Abstract
The prevalence of extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) and carbapenem-resistant Enterobacterales (CRE) has become a global public health problem. ESBL-E/CRE colonization can increase the risk of infection in patients and lead to poor disease prognosis. We conducted a systematic review and meta-analysis to evaluate current decolonization strategies regarding ESBL-E/CRE and their efficacy. A literature search was conducted until August 2023 on the five databases to review decolonization strategies associated with ESBL-E/CRE. A meta-analysis was conducted using RevMan 5.4 to compare differences in the decolonization strategy with placebo controls. The primary outcome was decolonization rates, with secondary outcomes of attributable death and adverse events. Quality of identified studies was determined using the Newcastle–Ottawa scale and cochrane risk assessment tool. Random and fixed effects meta-analyses were performed to calculate pooled value. A total of 25 studies were included. In five randomized controlled trial (RCT) studies, the decolonization effect of selective digestive decontamination(SDD) on ESBL-E/CRE at the end of treatment was significantly better in the experimental group than the controls [risk radio (RR): 3.30; 95% CI 1.78–6.14]. In three n-RCT studies, the decolonization effect in the experimental group was still better than the controls one month after SDD therapy [odds ratio (OR): 4.01; 95% CI 1.88–8.56]. The combined decolonization rates reported by six single-arm trial studies of SDD therapy ranged from 53.8 to 68.0%. Additionally, TSA analysis confirmed the effectiveness of SDD therapy. In studies on Faecal microbiota transplantation (FMT) therapy, the decolonization effect of the experimental group was significantly better than the controls 1 month after treatment (OR: 2.57; 95% CI 1.07–6.16). In studies without a control group and with varying follow-up times, the decolonization rates varied widely but indicated the effectiveness trend of FMT therapy (61.3–81.2%). Currently, research on the decolonization effect of probiotic therapy on ESBL-E/CRE is insufficient, and only a systematic review was conducted. SDD and FMT strategies have short-term benefits for ESBL-E/CRE decolonization, but long-term effects are unclear. The effect of probiotic therapy on ESBL-E/CRE decolonization is an interesting topic that still requires further investigation.
Similar content being viewed by others
Introduction
At present, the spread of infections caused by extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) and carbapenem-resistant Enterobacterales (CRE) in medical institutions have become a significant threat to global public health. ESBL-E/CRE infection has a large negative impact on society and economy. One study showed that in the United States, CRE infections cost hospitals $275 million, third-party payers $147 million, and society $553 million1. The study by Esteve-Palau et al. also found that total pharmacy costs and antibiotic costs for ESBL-producing Escherichia coli infections were much higher than for non-ESBL infections, as was the need for outpatient parenteral antibiotic treatment and its associated costs2. Moreover, due to the rapid spread trend and limited treatment options of ESBL-E/CRE related infections, high mortality rates and adverse outcomes often accompany clinical practice. In 2017, the World Health Organization (WHO) has identified ESBL-E/CRE as a major global threat or high-priority pathogen to guide the current development and management of antibiotics with new mechanisms of action. However, the process of antibiotic development and use is progressing very slowly to match the trend of resistant bacteria trends. Decolonization and other alternative strategies also play an integral role in combating the spread of ESBL-E/CRE resistance.
Colonization is a phenomenon in which microorganisms such as ESBL-E/CRE grow on the skin, gastrointestinal, respiratory, oral, and genital tracts of patients but have not yet caused clinical manifestations of the associated infection3. ESBL-E/CRE are currently prevalent globally, and it is reported that 8–28.2% of ICU patients are colonized with ESBL-E, while the colonization rate of CRE varies from 0.3 to 50%4. Decolonization is an effective measure to prevent colonized patients from progressing to ESBL-E/CRE infection and can control the spread of ESBL-E/CRE in the population at source.
