantages of traditional survey methods. This is because they can detect 34 species of fish that are not present in the eDNA results. This may be due to the incomplete database referenced in this study, thus comparing to other species with similar sequences53. It is also one of the limitations of eDNA technology.
Comparison of the fish species composition of the mainstem and tributaries revealed that at the species level, the fish species composition of the mainstem was highly similar to that of the tributaries, and the tributaries had even slightly more fish species. The Sorensen similarity index further confirms the high similarity in fish community structure between the mainstem and tributaries. This suggests that in addition to the mainstem, tributaries also play a crucial role in the survival and reproduction of fish in this region. Moreover, existing literatures have demonstrated that traditional surveys of the Nanguang River, a tributary of the Yangtze River, and the Zhougong River, a tributary of the Qingyi River, have found that tributaries play an essential role in fish communities54,55. In addition, the number of national protected fish species detected in the tributaries was the same as that in the mainstem, and the number of alien fish species in the upper Yangtze River was even higher, including S. prenanti, which was not found in the mainstem. This result highlights the important conservation value of the tributaries in the region, and emphasizes the unique roles of the tributaries in maintaining the local fish diversity and the ecological balance.
Alien fish species
Twelve alien fish species were found in this region, representing 5.34% of the total sequence of 123 identified fish species, with the highest proportion of O. niloticus. Oreochromis niloticus is an adaptable and economically valuable omnivorous fish that has been intentionally introduced into aquaculture due to its wide market demand56. However, the presence of O. niloticus in all sampling sections suggests that potential sources most likely include escapes from aquaculture and unintentional release. These unnatural routes of introduction may increase the chances of their expansion in the ecosystem, which may lead to the ecological displacement of native fish species19. Furthermore, certain alien fish species, such as I. punctatus, M. salmoides, and C. gariepinus, are known to be aggressive predators. These species tend to prey on some smaller-sized native fish, which may cause predation pressure and lead to changes in the trophic structure of local fish communities57,58,59. As a result, alien fish species may pose potential threats to local native fish communities and ecosystems, including competitive pressure, predation pressure, even disease spread60. To cope with these impacts, it is recommended that relevant authorities should strengthen monitoring and control measures for alien fish species. Simultaneously, efforts should be made to promote the ecological restoration and population growth of native fish species to maintain the diversity and ecological balance of fish communities61,62.
Diversity of fish species in the mainstem and tributaries
Diversity indices analysis showed that there was no significant difference in fish diversity among all the sampling sections, but seasonal factors had a significant effect on fish diversity. The Shannon index was significantly lower in spring than in winter and summer (P < 0.05), indicating lower species diversity in spring. In spring, C. carpio and C. idella had the highest sequence abundance, which coincides with their breeding season when they show more activity and may release more DNA into the water63. In summer, the Pielou index was significantly higher than in both winter and summer, suggesting that species abundance was more evenly distributed across the fish community during this season. Additionally, a comparison of diversity indices between the main and tributaries revealed that the Shannon index in tributaries was significantly higher than in the mainstem during summer., the Simpson index was significantly lower in the tributaries than in the mainstem during the spring and summer, suggesting that tributaries fish communities have higher species diversity and lower dominance. However, the species diversity indices calculated in this research were based on the number of sequences rather than the number of individuals, and it cannot fully reflect the abundance of a particular species. This is also one of the limitations of eDNA technology, where the number of reads does not represent the actual number of fish64.
Tributaries usually exhibit more microenvironmental variations, such as water flow conditions, substrate type, vegetation cover, and water nutrient status, which provide favorable conditions for fish community diversity65. Of the five tributaries, the Tang River and the Qijiang River are wide and flow slightly lower than the mainstem, with habitat characteristics similar to the mainstem. The Binan River has the highest degree of sinuosity, with mountain streams in its upstream area and rocky deep pools and bends in the downstream area, and has spawning ground, feeding ground and a diverse of habitats suitable for a wide range of fish species66. In contrast, there were no significant differences in fish species diversity between the mainstem and tributaries in winter, which likely due to the mainstem having more deep backwaters where certain fish migrate to warmer waters to overwinter67. An overall comparison of tributaries and mainstem shows that the tributaries play a crucial role in the overall biodiversity and ecological balance of aquatic ecosystems in this region. Therefore, protecting fish species and their habitats in tributaries is equally significant and contributes positively to the overall health of aquatic ecosystems.
