Abstract
Gas has been widely concerned due to its importance in the industrial and energy fields. In order to improve the inhibition effect of gas explosion, under the self-constructed pipe network system, the inhibition of gas explosion by composite inhibitor of fly ash and NaHCO3 with different ratios was investigated, and the microscopic inhibition mechanism of the two kinds of powders on the explosion of gas was investigated based on the method of density functional theory and transition state theory from the perspective of molecular dynamics. The results show that: the composite powder explosion suppression is better than a single powder, explosion suppression effect with the increase in the proportion of the mass of NaHCO3 significantly improve the 5 groups of conditions NaHCO3 loading of 40% (by mass) is the best, this time, the explosion of the peak overpressure, the peak flame propagation velocity, the peak flame temperature compared to the maximum reduction in the percentage of the measures taken for the maximum of 74.42%, 81.93%, 68.71%. In addition, NaHCO3, fly ash effectively inhibit the key primitive reaction of gas explosion. When the temperature reaches a certain point, the two and O*, O2, OH* and H* reaction rate higher than the rate of CH4 oxidation chain reaction, the dominant reaction, the maximum difference in rate constants of 70.95, 58.81, 60.06, 44.94. The study of the transition from the macro-scale to the molecular level of the study, in-depth understanding of the mechanism of explosion suppression, to reduce the risk of explosion from the ground up, and enriches the Gas explosion prevention and control of the theoretical system, for the operators to provide a strong technical guarantee for safe production.
Similar content being viewed by others
Introduction
The gas explosion accident seriously restricts the development of coal engineering, and the shock wave and flame generated by the explosion pose a serious threat to the surrounding environment, producing negative social effects1,2. There are four prevention and control methods involved in the control of gas explosions, which are explosion suppression, explosion arrest, explosion venting, and explosion isolation3,4,5. Relevant scholars in China have carried out extensive research on cost-effective explosion suppression materials. With the help of the performance of the explosion suppressant and the different materials of its materials, the explosion suppression effect and reaction mechanism are characterized, so as to reduce the occurrence of accidents. Combined with related materials, it is found that powder has always been a research hotspot for scholars at home and abroad because of its advantages of efficient inhibition ability, easy operation and zero energy supply6. Such as (NH4)2HPO4(ABC powder)7, NaHCO38, diatomaceous earth9, urea10, pozzolanic acid11, halide12 and so on. The study of different powder inhibitors can improve the level of prevention and control of gas explosions, reduce the risk of accidents, and ensure the safety of production in coal mines and other gas-related sites. Powder explosion suppression mainly includes two aspects: on the one hand, some components in the powder react with the free radicals or groups separated by the explosion chain13, and then reduce the concentration of gas mixture to achieve the inhibition effect. He et al.14 studied the interaction of two main silanols in diatomaceous earth (isolated silanols and congenial silanols) with the key intermediate products of gas explosion (H*,OH* and HCHO), and found that congenial silanols consume free radicals and adsorption of HCHO are equipped with a better effect, which in turn affects the chain reaction of the gas explosion to achieve the effect of suppression; Li et al.15 used a 20 L spherical explosion container to analyze the urea inhibition of gas explosion in the process of explosion pressure and the impact on the key free radicals and molecules in the explosive chain reaction, the results found that under the action of urea, NO, HNO content increases, CN, CHO, OH content decreases, so as to reduce the concentration of free radicals to inhibit the gas explosion. On the other hand, the powder can be used as a layer of cover in the gas environment to form a physical barrier, which in turn blocks the propagation of the gas explosion, thus preventing the gas from mixing with air and exploding. Ding et al.16 investigated the effects of the explosion suppression medium injection amount and the trigger time of the explosion suppression device on the explosion of the methane/air mixture by using nitrogen-driven ultrafine ABC powder in a 100 L explosion container, and found that the explosion suppression device was triggered within 50 ms after the powder volume was 40 g and the methane/air mixture was ignited, and the explosion flame was completely suppressed. Wang et al.17 investigated the suppression of CH4 in a 20 L spherical explosion container and 5 L Plexiglas pipe, using solvent-anti-solvent method to prepare the NaHCO3/red mud composite materials, and the results showed that the suppression of the NaHCO3/red mud composite powder is better than that of a single powder. When the optimum loading of NaHCO3 was 35%, the maximum pressure of explosion decreased by 44.9%, the maximum pressure rise rate decreased by 96.3%, and the time to peak pressure was delayed by 366.7%. In conclusion, more practical and stable powder inhibitor in gas explosion suppression should be further research.