In a systematic review conducted in 2018 by European Society of Clinical Microbiology and Infectious Diseases-European Committee of Infection Control (ESCMID-EUCIC), the research team analysed the existing literature on decolonization of MDR Gram-negative intestinal carriers (27 studies) and made recommendations against interventions routinely using Selective digestive decontamination (SDD) for CRE decolonization. For fecal microbiota transplantation (FMT) or other measures, the guidelines do not provide a recommendation due to the shortage of data5. In 2023, a research team from China proposed 16 clinical questions for carbapenem-resistant negative bacilli (CRGNB) infection, and used the PICO strategy (population, intervention, comparison, and outcome) to translate them into research questions for productive discussion. On the 15th key question (on whether CRE colonized patients, especially those with hematologic malignancies, should be recommended for CRE decolonization), the group also made recommendations that do not support or refute CRE decolonization in clinical practice due to the low quality of evidence6. However, studies in recent years may show positive results of decolonization measures for ESBL-E/CRE elimination. Considering the need to refine the system of evidence for decolonization strategies and to determine the validity of newly published studies, we conducted a meta-analysis to systematically review the potential roles of SDD, FMT and probiotic therapy in ESBL-E/CRE decolonization.
Methods
Design
This study was written in accordance with the Preferred Reporting Items for systematic review and meta-analysis (PRISMA) statement7. The protocol for this meta-analysis has been submitted to the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023454638.
Search strategy and selection criteria
A literature search was conducted on the PubMed, Web of Science, Medline, Embase, and Cochrane library to determine eligible human studies from the date of inception to August 1, 2023. The following MeSH Terms or keywords were used: Carbapenem-resistant Enterobacterales, extended spectrum β-lactamase, Carbapenemase producing, decolonization, Faecal transplantation, selective digestive decontamination, Bacteriophages. The final data search was performed on December 25, 2023, to identify newly published articles. Meanwhile, we checked references and manual searches of relevant literature to ensure the completeness of the included literature. The literature search strategy using PubMed as an example is shown in Supplementary Table S1.
PICOS strategy was used by us to structure the research question8. The population (P) was patients with ESBL-E/CRE colonization identified by any form of testing in a hospital or community setting. Intervention (I) was to perform bacterial decolonization, defined as any measure that unequivocally results in the removal of, or cancellation of adherence to, detected CRE colonization vectors, including the use of selective digestive decontamination (SDD), fecal microbiota transplantation (FMT), and other alternative therapies. Control (C): included without any decolonization measures, following the natural course of CRE. Primary outcome indicators (O) were assessed using decolonization rates; secondary outcomes included attributable mortality, and adverse event rates. Study design (S) were randomised or non-randomised controlled experimental studies, cohort studies and case–control studies. In addition, case-analysis studies with sample sizes greater than 5 were accepted. Missing primary outcome data were imputed based on the last available observation or data.
Exclusion criteria were (a) Studies that routinely performed decolonization for infection prevention (without microbiological methods of testing or screening to determine CRE colonization); (b) Observations of the effect of natural development of decolonization in individuals or studies of the mechanisms of decolonization; (c) Studies of combination therapies where antimicrobial drugs were more important than decolonization measures; (d) Studies of decolonization of study subjects, such as environments, devices, and animals, but not humans. (e) Review article, Conference abstracts category, letters to the editor, and studies with low quality literature.
Parameter definition
The following terms were defined prior to data collection:
-
CRE: Enterobacterales that meet any of the following conditions: (a) resistant to any of the carbapenems [(minimum inhibitory concentration (MIC) ≥ 4 mg/L for imipenem, meropenem), or ertapenem MIC ≥ 2 mg/L]; (b) carbapenemase-producing; (c) in the case of bacteria naturally resistant to imipenem (e.g., Morganella morganii) must be resistant to other carbapenems (e.g. meropenem, ertapenem)9.
-
Bacterial colonization: a phenomenon in which microorganisms, such as bacteria, grow on a patient’s skin, gastrointestinal tract, respiratory tract, oral cavity, and reproductive tract but have not yet caused clinical manifestations of the associated infection10.