Ecotypes of fish in the mainstem and tributaries
At the species level, the fish community in the Jiangjin section is characterized by a preference for quiet and slow-flowing waters, omnivorous feeding habits, the bottom layers behavior, and the production of demersal eggs. At the sequence level, the fish community is dominated by species that prefer quiet and slow-flowing waters, omnivorous feeding habits, the bottom layers behavior, and the production of sticky eggs. Sequence abundance of fish preferring quiet and slow-flowing waters in tributaries was significantly higher than that of fish preferring flowing waters, and the sequence abundance of fish preferring quiet and slow-flowing waters in the mainstem sections gradually increased from the upstream to downstream. Located the downstream of the reserve, the flow velocity gradually decreases in the Jiangjin section, especially in the lower reach sections (S1 and S2) adjacent to the tail of the Three Gorges Reservoir. The water conditions have transitioned from flowing to quiet and slow-flowing aquatic habitats. This hydrological transformation has, to some extent, provided a more suitable habitat for quiet and slow-flowing fish species like C. carpio68,69. Additionally, the establishment of the cascade hydropower stations on the upper reaches of the Jinsha River has reduced water connectivity, which may impede the migration of some flowing water fish species in the upper reaches, such as Jinshaia sinensis and Lepturichthys fimbriata16. In this complex and dynamic environment, omnivorous fish species such as C. carpio have showed strong adaptability. They can flexibly select food resources from the diverse and abundant environmental sources, occupying a larger ecological niche space than other feeding types, enabling them to survive and reproduce under different seasonal and habitat conditions70,71. For different water layers, the benthic zone provides abundant benthic organisms as food, while the crevices and vegetation on the bottom provide shelter from predators and fulfill the survival, breeding and reproduction needs of the bottom-dwelling fish species72. Furthermore, the fish community exhibited different proportions of spawning types at two levels, mainly influenced by fish species with relatively high sequence abundance, such as the genera Cyprinus, Carassius, Pelteobagrus, and Hemiculter, which tend to produce sticky eggs.
Comparing the mainstem and tributaries at the species level, the species richness of bivalve spawning fish and the upper layer habitat fish in the tributaries was significantly higher than in the mainstem, whereas the species richness of sticky eggs spawning fish, the bottom layer fish, and flowing water fish in the mainstem was significantly higher than in the tributaries. The slower water flow and fertile substrate of the tributaries provide suitable conditions for the survival of bivalve mollusks and create a conducive environment for the reproduction of bivalve spawning fish. In addition, the mainstem in this region has flowing water, wide water surface, deep water layers, and predominantly gravel, sand, and mud substrate, which provide suitable habitats for flowing water fish, the bottom layer fish, and sticky eggs spawning fish11,72.
Comparing the mainstem and tributaries at the sequence level, the sequence abundance of each ecotype was higher in the tributaries than in the mainstem. This may be due to the lower water flow and narrower river channels in the tributaries, resulting in higher fish densities that accumulate more eDNA in a given water area6. In contrast, the higher water flow in the mainstem can have a diluting effect on the released eDNA, and the broad river surface with less concentrated fish density may result in less detectable species-specific eDNA73,74. Ultimately, this could lead to a more pronounced difference in the sequence abundance of fish for various ecotypes between the tributaries and the mainstem. In summary, the differences in sequence abundance between the mainstem and tributaries may be due to environmental differences (the water flow and velocity in the tributaries are both less than those in the mainstem), leading to different concentrations of eDNA.
Community similarity of fish in the mainstem and tributaries
The results of PCoA analysis showed that there were significant differences in the fish community structure among different seasons in this region, with highly significant differences in pairwise comparisons between spring, summer, and winter. This suggests notable changes in fish community structure across different seasons. Furthermore, when comparing the fish community structures of the mainstem and tributaries in each season, significant differences were observed between spring and winter, while no significant differences were found between the mainstem and tributaries in summer. Significantly warmer water temperatures and greater abundance of food resources in the summer may lead to a more equitable utilization of resources (e.g., food and habitat) by different species and promote a more balanced relative proportion among species. However, the overwintering behavior of fish in winter and the reproductive activities in spring may result in aggregations migrating to more suitable water areas75. These differences may be influenced by environmental variables. There are significant differences in water temperature and dissolved oxygen levels between seasons, leading to changes in the fish community. In summary, seasonal climate changes can alter factors such as water temperature and nutrient levels, which can affect the ecological characteristics of fish, including their survival, foraging, and reproductive behaviors76,77,78.
