Abstract
Idiopathic pulmonary fibrosis (IPF) is a severe interstitial lung disease characterized by a grim prognosis, in which various forms of cell death are significant contributors to its development. The objective of this study is to explore diagnostic biomarkers and molecular subtypes associated with PANoptosis in IPF, and to develop reliable diagnostic models based on PANoptosis-related mechanisms. The peripheral blood transcriptomic data of IPF from the Gene Expression Omnibus (GEO) database and PANoptosis-related genes from the GeneCards database were utilized to conduct differential gene expression analysis and weighted gene co-expression network analysis (WGCNA), identifying PANoptosis-related differentially expressed genes (PDEGs). We yielded 9 PDEGs and employed machine learning algorithms to identify 3 key diagnostic biomarkers for PANoptosis in IPF: MMP9, FCMR, NIBAN3. Consensus clustering algorithm was applied to recognize two PANoptosis-related subtypes. Cluster 1 exhibited higher abundance of adaptive immune response cells and significant enrichment in DNA damage and repair-related pathways. Cluster 2 exhibited greater prevalence of innate immune response cells and predominant enhancement in pathways related to lipid cholesterol metabolism and vascular remodeling. Diagnostic models were developed with the aid of clinical decision-making and a novel approach to the diagnosis and treatment for IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic pulmonary disease of uncertain origin characterized by progressive and irreversible lung fibrosis, resulting in a gradual deterioration of pulmonary function1. The development of IPF is believed to involve complex interactions among different cell types and signaling pathways, with alveolar epithelial cell injury playing a central role in its pathogenesis2,3. IPF predominantly impacts middle-aged and elderly males, presenting initially with symptoms such as dyspnea and dry cough. In advanced stages, the presence of pulmonary arterial hypertension and right heart failure complicates the prognosis of IPF4. In addition to lung transplantation, only two drugs-nintedanib and pirfenidone, are currently approved worldwide for the treatment of IPF, but neither of these drugs can stabilize lung function or improve quality of life, and there is a problem of resistance1,5. Therefore, it is imperative to identify reliable biomarkers for differentiating disease subtypes in patients with IPF to facilitate the implementation of tailored drug treatment protocols based on the unique characteristics of different molecular subtypes.
The process of cell death is essential for maintaining tissue homeostasis in humans, especially in instances of tissue damage or the resolution of infections, as it aids in the elimination of inflammatory and stromal cells involved in tissue repair mechanisms6. This process is essential for the restoration of cellular homeostasis and the maintenance of tissue structure and organ function7. PANoptosis, a form of cell death that involves pyroptosis, apoptosis, and necroptosis, plays a crucial role in this process8. PANoptosis is closely related to the development of acute lung injury, ARDS, COPD, and other lung diseases9,10,11,12. The activation of inflammation is detected in pulmonary fibrosis, affecting the epithelial-mesenchymal transition and the pyroptotic process of epithelial cells13. It is possible to improve disease outcomes with compounds and endogenous molecules targeting PANoptosome formation specifically14. Therefore, our research aims to find compounds that target PANoptotic molecules for better understanding and treatment development of IPF.
The application of machine learning algorithms in the field of bioinformatics has emerged as a powerful tool for the identification of biomarkers associated with complex diseases in recent years15,16,17. By leveraging proteomic data, previous research identified and validated 140 protein biomarkers through machine learning algorithms as potential drivers of IPF progression18. Support vector machine(SVM), least absolute shrinkage and selection operator regression (LASSO), and random forest(RF) machine learning models were used to construct diagnostic classifiers for interstitial lung disease and IPF19. Machine learning not only facilitates the discovery of potential therapeutic targets but also aids in the stratification of patients based on molecular subtypes, ultimately contributing to personalized medicine strategies in the management of IPF.