Data extraction and quality assessment
Screening of the titles and abstracts was independently conducted and cross-checked by two authors (H J and H W). Moreover, the two authors carried out full-text readings for the eligible studies. Corresponding authors were consulted to assist in judgement when disagreements were encountered until agreement was reached (F Y). Excel was used to create data extraction tables for extracting information. The following information was included in the data extraction form: study characteristics, clinical data, and outcomes. Due to the lack of relevant international standards for decolonization, we also documented the study methodology and relevant variables used to monitor the quality of decolonization strategies in the study, including decolonization regimen (content, route, dosage), duration/number of measures, and definition of eradication. Disagreements were resolved by consensus or referral to senior researchers (M Z and F Y) when needed.
RCT studies were assessed using the Cochrane Risk of Bias assessment tool, including selection bias, performance bias, measurement bias, attrition bias, reporting bias and other bias, and the results were added to the right side of the forest plot11. Included n-RCT studies were assessed using the Newcastle–Ottawa scale (NOS)12.
Data analysis
Meta-analyses were conducted using RevMan (version 5.4.1) and R (version 4.3.1) software. We compared eradication rates at different time points after decolonization treatment and presented them according to the time frame. Studies that could not be meta we described qualitatively. For RCTs, dichotomous variable results were calculated as risk ratios (RR), and 95% confidence intervals (CI) were calculated for both. The pooled odds ratio (OR) with 95% CI was calculated for case–control studies. Given the anticipated heterogeneity across trials, Heterogeneity among studies was assessed using the I2 test. If I2 < 50%, we used fixed models to determine the pooled results. However, if I2 ≥ 50% we used the random-effects model13. Publication bias in randomised controlled trials assessed using funnel plots.
Trial sequential analysis (TSA)
We performed TSA analysis of CRE decolonization data using SDD therapy. The TSA was conducted using TSA version 0.9.5.10 beta (The Copenhagen trial unit). TSA forms a TSA bounding curve by correcting for random error, which, together with a horizontal line at Z = 1.96 (the traditional significance level; alpha = 0.05), determines the risk of a study reaching a false-positive or false-negative conclusion by looking at the cumulative Z-value from the meta-analysis. When the cumulative Z-curve crosses the trial sequential monitoring boundary, the intervention effect may reach a sufficient level of evidence. If the Z-curve does not cross any of the boundaries and the required information size has not been reached, the evidence is rendered inadequate to conclude.
Results
Study characteristics and quality assessment
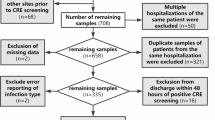
The initial search yielded 1677 results. Additional 6 articles were retrieved by cross-checking the reference lists and through manual searches. After removing duplicates using Note express (version 3.9), 1440 studies remained. A total of 105 full texts were read and, ultimately, 25 texts were selected for inclusion (14 SDD, 8 FMT, 3 probiotics, Fig. 1). Of the 14 studies that reported on SDD decolonization measures, 5 RCT studies were included. The quality assessment of eligible RCT studies is shown in Fig. 2. The included uncontrolled trial were of high quality (NOS score 6–8), as shown in Supplementary Table S2. The basic characteristics of the selected studies are shown in Table 1. The decolonization protocols (route, type), duration/number of measures, and definition of eradication for each study are shown in Supplementary Table S3.
Pooled results of RCT studies on the effectiveness of SDD therapy
We identified five RCT studies that evaluated the effectiveness of decolonization with SDD therapy14,15,16,17,18. However, the reported follow-up time points were inconsistent and we did the available synthesis. Overall, SDD had a significantly better rate of decolonization at the end of treatment than controls (risk radio (RR) 3.30; 95% CI 1.78–6.14, I2 = 0%, Fig. 2.1), and a higher rate of decolonization at two weeks and 1 month than controls (RR 1.76; 95% CI 0.88–3.50, I2 = 71%) (Fig. 2.2) and (RR: 1.67; 95% CI 1.19–2.35, I2 = 0%) (Fig. 2.3).