Relationship between fish community and environmental factors
The analysis of the relationship between environmental factors and fish communities showed that the fish communities in this region were mainly affected by environmental factors such as WT, DO, EC, and NH3-N. WT and DO are directly associated with the survival and reproduction of fish, which serve as crucial factors that limit the distribution of many fish species79,80. The fish community in S9 (tributary) was significantly influenced by EC and NH3-N in winter and spring, and by WT in winter. NH3-N is significantly higher in S9 than other sections, except in S8, where the EC is also significantly higher in S9 than other sections. The narrow channel and the presence of industrial zones such as paper mills and sand fields near the banks can lead to the discharge of industrial wastewater and pollutants into the river. This could include chemicals and heavy metals that lead to the dissolution of large amounts of ions and dissolved nitrogen substances (NH4+ & NH3) in water. Additionally, the limited water flow hinders the dilution of the above soluble substances, leaving them to remain in the water for an extended period, resulting in water quality degradation that ultimately affects the survival and reproduction of fish81. Moreover, the water temperature at S9 in winter was 19°C, which was approximately 4°C higher than the average water temperature at other sections during this season. The sequence abundance of O. niloticus in the fish composition was as high as 45.24%, indicating that this section may serve as a wintering ground for O. niloticus. Furthermore, there was a significant correlation between the fish community and DO at S11 (tributary) in winter. The S11 (tributary) has an open river surface, shallow water depth, and swift water flow, which may enhance the oxygen circulation between water and air. The abundant DO levels have a positive impact on fish82. However, for the mainstem, correlations with key environmental factors were relatively weak across seasons. This may be due to the dispersion of environmental pressures across a larger water body, resulting in less pronounced effects of localized environmental factor variations on the entire water system at a single point. In conclusion, the synergistic effects of these factors help explain the correlation between the fish community and environmental factors. Further research could delve into the mechanisms underlying these associations and their impact on aquatic ecosystems.
Conclusion
In summary, within the Jiangjin section, both the mainstem and tributaries collectively revealed a diverse fish community comprising 123 species across 7 orders, 19 families, and 77 genera, with Cyprinidae being the most abundant. Despite the richness in fish community structure, the presence of 12 invasive species signals a potential risk of ecosystem invasion. Comparative analysis between mainstem and tributaries indicated similarities in the composition of fish species, with higher fish community diversity in tributaries during the spring and summer. At the species level, the dominant ecological traits of the fish community in the region include a preference for quiet and slow-flowing water, omnivorous feeding habits, inhabiting the bottom layer, and demersal eggs spawning. Significant differences between mainstem and tributaries exist in terms of flow velocity preferences, preferred habitat layers, and spawning types. At the sequence level, the ecological traits of the fish community are primarily characterized by a preference for quiet and slow-flowing water, omnivorous feeding habits, inhabiting the bottom layer, and spawning sticky eggs, with tributaries exhibiting significantly higher ecological trait values than mainstem. PCoA analysis revealed spatiotemporal differences in fish communities, with significant distinctions between mainstem and tributaries during the spring and winter seasons. The fish community is mainly influenced by key environmental factors including water temperature (WT), dissolved oxygen (DO), electrical conductivity (EC), and ammonia nitrogen (NH3-N), with these factors having a greater impact on tributaries than on mainstem. In conclusion, similar to mainstem, the protection of tributaries and their fish habitats is crucial. This contributes to promoting the overall health of the aquatic ecosystem and underscores the need to take necessary measures to prevent potential threats from invasive fish species to the local ecosystem.
Data availability
The reference sequences are available on NCBI (https://dataview.ncbi.nlm.nih.gov) under the following accession numbers SRR25549005- SRR25549103.