Fly ash (FA) is a waste product of coal combustion, as an inert material, its internal unburned carbon particles are loose and porous, and the explosion-suppressing components, such as SiO2, CaO, Al2O3, MgO, and metal chlorides18, have been a hot research topic for many scholars. Wang et al.19 carried out explosion flame propagation and explosion suppression experiments with urea/fly ash hollow ball composite explosion suppressant, and the explosion suppression effect could be achieved at 40wt% urea/fly ash composite explosion suppressant. Guo20 and others in the 20 L spherical explosive device to fly ash (FA) and modified fly ash (MFA) suppression of the effect of the different concentrations of gas, the results show that FA suppression of the effect of gas, the results show that the FA of gas, the results show that the FA of gas, the results show that the FA of the effect of gas suppression, the results show that the FA of the effect of gas suppression, the results show that the FA of the effect of gas suppression. The results showed that FA had the optimal effect of explosion suppression. Under the same conditions, FA’s explosion suppression efficiency is up to 4.05% higher than MFA. Therefore, the consideration of fly ash as a blast suppression material not only reduces the waste of resources caused by the waste, but also is an efficient use of resources. Sodium bicarbonate (NaHCO3) decomposition process has a high thermal sensitivity and natural anti-caking properties, dispersed in the inert components, with the help of its anti-caking properties, the composite suppressant can reach a good state of dispersion by virtue of its own composition, without the need to add additional hydrophobic substances21,22.
Considering the complexity of the actual underground tunnel, it is difficult to qualitatively and quantitatively analyze the shock wave and flame wave propagation, and the conclusions of the study are not universal. Therefore, in this study, different ratios of fly ash and NaHCO3 were selected as the explosion-suppressing powders, and the inhibition characteristics of the two were investigated in the self-constructed pipeline network system to suppress the gas explosion. Based on the density functional theory (DFT) and the reaction rate constant, the microscopic inhibition mechanism of fly ash and NaHCO3 powders on gas explosion was investigated from the molecular dynamics point of view, which will promote the theoretical innovation in the field of explosion suppression, and open up a new way for the development of more advanced gas explosion suppression technology.
Experimental system and methods
Experimental pipeline network system
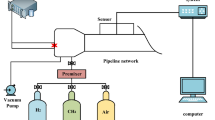
The research carried out the research lab on the Internet, the satellite system, the dynamic data sets, the fire system. The geometry of the experimental pipe network was 8100 mm × 5500 mm, with an inner diameter of 500 mm, and the pipes were equipped with threaded holes for the installation of sensors, and gaskets and screws were installed at the connection points of the individual pipes to ensure the airtightness of the network.
Experimental scheme
The experimental physical device is shown in Fig. 1. Before the test, a certain mass of composite powder was evenly spread in the pipe in the position as in Fig. 2, and a Poly tetrachloroethylene film was used to separate the explosion chamber from the pipe and to test the airtightness of the pipe. Pipeline for the atmospheric pressure state. After the explosion chamber is vacuumed, the control flow meter is energized with 9.5% by volume of gas test gas. Turn on the ignition device, and at the same time to ensure that the igniter and data acquisition system synchronization began to work. The ignition system mainly includes DX-GDH high-energy igniter, high-energy spark plugs at the front end of the explosion chamber, high-voltage and high-temperature resistant cables, power supply cables and ignition control box through the wire connected to the external trigger device. Ignition voltage is 2200 V, single stored energy is 30 J; in the computer terminal using TST6300 data acquisition system to collect dynamic data, the system mainly includes CYG1721 high-precision pressure sensors, NANMAC-E6 series of fast-response thermocouples with erosive probes, CKG100-type photosensitive flame sensor. The response time of the sensors is 1 ms and the data accuracy is 0.2%FS (full scale). At the end of the explosion, both ends of the pipe were opened to discharge the residual exhaust gas and clean the pipe in preparation for the next test. The test was repeated three times for each sample group to ensure the accuracy and reasonableness of the data obtained.
Experimental materials
Fly ash is the solid waste from the high temperature combustion of inorganic coal and is the tiny ash particles discharged during the combustion process. In this study, fly ash obtained from a power plant in Chifeng City, Inner Mongolia, China, was used as the test material, and different ratios of NaHCO3 and fly ash (10%NaHCO3/20% NaHCO3/30%NaHCO3/40%NaHCO3/50%NaHCO3) were placed in a constant temperature oven and dried at 60 °C for 9 h to remove the moisture before the experiment. The obtained composite powder was put into a planetary ball mill for grinding and sieved for 125 mesh to give it a larger specific surface area. On this basis, the composite powders were discussed in 5 groups of conditions on the explosion suppression of methane-air mixtures with a volume fraction of 9.5%. The mass of explosion suppression powder is 40 g23, and the particle size distribution of NaHCO3and fly ash is shown in Fig. 3.