Pooled results of n-RCT studies on the effectiveness of SDD therapy
In addition, the combined results of three n-RCT studies showed a higher rate of decolonization at 1 month after the end of treatment in SDD than in the control group (OR 4.01; 95% CI 1.88–8.56, I2 = 0%, Fig. 3) with a low degree of heterogeneity19,20,21.
Pooled results of uncontrolled trial studies on the effectiveness of SDD therapy
Furthermore, six uncontrolled trial studies with combined decolonization rates of 0.538 (95% CI 0.372–0.699) to 0.680 (95% CI 0.533–0.805) at different time points after SDD therapy were described (Fig. 4)22,23,24,25,26,27. The pooled results of three studies showed that at the end of treatment, the decolonization rate of SDD therapy was 0.555 (95% CI 0.338–0.773). The overall heterogeneity was large, which may be related to differences in study size and study ___location.
Trial sequential analysis (TSA)
The TSA results based on one month data after SDD decolonization are shown in Fig. 5. A total of seven studies with 542 case counts were included14,15,16,17,19,20,21, with an actual required sample size RIS of 970, and the RIS was estimated based on the following statistical indicators: probability of a category I error (α = 0.05); probability of a category II error (β = 0.2); relative risk reduction (RRR = 30 per cent) and incidence of decolonization in the control group (20 per cent, derived from historically reported data). Our results show that the cumulative Z-value of the current study (blue line) crosses the conventional horizontal line (green line) and also the TSA cut-off line (red line), and the figure indicates that the current study is still not as informative as hoped for. However, it can be concluded with certainty that the SDD therapy has a superior decolonization effect in patients colonized with ESBL-E/CRE compared to natural decolonization and placebo controls.
Faecal microbiota transplantation (FMT)
We identified eight studies, which focused on the efficacy of FMT decolonization for ESBL-E/CRE. In three n-RCT studies, 1 month after FMT therapy, the experimental group had a significantly better decolonization effect than the controls (OR 2.57; 95% CI 1.07–6.16, I2 = 6%, Fig. 6)28,29,30.
In the five single-arm trial studies, the combined decolonization rate one month after the end of FMT therapy was 61.3% (95% CI 38.1% to 84.4%, I2 = 47.17%, Fig. 7)31,32,33,34,35. In the study by Seong H et al., three months after the end of FMT therapy, the decolonization rate was 81.2% (13/16). In the study by Battipaglia et al., the decolonization rate was 70% (7/10) 14 months after the end of FMT therapy. Although there were insufficient included studies, this may have some trend and it is recommended that more cohort and randomized trial studies come in the future to focus on the effect of FMT on ESBL-E/CRE decolonization.
Probiotics
We identified three studies that evaluated the efficacy of probiotics as a measure for the decolonization of ESBL-E/CRE36,37,38. However, a meta-analysis could not be performed could to the low number of studies. The study by Ljungquist et al. intervened using a preparation containing eight live bacterial strains, which patients took once daily in the morning and evening for 2 months37. In the per-protocol analysis, no conclusion was drawn that the probiotic group was superior to placebo in terms of decolonization in ESBL-E carriers, according to the definition of primary outcome, when compared to the placebo control group (OR 2.71; 95% CI 0.49–19.4, P = 0.24). However, more participants in the probiotic group met the definition of elimination and the study may have been influenced by factors such as antibiotic treatment, small sample size, etc. The study by Choi E et al. used a comprehensive decolonization protocol to eradicate CRE carriage, including glycerol enemas, probiotic support containing Bacillus subtilis and Enterococcus faecalis, and skin and environmental cleansing. At the end of the trial, 13 CRE colonized nine of the patients (69.2%) were successfully decolonized, but no control group was identified which may limit the validity of the results36. Therefore, there is a need to continue to investigate the role of probiotics in ESBL-E/CRE decolonization38.