References
Magurran, A. E., Khachonpisitsak, S. & Ahmad, A. B. Biological diversity of fish communities: pattern and process. J. Fish Biol. 79, 1393–1412 (2011).
Margules, C. R. & Pressey, R. L. Systematic conservation planning. Nature. 405, 243–253 (2000).
Rice, S. P. & Ferguson, R. I. Tributary control of physical heterogeneity and biological diversity at river confluences. Can. J. Fish. Aquat. Sci. 63, 2553–2566 (2006).
Kiffney, P. M., Greene, C. M., Hall, J. E. & Davies, J. R. Tributary streams create spatial discontinuities in habitat, biological productivity, and diversity in mainstem rivers. Can. J. Fish. Aquat. Sci. 63, 2518–2530 (2006).
Argent, D. G. & Kimmel, W. G. Fish assemblage connectivity in the Monongahela River Basin. Northeastern Naturalist. 16, 607–620. https://doi.org/10.1656/045.016.n410 (2009).
Wang, K. Spatial and Temporal Distribution of fish in the Three Gorges Reservoir area and its Relationship with Related Factors (China Institute of Water Resources and Hydropower Research., 2014).
Starcevich, S. J., Howell, P. J., Jacobs, S. E. & Sankovich, P. M. Seasonal movement and distribution of fluvial adult bull trout in selected watersheds in the Mid-columbia River and Snake River basins. PLoS ONE. 7, e37257 (2012).
He, T., Li, X. L., Zheng, Y. H. & Liu, J. H. Comparative analysis of fish distribution between mainstem and tributaries in Chongqing district of the Yangtze River. Freshw. Fisheries. 46, 47–51. https://doi.org/10.13721/j.cnki.dsyy.2016.03.008 (2016).
Bunn, S. E. & Arthington, A. H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manage. 30, 492–507 (2002).
Fausch, K. D., Torgersen, C. E., Baxter, C. V. & Li, H. W. Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. BioScience. 52, 483–498 (2002).
Wei, Q. W. Scientific Investigation Report on National Nature Reserve for the Rare and Endemic Fishes in the Upper Reaches of the Yangtze River (Science, 2012).
He, T., Wei, Y. D., Lu, Q., Liao, Y. & Liu, J. H. Investigation on fishery resources in Chongqing section of rare and endemic fish Nature Reserve in the upper reaches of Yangtze River. Aquac. Res. 5, 85–97 (2018).
Tian, H. W. et al. Study on catch structure of three-layer gillnet in the upper reaches of Yangtze River. Freshw. Fish. 46, 37–42 (2016).
Zou, J. X. & Zhai, H. J. Impacts of Three Gorges Project on water environment and aquatic ecosystem and protective measures. Water Resour. Prot. 32, 136–140 (2016).
Xiong, F., Liu, H. Y., Duan, X. B., Liu, H. P. & Chen, D. Q. Fish community structure and resource utilization in the Upper reaches of the Yangtze River. J. Anhui Univ. (Natural Science). 38, 94–102 (2014).
Yang, Z. et al. Interannual variation of fish community structure in the Upper reaches of the Three Gorges Reservoir area, Jiangjin River. Chin. J. Ecol. 33, 1565–1572 (2014).
Liu, M. D., Gao, L., Tian, H. W., Zhang, F. Y. & Duan, X. B. Status of fishes at the early life history stage in the Yichang section in the middle reaches of the Yangtze River. J. Fish. Sci. China. 25, 147–158 (2018).
Chen, D. Q., Xiong, F., Wang, K. & Chang, Y. Status of research on Yangtze fish biology and fisheries. Environ. Biol. Fish. 85, 337–357. https://doi.org/10.1007/s10641-009-9517-0 (2009).
Ba, J. W. & Chen, D. Q. Preliminary study on invasive fishes in the three gorges reservoir area and the impact of water storage in the reservoir. J. Lake Sci. 24, 185–189. https://doi.org/10.18307/2012.0203 (2012).
Rees, H. C. et al. The application of eDNA for monitoring of the Great Crested Newt in the UK. Ecol. Evol. 4, 4023–4032 (2014).
Deiner, K., Walser, J. C., Mächler, E. & Altermatt, F. Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA. Biol. Conserv. 183, 53–63 (2015).