To investigate the interplay and impact of PANoptosis on IPF, this research employed transcriptomic sequencing data obtained from the Gene Expression Omnibus (GEO) database. Differential gene expression analysis, weighted gene co-expression network analysis (WGCNA), and machine learning algorithms were utilized to identify potential diagnostic biomarkers and to develop diagnostic models for PANoptosis in individuals with IPF. Additionally, consensus clustering analysis was employed to categorize IPF subtypes based on PANoptosis-related characteristics, followed by an examination of variations in the immune microenvironment and signaling pathways across these subtypes. The results of our study contributed to the identification of diagnostic biomarkers related to PANoptosis for IPF, and offer a theoretical foundation for the precise treatment and management in the clinic.
Materials and methods
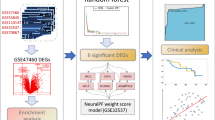
The flowchart of the study was shown in Fig. 1.
Data collection and preprocessing
The peripheral blood transcriptomic data was obtained from the GEO database using specific search criteria, including “Series” as the Entry type, “Homo sapiens” as the Top Organism, “Expression profiling by array” as the Study type, “peripheral blood” as the tissue, and a minimum sample size of 50. Ultimately, four datasets were selected (GSE28042, GSE33566, GSE93606, and GSE132607) (Table 1). The dataset of GSE33566 consisted of 93 IPF samples and 30 healthy samples. GSE28042 included 74 IPF samples and 19 healthy samples. GSE93606 contained 57 IPF samples and 20 healthy samples. GSE132607 comprised 74 IPF samples. GSE33566 was designated as the training set, and the rest were validation sets. The “SVA” R package was utilized to mitigate batch effects across the datasets.
Identification of core module genes in IPF by weighted gene co-expression network analysis
WGCNA was a bioinformatics algorithm utilized for the identification of co-expression networks linked to clinical traits of diseases20. WGCNA established a scale-free network through the application of weighted thresholds to genes, categorizing genes with comparable expression patterns based on expression matrices. Genes were segregated into distinct expression modules, and target genes that played a core role in the module could be more efficiently and accurately provided for biological research21. WGCNA was utilized to construct an unsupervised co-expression network to identify module genes associated with IPF. The dataset GSE33566 was transformed into a scale-free network using soft threshold β = 0.85, height cutoff = 0.25, and a minimum module gene size of 50. Pearson correlation analysis and p-values were employed to identify module genes that exhibited the strongest correlation with clinical features of the disease, which were then subjected to further core gene analysis.
Differential gene expression and enrichment analyses in IPF
In the GSE33566 dataset, differential gene expression analysis (DEGs) between IPF and healthy controls (HC) was performed by the “limma” package in R with criteria of |Log2fold change| > 0.58 and adjusted P value < 0.05. Subsequently, volcano plots and heatmaps for the DEGs were generated by the “ggVolcano” and “ComplexHeatmap” R packages. Enrichment analysis for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG22) pathways of the DEGs were carried out by the “clusterProfiler” R package, with a significance threshold of adjusted p < 0.05.
Identification of PANoptosis-related differentially expressed genes
By utilizing the GeneCards database, a total of 1324 PANoptosis-related genes were identified through the selection of genes with a relevance score exceeding 3(Table S1). Among these genes, there were 1313 apoptosis-related genes, 11 necroptosis-related genes, and 31 pyroptosis-related genes after eliminating duplicates. The intersection of PANoptosis-related genes, DEGs of IPF, and core module genes identified through WGCNA were referred to as PANoptosis-related differentially expressed genes (PDEGs).