Discussion
ESBL-E/CRE colonization is an independent risk factor for ESBL-E/CRE infection. It poses an even greater threat in immunocompromised patients, such as solid organ and blood transplant recipients32,39. Well-designed decolonization strategies are currently a fascinating topic, and these include SDD, FMT, probiotics, and other novel therapies that we have not reviewed. To our knowledge, this is the first study to make a meta-analysis of decolonization strategies, and we assessed the role of the above three decolonization strategies in eliminating ESBL-E/CRE colonization. A systematic literature search identified 25 eligible articles. In five RCT studies comparing SDD therapy with placebo, the effect of decolonization at the end of treatment, after two weeks, and after 1 month was superior to that of placebo treatment. In addition, the rate of decolonization at one month after the end of SDD therapy was higher than in the controls in the n-RCT studies (OR: 4.01; 95% CI 1.88–8.56). After a single-group rate meta-analysis of uncontrolled trial studies, the results showed a combined decolonization rate of 53.8–68% at different time points. The TSA analysis also concludes that the evidence is credible and that there is reason to believe in the short-term benefits of SDD therapy for ESBL-E/CRE decolonization.
However, given the lack of evidence for long-term assessment, we cannot rule out a long-term failure of SDD decolonization, as our current pooled microbiological results also show a decreasing decolonization benefit with longer follow-up. In the RCT by Saidel-Odes et al., the difference in decolonization between the treatment and control groups at the sixth week of follow-up was no longer significant (RR: 1.71; 95% CI 0.85–3.44)14, and in the cohort study by Park et al., among 47 patients followed up for > 90 days, the difference in CRE decolonization rate between the two groups was not statistically significant (94.1% vs. 76.775%; P = 0.228)19. In contrast, the results of favourable short-term benefits of SDD decolonization are more encouraging. Early decolonization of ESBL-E/CRE can prevent the spread and outbreak of ESBL-E/CRE infection at an early stage, rapidly release colonised patients from single-room isolation and contact precautions, shorten the length of patients’ hospital stay, and reduce the burden of healthcare, which is critically important in areas with strained healthcare resources19,40.
As of now, another major factor hindering the adoption of SDD is the concern about antimicrobial resistance and clinical outcomes. The ESCMID-EUCIC study group evaluated the development of resistance in four studies5. Machuca et al. found that the proportion of patients who underwent decolonization who had gentamicin-resistant isolates in follow-up cultures was significant (13% (6/44) vs. 3% (1/33); P = 0.008)41. In the Oren et al. and Lubbert et al. studies, 14% (7/50) and 28% (4/14) of patients, respectively, developed secondary resistance to decolonizing agents16,21. Increased resistance was not observed by Saidel-Odes et al.14. However, newer studies to date give positive direction. Plantinga et al. established four cohorts to study CRGNB eradication, including 1314 unique microorganisms from 936 unique patients, and showed that SDD was more effective at eradicating CRGNB from either the rectum or respiratory tract compared to standard treatment at baseline. Moreover, during all study periods, colistin-resistant Enterobacteriaceae colonization rates were low (< 2.4% in the rectum and < 1.0% in the respiratory tract during unit-wide point prevalence surveys)40. In the Park SY et al. Studies, no significant difference in the acquisition of gentamicin resistance was detected between the two groups (23.1% vs 26.1%)19. In addition, Fariñas MC et al. detected mucin resistance in rectal flora in three liver recipients in the experimental group and one liver recipient in the control group group, but none of them developed multidrug-resistant infections17.
In addition to SDD, we have an usable combination of FMT and probiotic therapies. The three studies on FMT combined had a higher 1-month decolonization rate than the control group (OR: 2.57; 95% CI 1.07–6.16, P = 0.34), but further studies are needed to draw reliable conclusions. In addition, association studies with genetic analyses have reported changes in microbial composition and function before and after FMT, and a study by Bar-Yoseph et al. found that FMT may be an ecological strategy with long-lasting benefits that sustains the fight against gut microbes rather than temporarily eradicating ESBL-E/CRE carriage28. For probiotics, the RCT by Ljungquist et al. did not yield significant efficacy in their study, but gives us more insight. Gut bacterial diversity is often favourable and the efficacy of probiotic interventions may depend on the specific bacterial strains in the formulation, the current composition of the gut microbiota and the current and future dietary habits of the person taking it. To identify the few bacterial species with pre-specified health benefits for the general population is a major challenge and deserves further discussion37.