Franklin, T. W. et al. Using environmental DNA methods to improve winter surveys for rare carnivores: DNA from snow and improved noninvasive techniques. Biol. Conserv. 229, 50–58 (2019).
Ogram, A., Sayler, G. S. & Barkay, T. The extraction and purification of microbial DNA from sediments. J. Microbiol. Methods. 7, 57–66 (1987).
Valentin, R. E. et al. Moving eDNA surveys onto land: strategies for active eDNA aggregation to detect invasive forest insects. Mol. Ecol. Resour. 20, 746–755 (2020).
Valentini, A. et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 25, 929–942 (2016).
Weitemier, K. et al. Estimating the genetic diversity of pacific salmon and trout using multigene edna metabarcoding. Mol. Ecol. (2021).
Ficetola, G. F., Miaud, C., Pompanon, F. & Taberlet, P. Species detection using environmental DNA from water samples. Biol. Lett. 4, 423–425. https://doi.org/10.1098/rsbl.2008.0118 (2008).
Hänfling, B., Handley, L., Read, L., Hahn, D. S., Jianlong, N. & C. & Environmental DNA metabarcoding of lake fish communities reflects long-term data from established survey methods. Mol. Ecol. 25, 3101–3119. https://doi.org/10.1111/mec.13660 (2016).
Cheng, R. L. et al. Application of eDNA metabarcoding for monitoring the fish diversity of the Jiangjin to Fuling section of the upper reaches of the Yangtze River. Hydrobiologia. 850, 4067–4088. https://doi.org/10.1007/s10750-023-05297-1 (2023).
Bessy, C. et al. Maximizing fish detection with eDNA metabarcoding. Environ. DNA. 2, 493–504. https://doi.org/10.1002/edn3.74 (2020).
Pilliod, D. S., Goldberg, C. S., Arkle, R. S. & Waits, L. P. Estimating occupancy and abundance of stream amphibians using environmental DNA from filtered water samples. Can. J. Fish. Aquat. Sci. 70, 1123–1130 (2013).
Janosik, A. M. & Johnston, C. E. Environmental DNA as an effective tool for detection of imperiled fishes. Environ. Biol. Fish. 98, 1889–1893 (2015).
Luo, J. S. Fish Diversity in Lakes of Central Yunnan Plateau Using Environmental DNA (Yunnan University, 2019).
Xu, N. & Chang, J. Preliminary study on fish species detection in the middle and lower Yangtze River using environmental DNA. J. Hydroecology. 37, 49–55 (2016).
Hao, Y., Zhang, A., Liu, J. & Gu, Z. Application of environmental DNA technology in the study of fish resources. Biotechnol. Bull. 34, 56 (2018).
Taberlet, P., Bonin, A. & Coissac, E. Environmental DNA: for Biodiversity Research and Monitoring (Oxford University Press, 2018).
Bolger, A. M., Lohse, M., Usadel, B. & Trimmomatic A flexible trimmer for Illumina sequence data. Bioinformatics. 30, 2114–2120 (2014).
Magoč, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27, 2957–2963 (2011).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7, 335–336 (2010).
Zhang, C. G. & Zhao, Y. H. Species Diversity and Distribution of Inland Fishes in China (Science, 2016).
Fish Research Laboratory of Hubei Institute of Hydrobiology. The Fishes of the Yangtze River (Science, 1976).
Ding, R. H. The Fishes of Sichuan (Sichuan Science and Technology, 1994).
Liu, L. G. et al. Status and diversity of fish resources of the Lishui river in Hunan Province. Resour. Environ. Yangtze Basin. 22, 1165–1171 (2013).
Zhao, S. S., Ye, S. W., Xie, S. G. & Cheng, F. The current situation of fishery resources in the Xiangxi River of the Three Gorges Reservoir and advices on the management. Acta Hydrobiol. Sin. 39, 973–982 (2015).
Chen, S. B. Studies on Fishery Capture and Fishery Biology in the Three Gorges Reservoir (University of Chinese Academy of Sciences, 2016).
Sorensen, T. A. Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content, and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskabs Biologiske Skrifter. 5, 1–34 (1948).
Shannon, C. E. A mathematical theory of communication. BellSystem Tech. J. 27, 379–423 (1948).