Identification core diagnostic markers of IPF
The study utilized a combination of four machine learning algorithms, namely Least Absolute Shrinkage and Selection Operator (LASSO), Support Vector Machines-Recursive Feature Elimination (SVM-RFE), Boruta, and Random Forest (RF), to identify key diagnostic biomarkers for IPF based on PDEGs. The LASSO algorithm, implemented by the “glmnet” R package, was a statistical technique employed for feature selection and the creation of sparse models. The process of feature selection and elimination of irrelevant features was accomplished through the incorporation of penalty terms in the loss function of the model, leading to the contraction of model coefficients towards zero and the automatic identification of significant features. The SVM-RFE algorithm, implemented by the “e1071” R package, was a widely utilized machine learning technique for conducting feature selection analysis. This algorithm systematically eliminated underperforming features and refitted the model with the remaining features, culminating in the identification of an optimal subset that enhanced the overall performance of the model. The Boruta algorithm, employed through the “Boruta” R package, served as a machine learning technique specifically crafted for feature selection, with the primary objective of pinpointing the most pertinent features within a given dataset. An advantageous aspect of this algorithm lay in its capacity to effectively manage highly correlated features through the comparison of original features with randomly permuted counterparts. RF was utilized as a classifier, entailing the training of numerous decision trees on the provided samples to facilitate accurate predictions. Utilizing the “randomForest” package, this study employed multiple decision tree classifiers to determine the output class based on the majority class of individual tree outputs.
The intersection of these four machine learning algorithms was utilized to identify the core diagnostic biomarkers for IPF. The study further validated the differential expression of the core diagnostic biomarkers between IPF and HC groups in both the training and validation sets. Furthermore, spearman correlation analysis was utilized to investigate the relationship between core diagnostic biomarkers and immune cells, with a significance threshold established at p < 0.05 for inclusion. Lastly, a nomogram was generated from the core diagnostic biomarkers and clinical information utilizing the nomogram function from the rms library and the receiver operator characteristic(ROC) curve was drawn to assess the prediction accuracy of the nomogram.
Identification of different subtypes in IPF driven by PDEGs
The study employed the expression matrix of 9 PDEGs as input data for consensus clustering analysis using the “ConsensusClusterPlus” package, with the maximum number of subgroups set to 9. This approach resulted in the identification of distinct molecular subtypes of PANoptosis-related IPF based on various features. Principal component analysis was subsequently utilized to evaluate the classification efficacy, and a heatmap was constructed to visualize the expression patterns of PDEGs across the different subtypes. Kaplan-Meier survival curve (KM) was used to assess the survival differences between distinct subtypes. In addition, we compared the differences in clinical indexes-percent predicted forced vital capacity(FVC), diffusing capacity of the lung for carbon monoxide(DLCO), gender, and age between distinct subtypes.
The immune status of different subtypes of IPF patients was characterized by ssGSEA and GSVA enrichment analysis
The Single Sample Gene Set Enrichment Analysis (ssGSEA) method was frequently employed for immune infiltration analysis. It entailed the ranking of genes according to their expression levels and the computation of the cumulative distribution function for genes exhibiting elevated expression within immune gene sets, denoted as the gene set enrichment score23. We performed an evaluation of the ssGSEA scores for individual samples within specific gene sets, facilitating a comparison of the disparities in the relative abundance of immune cell types among distinct subtypes. Gene Set Variation Analysis (GSVA) was a computational method utilized to analyze gene set variations, implemented through the R package “GSVA”24. Pathway sets sourced from the molecular signatures database including hallmark, reactome, go, and kegg were employed as background gene sets. The R package “limma” was utilized to assess the biological functional disparities among distinct subtypes.
Construction and evaluation of diagnostic models of IPF
Utilizing the expression profile of PDEGs in IPF samples, three machine learning algorithms-SVM, RF, and GLM, were employed to develop diagnostic models for IPF. Subsequently, the diagnostic performance of the models was evaluated using the area under the receiver operating characteristic curve (ROC), where a larger area indicated superior diagnostic capability.
Screening of candidates for IPF
The Comparative Toxicogenomics Database (CTD) provided information on gene-drug-disease interactions and was commonly utilized for drug screening purposes. PDEGs were used to screen out potential target drugs for IPF in the CTD database.