Results and discussion
Effect on peak shock wave pressure
Figure 4 shows no powder (blank control), a single powder and different quality ratios of NaHCO3 and fly ash composite powder to inhibit the explosion of the peak shock wave pressure and its law of change of the measurement points in the pipe network. From Fig. 4, the peak shock wave pressure observed with the composite powder NaHCO3 load increases and shows a decreasing trend. In the absence of the addition of explosion-suppressing powder, the maximum pressure peak measured at the measurement point 1 in the branch pipe 1 is 0.53 MPa, the pressure peak for the entire explosion process pressure peak. This is because the shock wave propagation process, due to the special characteristics of the explosion pipe network, the shock wave in the pipe network will be repeatedly superimposed and constantly attenuated24. When the shock wave along the branch pipe 1 forward propagation to reach the measurement point T2, due to energy loss and pipeline heat dissipation and other impacts, the pressure peak at the measurement point T2 is less than T1; to reach the branch pipe 2 when the pressure of the shock wave attenuation phenomenon, which may be the propagation path is too long as well as the collision of vertical pipelines leading to the measurement of the point T3, T4 pressure peak continues to decrease; when the shock wave to reach the branch pipe 3, in the corner after the measurement of the point T5, T6, T7 when the shock wave superposition phenomenon occurs, resulting in a larger pressure peak; due to the measurement point T8 near the explosion source, in the event of an explosion in the pressure of the shock wave after the “T” type pipe network when part of the diversion, the pressure peak is slightly smaller compared to the measurement point 1. When the addition of NaHCO3 and fly ash, compared to not take measures to suppress the explosion, the explosion of the overpressure of each measurement point have shown a substantial reduction, the maximum drop of 0.16 MPa, 0.19 MPa. Along with the composite powder in the increase in the amount of loaded NaHCO3, the pipeline network of the peak pressure of each measurement point compared to a single NaHCO3 and fly ash of the peak pressure of the explosion is a downward trend, may be due to The addition of NaHCO3 improves the phenomenon of fly ash surface agglomeration and adhesion. When the NaHCO3 loading increased to 40%, the composite powder explosion overpressure compared to the NaHCO3 loading of 10% decreased by 0.11 MPa, indicating that the chemical inhibition of NaHCO3 explosion suppression effect is better than the main physical inhibition of fly ash. When the NaHCO3 loading of 50%, the peak overpressure at each monitoring point compared to the NaHCO3 loading of 40% slightly decreased, but the peak value does not change much. Therefore, comparing the five conditions, the synergistic inhibition effect of the two was best at 40% NaHCO3 loading.
Different ratios of NaHCO3 and fly ash to inhibit the gas explosion at each point of the flame wave propagation velocity peak change rule is shown in Table 1 and Fig. 5. It can be seen from Fig. 5 and Table 1, before the addition of powder, each point of the flame wave propagation velocity peak in descending order of T5, T4, T6, T3, T1, T7, T2, T8, the highest to reach 340.23 m/s. When the flame wave enters the pipeline network system, the flame wave propagation velocity appears to be significantly reduced through the “T” shaped bifurcation. “T”-shaped bifurcation at the flame wave propagation speed has decreased significantly, which is due to the sudden increase in the flame wave propagation area resulting in a reduction in speed, and the flame wave in the propagation process by the collision between the inner wall of the pipeline, the capture of heat resulting in energy loss, making the propagation speed reduced25. At the same time, the flame wave propagation velocity peak at measurement point T1 in branch pipe 1 is higher than that at measurement point T8 in branch pipe 4, indicating that at the “T”-shaped bifurcation, the pressure loss of the flame wave velocity in the vertical direction is higher than that in the horizontal direction. When the flame wave along the horizontal direction to reach the measurement point 2 at right-angle bend, the flame wave due to successive phenomena such as reflection superposition, energy loss, resulting in a gradual decrease in the speed of flame wave propagation. When added to the explosion suppression powder, different ratios of NaHCO3 and fly ash suppression of gas explosion flame front velocity peak show similar trends, are with the increase in the concentration of NaHCO3 downward trend. Since the pressure increase in the explosion vessel is caused by the volume expansion of isocratic combustion products, the change of explosion pressure also shows a similar trend26. When the NaHCO3 loading gradually increased, the peak flame wave propagation velocity at measurement point 1 decreased by 103.51 m/s, 149.19 m/s, 172.37 m/s, 235.36 m/s, 257.70 m/s, 255.81 m/s respectively compared with that before the addition of the explosion-suppressing powder, which confirms the inhibitory effect of the composite powder of NaHCO3 and fly ash on the propagation of the flame wave of the gas explosion. When the NaHCO3 loading is 50%, the flame wave propagation velocity peak data measured at each measurement point is slightly attenuated, basically the same as the peak velocity when the loading is 40%, indicating that the composite powder to inhibit the gas explosion in the pipeline network system NaHCO3 optimal loading is 40%.