Simpson, E. H. Measurement of diversity. Nature. 163, 688 (1949).
Pielou, E. C. J. The measurement of diversity in different typesof biological collections. J. Theor. Biol. 13, 131–144 (1966).
Gao, T. H., Tian, H. W., Ye, C. & Duan, X. Fish composition and diversity in the mainstem of the national Nature Reserve of rare and endemic fishes in the upper reaches of the Yangtze River. Freshw. Fish. 43, 36–42 (2013).
Wang, M. et al. Study on fish diversity in Chongqing section of national Nature Reserve of Rare and endemic fish in the upper reaches of Yangtze River based on eDNA technique. Acta Hydrobiol. Sin. 46, 2–16 (2022).
Li, H. X., Huang, X. N., Li, S. G. & Zhan, A. B. Early detection and early warning of invasive organisms in aquatic ecosystems based on environmental DNA-macro barcoding technology. Biodiversity. 27, 491–504 (2019).
Kumar, G. et al. Comparing eDNA metabarcoding primers for assessing fish communities in a biodiverse estuary. PloS One. 17, e0266720–e0266720 (2022).
Dai, M. M. et al. Fish diversity and resource status in the Nanguang River, a tributary of upper Yangtze River. Biodivers. Sci. 27, 1081–1089 (2019).
Zhou, Y. et al. Status of Fish resources in the Zhougong River, a Tributary of the Qingyi River. Sichuan J. Zool. 37, 592–600 (2018).
Wu, F. H., Qiu, Q. R., Hu, M., Wang, L. L. & Zhang, Z. Y. Oreochromis niloticus cultivation. Freshw. Fisheries, 17–21 (1980).
Xiang, J. G., Zhou, J. & Jin, H. A study on the Biological and biochemical characteristics of the ictalurs Punctatus. J. Hunan Agricultural University: Nat. Sci. Ed. 30, 355–358 (2004).
Zhang, Q. et al. Effects of Captive Density on Growth Performance and Health Status of Largemouth Bass (Micropterus salmoides). Acta Hydrobiol. Sin. 46, 671–678 (2022).
Zhao, Y. et al. Species diversity and conservation of freshwater fishes in the Yellow River basin. Biodivers. Sci. 28, 1496–1510 (2020).
Yan, T. M. et al. Assessment of ecosystem health on the upper and middle reaches of Nanhe River with the fish-based biotic integrity index. Freshw. Fisheries. 51, 3–12 (2021).
Cheng, R. L. et al. eDNA metabarcoding reveals differences in fish diversity and community structure in heterogeneous habitat areas shaped by cascade hydropower. Ecol. Evol. 13, e10275. https://doi.org/10.1002/ece3.10275 (2023).
Cheng, R. L. et al. Research on fish diversity of the cascade hydropower reservoir area of the Wujiang River based on environmental DNA technology. J. Fisheries China, 1–19 (2023).
Stewart, K. A. Understanding the biotic and abiotic factors on sources of aquatic environmental DNA. Biodiversity. 28, 983–1001 (2019).
Bylemans, J., Gleeson, D. M., Duncan, R. P., Hardy, C. M. & Furlan, E. M. A performance evaluation of targeted eDNA and eDNA metabarcoding analyses for freshwater fishes. Environ. DNA. 1, 402–414 (2019).
Zou, X., Yang, R. H., Yang, Z., Zheng, Z. W. & Shi, F. Habitat health assessment of typical tributaries of the Yangtze River. J. Hydroecology. 42, 29–39 (2021).
Rhoads, B. L., Schwartz, J. S. & Porter, S. Stream geomorphology, bank vegetation, and three-dimensional habitat hydraulics for fish in midwestern agricultural streams. Water Resour. Res. 39, 2–13 (2003).
Sandersfeld, T., Mark, F. C. & Knust, R. Temperature-dependent metabolism in Antarctic fish: do habitat temperature conditions affect thermal tolerance ranges? Polar Biol. 40, 141–149 (2017).
Wei, N., Zhang, Y., Wu, F., Shen, Z. W. & Ru, H. J. Current status and changes in Fish assemblages in the Three Gorges Reservoir. Resour. Environ. Yangtze Basin. 30, 1858–1869 (2021).