Statistical analysis
Statistical analyses were performed using R software (version 4.2.1). Wilcoxon test was used to compare the differences among different molecular subtypes. Statistical significance was defined as p-value<0.05.
Results
Core module genes of IPF
In the GSE33566 dataset, the “WGCNA” R package was used to analyze the genes of IPF and HC group-related modules. The dataset included 93 samples from IPF patients and 30 samples from healthy controls, with a dendrogram illustrating the clustering of samples in Fig. 2A. A soft threshold of 5 was applied, resulting in an R2 value exceeding 0.9, indicating the presence of a scale-free network of gene connectivity as depicted in Fig. 2B. After the modules were merged, a total of 17 co-expression modules were identified, as shown in Fig. 2C. The brown and green modules displayed the strongest positive (r = 0.39, p = 7e-06) and negative (r = -0.32, p = 4e-04) correlations with IPF as depicted in Fig. 2D. The brown module consisted of 451 genes, while the green module consisted of 461 genes. 912 genes from the brown and green modules were identified as core genes linked to IPF for further examination.
WGCNA analysis of the GSE33566 dataset. (A) Sample clustering tree diagram. (B) Soft threshold analysis. (C) Genes with differential expression measured were clustered in the dendrogram. (D) Heatmap of the module–trait relationship. Each cell displays the corresponding correlation and p-value. IP: idiopathic pulmonary fibrosis; HC: healthy control.
Differentially expressed genes between IPF and HC
In the GSE33566 dataset, DEGs between IPF and HC were screened by the “limma” package. The results revealed 40 upregulated genes and 74 downregulated genes(Table S2). The volcano plot illustrated the top ten upregulated and downregulated genes (Fig. 3A), while the heatmap visualized the top 30 upregulated and downregulated genes (Fig. 3B). The GO and KEGG enrichment analysis indicated that a majority of the DEGs were enriched in various biological processes, including cytosolic ribosome, leukocyte activation, ribosome, cellular response to cytokine stimulus, positive regulation of cell death, regulation of immune response, and cytokine. The findings suggested a notable association between the pathogenesis and progression of IPF and immune inflammation, as illustrated in Fig. 3C and D.
Identification of differentially expressed genes in GSE33566. (A) Volcano map of the DEGs. Blue dots represented downregulated DEGs, red dots represented upregulated DEGs and grey dots showed genes with no significant difference. (B) Heatmap of the first 30 up-regulated and down-regulated DEGs. (C) GO enrichment analysis of the DEGs. (D) KEGG enrichment analysis of the DEGs.
Identification of PDEGs and core diagnostic markers of IPF
The overlap of PANoptosis-related genes, DEGs of IPF, and the most relevant module genes of WGCNA were defined as PANoptosis-related differentially expressed genes (PDEGs), consisting of 9 genes: MMP9, FCMR, TNFRSF25, HSPA1A, ADM, NIBAN3, CCR7, CD19, IL7R (Fig. 4A). The relationships among PDEGs were investigated through Spearman correlation analysis. The findings revealed a strong positive correlation between NIBAN3 and CD19, FCMR, as well as a notable negative correlation between MMP9 and TNFRSF25, FCMR, IL7R (Fig. 4B).
The Boruta algorithm was utilized to assess the significance of PDEGs, leading to the identification of the top five genes as MMP9, FCMR, HSPA1A, ADM, and NIBAN3 (Fig. 4C). Subsequently, SVM-RFE identified the four most significant targets as MMP9, FCMR, ADM, and NIBAN3 (Fig. 4D). The LASSO algorithm was then applied to reduce dimensionality, resulting in the selection of MMP9, FCMR, HSPA1A, and NIBAN3 as the key genes (Fig. 4E-F). Genes with a gini coefficient exceeding 5 were chosen as core genes through the RF approach (Fig. 4G). The final core diagnostic markers (MMP9, FCMR, and NIBAN3) were determined through the intersection of the four machine learning algorithms (Fig. 4H). GeneMANIA was employed to forecast genes that were functionally similar to the core diagnostic markers and to create a protein interaction network depicting genes that interacted with the core diagnostic markers. Each node in the diagram represented a gene, with various colors indicating different functions (Fig. 4I). A multivariate logistic regression model was used to develop a nomogram of core diagnostic markers and clinical data to predict the diagnosis of IPF(Fig. 4J). ROC curve analysis was conducted to determine the diagnostic performance of core diagnostic markers, with AUC = 76.8%, gender + age + core markers AUC = 93.3% (Fig. 4K).