Effect on peak flame temperature
Gas explosion measured the peak temperature of the flame wave at each measurement point as shown in Fig. 6. Analysis of the various pipelines on the measurement point found that before the addition of explosion-suppressing powder, the temperature at the measurement point T1 is higher than the other measurement points, 1390 K; in the measurement point T4 to obtain the lowest temperature of 892 K. Along with the increasing propagation distance, the temperature shows a trend of decreasing. Comparing the peak change of flame front speed in Fig. 6, the temperature is not necessarily high where the flame wave speed is fast, because the flame front temperature has a certain hysteresis relative to the flame wave speed, and the collision pipe network energy consumption is high in the event of an explosion27. When the addition of NaHCO3 and fly ash powder, respectively, the peak flame wave temperature of each measurement point in the pipe network has the same trend of change, are measured at the measurement point 1 of the peak flame wave temperature, compared with the peak temperature before the addition of powder were reduced by 331 K, 411 K. The reason for this is that the NaHCO3 in the gas explosion rapid thermal decomposition, in the process of completing the decomposition of heat absorption and cooling, reduce the ambient temperature. In addition, the porous structure of fly ash can cause the radiation dispersion of explosive substances, reducing the ambient temperature during the explosion while reducing the energy release of the shock wave28. Along with the NaHCO3 loading of 10%, the peak temperature of each measurement point showed different degrees of reduction, which confirms the superiority of composite powder explosion suppression compared with a single powder. When the NaHCO3 loading of 20%, T1, T6, T7 showed a small decrease, T4 compared to the NaHCO3 loading of 10% decreased by 37 K.At 30% NaHCO3 loading, the peak flame temperature decreased from 694 to 509 K at measurement point 1, and from 367 to 302 K at measurement point 4. At 40% NaHCO3 loading, the peak flame temperature decreased slightly compared with that at 30% NaHCO3 loading. When the NaHCO3 loading of 50%, compared to the NaHCO3 loading of 40% of the composite powder on the role of the explosion flame temperature is not significant, indicating that the two mixed to reach a certain threshold, the increase in the concentration of NaHCO3 on the explosion suppression does not play a significant role. Therefore, in the NaHCO3 loading of 40% compared to the other four conditions of inhibition is optimal.
Based on the analysis, the composite powder of NaHCO3/fly ash demonstrates superior performance in suppressing methane explosions in pipelines compared to the use of single powders. The explosion suppression effect of the composite powder significantly increases with the proportion of NaHCO3. In five experimental conditions, the optimal suppression effect is achieved when NaHCO3 loading is 40%, with maximum reductions in explosion overpressure peak, flame wave propagation speed peak, and flame temperature peak by 74.42%、81.93%、68.71% respectively, compared to conditions without any measures. The results from Wang et al.29 indicate that in enclosed conditions, both explosion overpressure and the rate of overpressure increase decrease with the addition of the urea/fly ash (FAC) composite suppressant, and the suppression effect improves progressively. These findings are consistent with the conclusions of this paper. Therefore, the NaHCO3/fly ash composite powder is effective in suppressing the propagation of methane explosion flame waves and shock waves, thus playing a suppressive role.
Explosion suppression mechanism analysis
On the basis of previous studies, O*, OH*, H* and O2 are the key elements to initiate the chain reaction of gas explosion30. Under high temperature conditions, NaHCO3 is susceptible to thermal decomposition, and the free radicals from pyrolysis can reduce the free radicals from CH4 fracture. The main metal atoms (Si, Ca, Al, Mg, Fe) and metal oxides (SiO, CaO, AlO, MgO, FeO, Al2O3) in fly ash can also react with the free radicals and gas molecules produced by methane explosion, which in turn impede the explosive chain reaction and serve to inhibit the gas explosion31,32. In order to investigate the targeting of NaHCO3 and fly ash on the free radicals of gas explosion fracture, respectively, the transition state simulation was carried out using the Dmol3 module of the Materials Studio software33, and the microscopic inhibition mechanism of NaHCO3 and fly ash on gas explosion was investigated.