Several limitations of this study should be considered. Caution must be taken when interpreting the summary estimates because of the heterogeneity of studies. The lack of an internationally recognised definition of decolonization may partly explain this discrepancy. Similarly, study populations varied, with some studies using the general population and others using organ and blood transplant populations. Third, although we kept detailed records of decolonization programmes, heterogeneity was always present. Fourth, the lack of long-term evaluation of microbiological outcomes and clinical outcomes of decolonization strategies is our main limitation. Finally, the implementation of the ESBL-E/CRE decolonization strategy still needs to be further evaluated in terms of cost and practical benefits, and more research is needed.
Conclusion
Overall, as of December 2023, the currently available evidence-based evidence can only support our conclusion that SDD and FMT strategies have short-term benefits for ESBL-E/CRE decolonization. The effect of probiotic therapy on ESBL-E/CRE decolonization is an interesting topic that still requires more investigation. Further evaluation of the health economic benefits of decolonization strategies, as well as the long-term impact on microbiology, epidemiology, clinical outcomes, and development of drug resistance in the general or at-risk patient population through well-designed, multicentre, randomised controlled trials is necessary in the future.
Data availability
The datasets used and analysed in this meta-analysis are available from the corresponding author on reasonable request. The study protocol and statistical analysis plan are accessible on PROSPERO under the registration number CRD42023454638.
References
Bartsch, S. M. et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin. Microbiol. Infect. 23, e9–e16. https://doi.org/10.1016/j.cmi.2016.09.003 (2017).
Esteve-Palau, E. et al. Clinical and economic impact of urinary tract infections caused by ESBL-producing Escherichia coli requiring hospitalization: A matched cohort study. J. Infect. 71, 667–674. https://doi.org/10.1016/j.jinf.2015.08.012 (2015).
Siegel, S. J. & Weiser, J. N. Mechanisms of bacterial colonization of the respiratory tract. Annu. Rev. Microbiol. 69, 425–444. https://doi.org/10.1146/annurev-micro-091014-104209 (2015).
Bar-Yoseph, H., Hussein, K., Braun, E. & Paul, M. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J. Antimicrob. Chemother. 71, 2729–2739. https://doi.org/10.1093/jac/dkw221 (2016).
Tacconelli, E. et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 25, 807–817. https://doi.org/10.1016/j.cmi.2019.01.005 (2019).
Zeng, M. et al. Guidelines for the diagnosis, treatment, prevention and control of infections caused by carbapenem-resistant gram-negative bacilli. J. Microbiol. Immunol. Infect. 56, 653–671. https://doi.org/10.1016/j.jmii.2023.01.017 (2023).
Page, M. J. et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160. https://doi.org/10.1136/bmj.n160 (2021).
Yu, S. Y. et al. Clinical efficacy and safety of SARS-CoV-2-neutralizing monoclonal antibody in patients with COVID-19: A living systematic review and meta-analysis. J. Microbiol. Immunol. Infect. 56, 909–920. https://doi.org/10.1016/j.jmii.2023.07.009 (2023).
Centers for Disease Control and Prevention (CDC). Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE) 2015 Update. https://www.cdc.gov/hai/pdfs/cre/cre-guidance-508.pdf (2015).
Logan, L. K. & Weinstein, R. A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 215, S28–S36. https://doi.org/10.1093/infdis/jiw282 (2017).
Higgins, J. P. et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. https://doi.org/10.1007/s10654-010-9491-z (2010).
Huang, Y., Cai, X., Mai, W., Li, M. & Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 355, i5953. https://doi.org/10.1136/bmj.i5953 (2016).
Saidel-Odes, L. et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect. Control Hosp. Epidemiol. 33, 14–19. https://doi.org/10.1086/663206 (2012).