Identifying of core diagnostic markers of IPF. (A) Venn diagram of PDEGs. (B) Correlation map of PDEGs. (C-G) Core diagnostic markers screening in Boruta, SVM, LASSO and RF algorithms. (H) Venn diagram of four algorithms selected core diagnostic markers of IPF. (I) PPI network of core diagnostic markers. (J) Nomogram of core diagnostic markers and clinical data. (K) ROC of the predictive capacity of core diagnostic markers.
Validation of core diagnostic markers expression in IPF
We validated the expression and diagnostic performance of three core diagnostic biomarkers in both training and validation datasets. Specifically, MMP9 was upregulated in IPF, while FCMR and NIBAN3 were downregulated in the training dataset GSE33566. The differences were statistically significant (Fig. 5A). Receiver operating characteristic (ROC) curve analysis revealed that MMP9 (AUC = 0.834), FCMR (AUC = 0.812), and NIBAN3 (AUC = 0.747) exhibited notable diagnostic values between IPF and HC groups (Fig. 5B). Furthermore, we confirmed the expression of the three genes in the validation set GSE93606, and the results were consistent with those from the training set. Although the expression of NIBAN3 in the validation set lacked statistical significance, its expression trend was consistent (Fig. 5C). ROC curve analysis demonstrated the reliability of MMP9 (AUC = 0.817), FCMR (AUC = 0.792), and NIBAN3 (AUC = 0.591) as core diagnostic biomarkers for IPF (Fig. 5D).
Correlation analysis between core diagnostic markers and immune cells
We examined the relationship between core diagnostic biomarkers and immune infiltrating cells by Spearman correlation analysis. Our findings revealed a statistically significant positive correlation between MMP9 and innate immune response cells, such as neutrophils, eosinophils, megakaryocytes, monocytes, and basophils (p < 0.001), as well as a significant negative correlation with adaptive immune response cells, including T cells, B cells, and Plasma cells (p < 0.001) (Fig. 6A). On the other hand, FCMR and NIBAN3 displayed divergent trends, showing a notable positive association with adaptive immune cell types such as T cells and B cells (p < 0.001), as well as a significant negative relationship with innate immune cells such as monocytes, neutrophils, and macrophages (p < 0.001) (Fig. 6B-C).
Subtypes driven by PDEGs expression in IPF
The ComBat algorithm was utilized for the integration and batch correction of blood transcriptomic sequencing samples from four datasets (GSE33566, GSE132607, GSE28042, GSE93606), leading to the formation of a substantial cohort consisting of 298 patients of IPF. The effectiveness of batch correction was assessed through principal component analysis (PCA) (Fig. 7A-B). Subsequently, cluster analysis was performed using the k-means clustering method within the ConsensusClusterPlus function, with the optimal clustering identified at k = 2 based on Cumulative Distribution Frequency (CDF) values and relative changes in the area under the CDF curve (Fig. 7C-D). PCA identified notable variations in transcriptomic expression between the two subtypes(Fig. 7E). Furthermore, the heatmap illustrated distinct expression patterns of PDEGs between the two subtypes. Specifically, NIBAN3, FCMR, IL7R, CD19, CCR7, and TNFRSF25 were found to be upregulated in cluster 1, while ADM, MMP9, and HSPA1A were upregulated in cluster 2 (Fig. 7F). We compared the survival differences between the two clusters and found that the overall survival rate of cluster 1 was higher than that of cluster 2 (p = 0.0041)(Fig. 7G, Table S3). Finally, we also analyzed the differences in clinical indexes-FVC, DLCO, gender, and age between the two clusters by Sankey diagram. The patients in the cluster 2 cohort exhibited moderate to severe FVC and severe DLCO, predominantly consisting of elderly male individuals(Fig. 7H, Table S4).