Reaction explosion suppression mechanism
Density Functional Theory (DFT) is a quantum mechanical computational method used to investigate the electronic structure of many-electron systems. It is commonly applied to calculate molecular geometries, energies, and reaction barriers. Transition State Theory (TST) is a theoretical framework used to describe the rates of chemical reactions, requiring the calculation of the energies of reactants, transition states, and products to determine the kinetic characteristics of a reaction. These two approaches are often complementary in the study of chemical reactions. In the investigation of reaction dynamics, DFT can aid in identifying transition states, which can then be analyzed using TST to evaluate reaction rates. Therefore, this study explores the microscopic suppression mechanism of the interaction between NaHCO3 and fly ash powder on gas explosion based on these two methods. Gibbs free energy is an important characterization parameter for characterizing the active energy required for a chemical reaction and the activation energy of all active factors in the reaction system, the lower the value of the Gibbs free energy barriers required to complete the chemical reaction process, the easier the reaction occurs34. For NaHCO3 and fly ash in this paper, the structure and energy of both molecules were simulated by the Dmol3 module of Materials Studio software. In the Dmol3 module, select PBE in GGA for functional and LST/optimization for search protocol. RMS conversion is set to 0.01A, Basis set to DND, Base file is set to 3.5, SCF tolerance is set to 1.0e-5, Max.SCF cycles is set to 50, and smoothing is set to 0.05 Ha.
By searching and optimizing the structures of reactants, transition states, and products, the reaction pathway of NaHCO3 participating in the explosion was obtained as shown in Table 2. The Gibbs free energy barrier ranges from -78.66 to 136.64 kJ/mol. Based on Fig. 7, in reactions 1, 3, 4, and 6, NaHCO3 absorbs ambient temperature and undergoes pyrolysis. During the pyrolysis process, Na* ions exhibit a strong reaction trend, mainly through oxidation reactions. Reactions 2, 5, and 7 are non-barrier reactions, which may be due to excessive free radical activity, making it difficult to obtain intermediate states.
By searching for transition states and determining the connection between reactants, products and intermediate transition states, the main reactions of key components in fly ash involved in the explosion are obtained as shown in Table 3 below. Reaction 1 to reaction 5 are the reaction paths of metal oxides (AlO, FeO, CaO, MgO, SiO) in fly ash with free O* during the explosion. The Gibbs free energy barriers generated during the reaction are between 12.48 and 55.12 kJ/mol, all of which are heat-absorbing reactions, in which no intermediate transition state is found between SiO and O*. Combined with Fig. 8a, it can be seen that the metal oxides in the fly ash have a certain consumption effect on O*, in which the relative energy barriers of AlO and O* are lower and more likely to occur; Reaction 6 to Reaction 12 are the reaction paths of the metals in the fly ash (Ca, Si, Al, and Mg) and the metal oxides (FeO, CaO, and MgO) in the encounter with O2. The Gibbs free energy barriers generated by analyzing the metal elements involved in the reaction in fly ash ranged from -202.98 to -448.72 kJ/mol, all of which were exothermic reactions. The transitional structure between Mg and O2 was not found, indicating that the rate of transitional state formation is likely much lower than the rate of Mg oxidation and decomposition, resulting in an unstable reaction process that makes intermediate states difficult to obtain. The Gibbs free energy barriers produced by the reaction of free metal oxides with O2 ranged from -297.76 to -350.87 kJ/mol, which were exothermic. Combined with Fig. 8b, it is intuitively obvious that, in comparison, Ca mainly takes the role of consuming O2; Reaction 13 to Reaction 17 is the reaction path of fly ash and OH*. In the gas explosion process only the Si element was found in the transition state structure as shown in Fig. 8c, the Gibbs free energy barrier is -227.22 kJ/mol, an exothermic reaction. This phenomenon may occur because the activity of OH* radicals is too high leading to the fact that the fly ash is not easy to react with it; Reactions 18 to 23 are the reaction paths of the metal oxides (AlO, FeO, CaO, MgO, SiO) in the fly ash and the H* produced during the explosion. The Gibbs free energy barriers generated during the reaction range from -501.46 to 4.14 kJ/mol, and most of them are exothermic. Among them, the reaction between CaO and H* is an adsorptive reaction, where H* seizes an O* on CaO to generate Ca and OH*, as shown in Fig. 8d, which has the lowest free energy barrier and is more likely to occur at high temperatures.