Huttner, B. et al. Decolonization of intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: A randomized, double-blind, placebo-controlled trial. J. Antimicrob. Chemother. 68, 2375–2382. https://doi.org/10.1093/jac/dkt174 (2013).
Oren, I. et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: A prospective controlled trial. Am. J. Infect. Control 41, 1167–1172. https://doi.org/10.1016/j.ajic.2013.04.018 (2013).
Fariñas, M. C. et al. Oral decontamination with colistin plus neomycin in solid organ transplant recipients colonized by multidrug-resistant Enterobacterales: A multicentre, randomized, controlled, open-label, parallel-group clinical trial. Clin. Microbiol. Infect. 27, 856–863. https://doi.org/10.1016/j.cmi.2020.12.016 (2021).
Stoma, I. et al. Decolonization of intestinal carriage of MDR/XDR gram-negative bacteria with oral colistin in patients with hematological malignancies: Results of a randomized controlled trial. Mediterr. J. Hematol. Infect. Dis. 10, e2018030. https://doi.org/10.4084/MJHID.2018.030 (2018).
Park, S. Y., Lee, J. S., Oh, J., Lee, S. H. & Jung, J. Effectiveness of selective digestive decolonization therapy using oral gentamicin for eradication of carbapenem-resistant Enterobacteriaceae carriage. Infect. Control Hosp. Epidemiol. 43, 1580–1585. https://doi.org/10.1017/ice.2021.492 (2022).
Pellicé, M. et al. Factors associated with short-term eradication of rectal colonization by KPC-2 producing Klebsiella pneumoniae in an outbreak setting. Front. Microbiol. 12, 630826. https://doi.org/10.3389/fmicb.2021.630826 (2021).
Lübbert, C. et al. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: A single-centre experience. Int. J. Antimicrob. Agents 42, 565–570. https://doi.org/10.1016/j.ijantimicag.2013.08.008 (2013).
Rieg, S. et al. Intestinal decolonization of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBL): A retrospective observational study in patients at risk for infection and a brief review of the literature. BMC Infect. Dis. 15, 475. https://doi.org/10.1186/s12879-015-1225-0 (2015).
Abecasis, F., Sarginson, R. E., Kerr, S., Taylor, N. & van Saene, H. K. Is selective digestive decontamination useful in controlling aerobic gram-negative bacilli producing extended spectrum beta-lactamases?. Microb. Drug Resist. 17, 17–23. https://doi.org/10.1089/mdr.2010.0060 (2011).
Tascini, C. et al. Oral gentamicin gut decontamination for prevention of KPC-producing Klebsiella pneumoniae infections: Relevance of concomitant systemic antibiotic therapy. Antimicrob. Agents Chemother. 58, 1972–1976. https://doi.org/10.1128/AAC.02283-13 (2014).
Zuckerman, T. et al. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 46, 1226–1230. https://doi.org/10.1038/bmt.2010.279 (2011).
Buehlmann, M., Bruderer, T., Frei, R. & Widmer, A. F. Effectiveness of a new decolonisation regimen for eradication of extended-spectrum β-lactamase-producing Enterobacteriaceae. J. Hosp. Infect. 77, 113–117. https://doi.org/10.1016/j.jhin.2010.09.022 (2011).
Troché, G., Joly, L. M., Guibert, M. & Zazzo, J. F. Detection and treatment of antibiotic-resistant bacterial carriage in a surgical intensive care unit: A 6-year prospective survey. Infect. Control Hosp. Epidemiol. 26, 161–165. https://doi.org/10.1086/502521 (2005).
Bar-Yoseph, H. et al. Oral capsulized fecal microbiota transplantation for eradication of carbapenemase-producing enterobacteriaceae colonization with a metagenomic perspective. Clin. Infect. Dis. 73, e166–e175. https://doi.org/10.1093/cid/ciaa737 (2021).
Huttner, B. D. et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 25, 830–838. https://doi.org/10.1016/j.cmi.2018.12.009 (2019).