Shakman, E. & Kinzelbach, R. Commercial fishery and fish species composition in coastal waters of Libya. Rostocker Meeresbiologische Beitr. 18, 63–78 (2007).
Chen, J. L. Reservoir Communities in the Qingshitan web Structure and Energy Sources of fish Spatial and Temporal Distribution, food (Guilin University of Technology, 2023).
Wang, Y. P. et al. Community structure and species diversity of fish around the Xinzhou shoal in the Anqing section of the Yangtze River, China. Acta Ecol. Sin. 40, 2417–2426 (2020).
Tang, C. et al. Analysis on the characteristics of fish community structure in the mainstem section of the National Nature Reserve for Rare and endemic fish in the upper reaches of the Yangtze River. J. Fisheries China. 42, 81–100 (2023).
Morita, K., Fukuwaka, M. A., Tanimata, N. & Yamamura, O. Size-dependent thermal preferences in a pelagic fish. Oikos. 119, 1265–1272 (2010).
Ficetola, G. F., Pansu, J., Aurélie Bonin, Coissac, E. & Taberlet, P. Replication levels, false presences, and the estimation of presence / absence from eDNA metabarcoding data. Mol. Ecol. Resour. 15, 543–556 (2014).
Li, X. Y. Spatial and Temporal Distribution Characteristics of Fish in the Mainstem of the Upper Yangtze River Reserve Jiangan-Chongqing Section in the Early Period of Closed Fishing (Southwest University, 2023).
Bondarev, D. et al. Temporal dynamics of the fish communities in the reservoir: the influence of eutrophication on ecological guilds structure. Ichthyol. Res. 70. https://doi.org/10.1007/s10228-021-00854-x (2023).
Keast, A. Trophic and spatial interrelationships in the fish species of an Ontario temperate lake. Environ. Biol. Fish. 3, 7–31 (1978).
Ye, S. W. et al. Community structure of small fishes in a shallow macrophytic lake (Niushan Lake) along the middle reach of the Yangtze River, China. Aquat. Living. Resour. 19, 349–359 (2006).
Maes, J., Stevens, M. & Breine, J. Modelling the migration opportunities of diadromous fish species along a gradient of dissolved oxygen concentration in a European tidal watershed. Estuar. Coast. Shelf Sci. 75, 151–162 (2007).
Kadye, W. T., Magadza, C. H. D., Moyo, N. A. G. & Kativu, S. Stream fish assemblages in relation to environmental factors on a montane plateau (Nyika Plateau, Malawi). Environ. Biol. Fish. 83, 417–428 (2008).
Jensen, F. B. & Hansen, M. N. Differential uptake and metabolism of Nitrite in normoxic and hypoxic goldfish. Aquat. Toxicol. 101, 318–325 (2011).
Staub, E. Effects of sediment flushing on fish and invertebrates in Swiss Alpine Rivers. In International Workshop and Symposium on Reservoir Sedimentation Management, 185–193 (2000).
Acknowledgements
The authors sincerely thank all the crew members for their help with manuscript writing and data analysis. This work was supported by the National Natural Science Foundation of China (Grant No. 32202939) and the Project of Innovation Team of Survey and Assessment of the Pearl River Fishery Resources (2023TD-10).
Author information
Authors and Affiliations
Contributions
Writing – review & editing, Yanjun Shen; Data curation, Xinxin Zhou; Investigation, Yufeng Zhang and Jiaming Zhang; Software, Qinghua Li; Funding acquisition, Yingwen Li and Yanjun Shen; Project administration, Yingwen Li; Supervision: Qiliang Chen and Zhihao Liu; Formal analysis, Ruli Cheng and Yang Luo.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical declarations
This study did not involve any live animals, and fish species were obtained by collecting river water samples for environmental DNA analysis. All experimental protocols were approved by the Ethics Committee of Chongqing Normal University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, Y., Zhou, X., Zhang, Y. et al. Important fish diversity maintenance status of the tributaries in a hotspot fish conservation area in the upper Yangtze River revealed by eDNA metabarcoding. Sci Rep 14, 24128 (2024). https://doi.org/10.1038/s41598-024-75176-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75176-9