Reaction rate constant
Since the energy barrier is not the only quantity that characterizes the reaction speed, after determining the intermediate state of the primordial reaction, the rate speed of each reaction is judged by comparing the magnitude of the rate constant (KTST)14,35. This article uses Materials Studio simulation software to calculate the dynamics. During the simulation process, the temperature is set at 273.15 K to 1000 K, every 25 K. Since problems involving hydrogen transfer at low temperatures cause strong tunneling effects, the tunneling correction factor k is calculated (the imaginary frequency of the reactions involved in this paper ω < 1000):
where: \(k\) is the tunneling effect correction coefficient; \({K}_{b}\) is Boltzmann’s constant taken as 1.381 × 10–23 J/K; \(T\) is the absolute temperature in K; \(h\) is Planck’s constant taken as 6.626 × 10–34 J·s; R is the ideal gas constant taken as 8.31447 J/(mol·K); P is the gas-phase standard-state pressure; ω is the imaginary frequency, cm-1; ∆F is the difference between the free energy of the transition state and that of the reactants at the gas-phase standard state pressure, kJ/mol.
The Fig. 9 show the rate constants of the reactions of NaHCO3 and fly ash with O*, O2, OH* and H*, respectively, at different temperatures (273.15 K ~ 1000 K). In addition, the methane oxidation chain reaction is introduced in this paper to determine the dominant reaction. To facilitate the comparison of the rate of each reaction, the vertical axis is taken as the logarithm of LnK. From Fig. 9, at the same temperature, the reaction rate of different reactions is different, but the overall trend is upward with the increase of temperature. Comparing the rate constants KTST, the reaction rate of NaHCO3 with fly ash is higher than the rate of oxidative chain reaction of CH4 with O*, O2, OH* and H*.
Analyzing Fig. 9a, the individual metal atoms in the fly ash reacted intensely with the metal oxides competing for O*, and finally the rate gradually leveled off with the increasing temperature. Among them, the reaction rate of CH4 itself and O* in the ambient temperature of 325 K and CaO and O* compared to the largest difference, the rate constant LnK difference of 70.95, in the temperature of 1000 K compared and AlO and O* the smallest difference in the rate of the reaction, 29.94; Fig. 9b reflects the rate constants of the reaction of NaHCO3, fly ash and O2. With the increase of temperature, each reaction is intense in the early stage, but then the reaction rate gradually pulls away compared with the CH4 oxidation chain reaction. The rate constants for the reaction of CH4 with Si and Ca were the same at 575 K, and the rate constant for the reaction of Al with CH4 in fly ash was the largest, 58.81, at 600 K. The rate constant for the reaction of Al with O2 was the same as that for the reaction of Na when the temperature was further increased to 775 K. The rate constants for the reaction of Na with O2 were the same at 1000 K. The rate constants for the reaction of Na with O2 were the same at 775 K. At a temperature of 1000 K, NaHCO3 and the metal in the fly ash and its metal oxides involved in the reaction rate constants Al > FeO > CaO > MgO > CH4 > Na > Ca > Si; Fig. 9c only analyzes the rate constants of the OH* radicals in the fly ash with the increase in temperature with the increase in the OH* radicals of the chain reaction of the gas explosion. Compared to CH4 on OH* scramble, fly ash encounter OH* rate constant in the ambient temperature of 325 K when the difference is the most, 60.06. Figure 9d in the reaction rate of Al2O3 > AlO > CaO > FeO > MgO > NaO > SiO. fly ash in Al2O3 with H* rate constant values with temperature changes with the temperature, the fastest reaction rate. Among them, when the temperature environment is higher than 575 K, Al2O3 and H* show dominant reaction, and SiO, which reacts with relative inferiority, reaches the same reaction rate at this temperature. When the temperature increases to 1000 K, the reaction rate gradually tends to stabilize, at this time AlO in the fly ash and CH4 compete for H* rate constant comparative difference is the largest, 44.97; and SiO encounter H* rate constant difference is the least, 6.92.
According to the analysis above, the collisions of key radical species O*, O2, OH* and H* in the chain reaction of methane oxidation involving NaHCO3 and fly ash increase the consumption of radicals. During this process, the relatively large specific surface area of residual carbon in fly ash also facilitates the adsorption of radicals, consistent with the microscale inhibition mechanism studied by Guo et al.20 Fly ash interrupts the gas explosion chain reaction, thereby exerting an inhibitory effect on gas explosions.