Saïdani, N. et al. Faecal microbiota transplantation shortens the colonisation period and allows re-entry of patients carrying carbapenamase-producing bacteria into medical care facilities. Int. J. Antimicrob. Agents 53, 355–361. https://doi.org/10.1016/j.ijantimicag.2018.11.014 (2019).
Bilinski, J. et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: Results of a prospective, single-center study. Clin. Infect. Dis. 65, 364–370. https://doi.org/10.1093/cid/cix252 (2017).
Battipaglia, G. et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 104, 1682–1688. https://doi.org/10.3324/haematol.2018.198549 (2019).
Seong, H. et al. Fecal microbiota transplantation for multidrug-resistant organism: Efficacy and response prediction. J. Infect. 81, 719–725. https://doi.org/10.1016/j.jinf.2020.09.003 (2020).
Dinh, A. et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J. Hosp. Infect. 99, 481–486. https://doi.org/10.1016/j.jhin.2018.02.018 (2018).
Davido, B. et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage?. J. Hosp. Infect. 95, 433–437. https://doi.org/10.1016/j.jhin.2017.02.001 (2017).
Choi, E. et al. Comprehensive, multisystem, mechanical decolonization of vancomycin-resistant Enterococcus and Carbapenem-resistant Enterobacteriacease without the use of antibiotics. Medicine 100, e23686. https://doi.org/10.1097/MD.0000000000023686 (2021).
Ljungquist, O., Kampmann, C., Resman, F., Riesbeck, K. & Tham, J. Probiotics for intestinal decolonization of ESBL-producing Enterobacteriaceae: A randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect. 26, 456–462. https://doi.org/10.1016/j.cmi.2019.08.019 (2020).
Nouvenne, A., Ticinesi, A. & Meschi, T. Carbapenemase-producing Klebsiella pneumoniae in elderly frail patients admitted to medical wards. Ital. J. Med. 9, 116–119. https://doi.org/10.4081/itjm.2014.476 (2015).
Wang, H. et al. Analysis of risk factors for carbapenem resistant Klebsiella pneumoniae infection and construction of nomogram model: A large case-control and cohort study from Shanxi, China. Infect. Drug Resist. 16, 7351–7363. https://doi.org/10.2147/IDR.S442909 (2023).
Plantinga, N. L., Wittekamp, B. H. J., Brun-Buisson, C., Bonten, M. J. M., R-GNOSIS ICU Study Group. The effects of topical antibiotics on eradication and acquisition of third-generation cephalosporin and carbapenem-resistant Gram-negative bacteria in ICU patients; a post hoc analysis from a multicentre cluster-randomized trial. Clin. Microbiol. Infect. 26, 485–491. https://doi.org/10.1016/j.cmi.2019.08.001 (2020).
Machuca, I. et al. Oral decontamination with aminoglycosides is associated with lower risk of mortality and infections in high-risk patients colonized with colistin-resistant, KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 71, 3242–3249. https://doi.org/10.1093/jac/dkw272 (2016).
Funding
This work was supported by the Natural Science Foundation of Shanxi Province (202303021211121), the Science and Technology Innovation Project of Colleges and Universities of Shanxi Province (2024L090), and the General Soft Science Project of Shanxi Province (2017041037-2).
Author information
Authors and Affiliations
Contributions
Zhang, Wang and Tian conducted the literature search and reviewed relevant literature. All authors participated in the data acquisition. Zhang and Wang analysed and interpreted the data and wrote the manuscript, and Zhao designed the study and revised the manuscript. All authors contributed to the final version of the manuscript and have approved its submission. The final version of the report was approved by all authors. All authors had full access to the study data and shared the final responsibility for the decision to submit the manuscript for publication. Author Zhang and author Wang contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Hj., Wang, Hw., Tian, Fy. et al. Decolonization strategies for ESBL-producing or carbapenem-resistant Enterobacterales carriage: a systematic review and meta-analysis. Sci Rep 14, 24349 (2024). https://doi.org/10.1038/s41598-024-75791-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75791-6