Conclusion
Through the self-constructed experimental system of the explosion pipe network, the inhibition characteristics of NaHCO3/fly ash powder on the gas explosion of the pipe network were investigated under different mass ratios. In addition, based on the DFT, reaction energy barriers and reaction rate constants with temperature changes in the explosion suppression mechanism, the following main conclusions were obtained:
-
1.
The analysis of the composite powder explosion suppression characteristics show that the NaHCO3/fly ash composite powder can effectively inhibit the methane explosion flame wave and shock wave propagation, and better than a single powder. When no measures are taken, a single NaHCO3, fly ash explosion peak overpressure decreased by 0.16 MPa, 0.19 MPa. composite powder explosion suppression effect with the increase in the proportion of NaHCO3 mass, 40% NaHCO3 is the best. Peak overpressure, peak flame propagation velocity, peak flame temperature compared to the blank control decreased by 74.42%, 81.93%, 68.71%, respectively. After reaching the critical value, the increase in the concentration of NaHCO3 has no significant effect on explosion suppression.
-
2.
Analysis of the composite powder explosion suppression mechanism shows that NaHCO3 and fly ash can effectively participate in and inhibit the key reaction of the gas explosion, the two and their metal oxides involved in the reaction of the energy barrier is low, the process is easy to occur. In addition, at a certain temperature, the reaction rates of NaHCO3 and fly ash with O*, O2, OH* and H* are higher than that of the oxidative chain reaction rate of CH4, with a maximum difference of 70.95, 58.81, 60.06 and 44.94, respectively, and they gradually become the dominant reaction with the increase of temperature and play an inhibitory role.
This paper reveals the interaction mechanism of composite powders on the key reaction of gas explosion, which provides a new perspective and method for the in-depth understanding of the explosion suppression mechanism of composite powders. In addition, the explosion suppression effect and mechanism changes of composite powders under different environmental conditions (e.g. pressure, humidity) can be considered to improve their reliability in practical applications and provide theoretical guidance for optimizing the explosion suppression performance.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].
References
Zhu, Y. et al. A statistical analysis of coalmine fires and explosions in China. Process Saf. Environ. Prot. 121, 357–366 (2019).
Yu, M. G. et al. Research progress and development trend of gas explosion suppression and disaster reduction technology in coal mines in China. J. Coal Sci. 45(01), 168–188 (2020).
Cheng, F. M. et al. Research progress and development trend of gas explosion suppression materials and mechanism. Coal Sci. Technol. 49, 114–124 (2021).
Cao, M. et al. Experimental study on water sealing of fire barriers and explosion venting of large-scale pipeline with low-concentration gas. J. Loss Prev. Process Ind. 83, 105041 (2023).
Li, A., Si, J. & Zhou, X. Experimental research on rapid fire zone sealing and explosion venting characteristics of an explosion venting door using a large-diameter explosion pipeline. ACS Omega 6(41), 27536–27545 (2021).
Yu, M. G. et al. Influence of modified kaolin explosion suppressant on the composite explosion pressure of gas and coal dust. J. China Coal Soc. 47(1), 348–359 (2022).
Zhao, Q. et al. Suppression characteristics and mechanisms of ABC powder on methane/coal dust compound deflagration. Fuel 298, 120831 (2021).
Wei, X. et al. Study on explosion suppression of coal dust with different particle size by shell powder and NaHCO3. Fuel 306, 121709 (2021).
Cheng, F. M. et al. Experimental study on the suppression of gas explosion by diatomaceous earth powder. J. Min. Saf. Eng. 27(04), 604–607 (2010).
Yu, M. G., Wang, T. Z. & You, H. Study on the influence of thermal properties of powder materials on gas explosion suppression effect. J. Coal Sci. 37(05), 830–835 (2012).
Zhang, H. et al. Experimental study on the effect of silicate mineral desiccants on the hygroscopicity and explosion suppression performance of explosion suppressive powders. Min. Saf. Environ. Prot. 43(3), 5–9 (2016).
Wang, T. et al. Experimental investigation and numerical analysis on the confined deflagration behavior of methane-air mixtures within the suppression of typical haloalkanes. Process Saf. Environ. Prot. 183, 87–98 (2024).
Wang, Y. et al. Study on the influence of inert gas on the methane explosion suppression performance of KHCO3 cold aerosol. Coal Sci. Technol. 49(2), 145–152 (2021).
He, W. H. et al. Microscopic mechanism analysis of diatomaceous earth in suppressing gas explosions. J. Coal Sci. 47(10), 3695–3703 (2022).
Li, X. B. et al. Coupling analysis of explosion pressure and free radical changes during methane explosion suppression by urea. Explos. Shock 40(03), 13–23 (2020).
Ding, C. et al. Inhibition and enhancement of gas explosion by spraying ultrafine ABC powder. J. Coal Sci. 46(06), 1799–1807 (2021).
Wang, Y. et al. Methane explosion suppression characteristics based on the NaHCO3/red-mud composite powders with core-shell structure. J. Hazard. Mater. 335, 84–91 (2017).
Wang, X. Research on the suppression characteristics and mechanism of composite powder explosion suppressants on coal dust explosion. Shandong University of Science and Technology (2020).
Wang, X. et al. Effectiveness and mechanism of carbamide/fly ash cenosphere with bilayer spherical shell structure as explosion suppressant of coal dust. J. Hazard. Mater. 365, 555–564 (2019).
Guo, C. et al. Suppression effect and mechanism of fly ash on gas explosions. J. Loss Prev. Process Ind. 74, 104643 (2022).
Zhang, Y. et al. Study on suppression of coal dust explosion by superfine NaHCO3/shell powder composite suppressant. Powder Technol. 394, 35–43 (2021).
Fan, R. et al. Investigation of the physical and chemical effects of fire suppression powder NaHCO3 addition on methane-air flames. Fuel 257, 116048 (2019).
Wang, F. X., Jia, J. Z. & Tian, X. Performance and mechanism of bentonite in suppressing methane explosions in a pipeline network. Geomech. Geophys. Geo-Energy Geo-Resour. 31(03), 41–46 (2021).
Zhu, C. J. et al. Propagation characteristics of gas explosion flames and shock waves in parallel tunnel networks. J. China Univ. Min. Technol. 40(03), 385–389 (2011).
Geng, J. J. et al. Experimental study on the propagation law of gas explosion shock waves in unidirectional bifurcated pipelines in non-combustible areas. J. Saf. Environ. 15(05), 108–111 (2015).
Qu, Z. M. et al. The attenuation law of gas explosion shock wave overpressure. J. Coal Sci. 23(4), 410–414 (2008).
Jie, B. J., Du, Y. J. & Wang, L. Experimental and numerical simulation of flame propagation law of gas explosion in bifurcated pipelines. J. Chongqing Univ. 42(06), 69–77 (2019).
Zhu, C. et al. Experimental study on the effect of bifurcations on the flame speed of premixed methane/air explosions in ducts. J. Loss Prev. Process Ind. 49, 545–550 (2017).
Jia, J. et al. Propagation characteristics of the overpressure waves and flame fronts of methane explosions in complex pipeline networks. Geomat. Natl. Hazards Risk 13(1), 54–74 (2022).
Gao, N. et al. Chain reaction kinetics analysis of gas explosion in confined spaces. Chin. J. Saf. Sci. 24(01), 60–65 (2014).
Zierold, K. M. & Odoh, C. A review on fly ash from coal-fired power plants: chemical composition, regulations, and health evidence. Rev. Environ. Health 35(4), 401–418 (2020).
Yang, K. et al. Research on the inhibitory effect and mechanism of waste incineration fly ash on gas explosion pressure and flame propagation. J. Chem. Eng. 74(8), 3597–3607 (2023).
Jia, J., Song, H. & Jia, P. Molecular simulation of methane adsorption properties of coal samples with different coal rank superposition states. ACS Omega 8(3), 3461–3469 (2023).
Luo, Z. et al. Thermodynamic effects of the generation of H*/OH*/CH2O* on flammable gas explosion. Fuel 280, 118679 (2020).
SOBEREVA. Excel spreadsheet for calculating reaction rate constants based on transition state theory [EB/OL]. (2015-12-10) [2021-9-10]. http://sobereva.com/310.
Acknowledgements
This research is conducted with financial support from the National Natural Science Foundation of China (No.52174183 and 52374203) and Liaoning Province Doctoral Research Start-up Fund Project (No.2023-BS-203).
Author information
Authors and Affiliations
Contributions
SHAN and JIA.J wrote the main manuscript text. JIA.P and ZHANG conducted the manuscript article revision and software guidance. JIA.J provided financial support.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jia, J., Shan, S., Jia, P. et al. The characteristics and mechanism of NaHCO3 compound fly ash in suppressing gas explosions in pipeline networks. Sci Rep 14, 24925 (2024). https://doi.org/10.1038/s41598-024-75600-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75600-0