To delineate the differences between the two subtypes of IPF, we performed differential gene expression analysis and enrichment study. A total of 203 DEGs were identified, with 137 genes showing upregulation and 66 genes showing downregulation(Table S5). Enrichment analysis of the DEGs indicated significant involvement in immune-inflammatory responses, such as leukocyte activation, inflammatory response, cellular response to cytokine stimulus, and positive regulation of immune response (Fig. 7I), as well as a notable enrichment in KEGG signaling pathways such as cytokine-cytokine receptor interaction, neutrophil extracellular trap formation, and T cell receptor signaling pathway (Fig. 7J), indicating a close association of both IPF subtypes with immune-inflammatory processes.
PANoptosis molecular features of IPF subtypes. (A) Principal component distribution map before batch correction of four data series. (B) Principal component distribution map after batch correction of four data series. (C) Heatmap of consensus matrix at k = 2. (D) CDF curve of clustered samples. (E) PCA plot showing two subtypes after consensus clustering. (F) Heatmap showing gene expression differences between subtypes. (G) Kaplan-Meier survival curve between subtypes. (H) Sankey diagram showed the difference of clinical indexes between different subtypes. (I) GO enrichment analysis of the DEGs between subtypes. (J) KEGG enrichment analysis of the DEGs between subtypes.
Characterization of PANoptosis-related subtypes in IPF
The ssGSEA algorithm was utilized to perform a comparative analysis of immune cell expression levels among different subtypes of IPF. The findings revealed distinct immune cell profiles within the identified clusters. Cluster 1 demonstrated adaptive immune cell compositions with elevated levels of T cells and B cells, while Cluster 2 exhibited innate immune cell profiles characterized by increased infiltration of Neutrophils, Natural Killer cells, Macrophages, and Mast cells (Fig. 8A). To assess the effectiveness of immunotherapy in two subtypes, the sensitivity was determined by examining the levels of various immune checkpoints, indicating that cluster 1 displayed heightened sensitivity to immunotherapy. This was supported by the elevated expression levels of BTLA, CD226, CD27, CD28, CD40, HLA-DQA1, TIGIT, and SIRPA in cluster 1 (Fig. 8B). The results might explain that Cluster1 had a higher survival rate than Cluster2.
The GSVA pathway enrichment analysis revealed that cluster 1 was primarily associated with DNA damage and repair. The results were supported by GO enrichment in ribosomal subunits (Fig. 8C), Hallmark enrichment in DNA repair and oxidative phosphorylation pathways (Fig. 8D), KEGG enrichment analysis showing DNA replication (Fig. 8E), and Reactome enrichment in mitochondrial RNA degradation (Fig. 8F). On the other hand, Cluster 2 was predominantly linked to lipid cholesterol metabolism and vascular remodeling pathways, as evidenced by Hallmark enrichment in cholesterol homeostasis and angiogenesis pathways (Fig. 8D), KEGG enrichment analysis indicating glycerophospholipid metabolism (Fig. 8E), and Reactome enrichment in alpha-linolenic omegas and linoleic omega acid metabolism (Fig. 8F).
Molecular features of subtypes. (A) Expression abundance of immune cells in different subtypes. (B) Sensitivity analysis of immune checkpoints in different subtypes. (C) GO analysis in different subtypes. (D) Hallmark gene sets analysis in different subtypes. (E) KEGG analysis in different subtypes. (F) Reactome gene sets analysis in different subtypes. * P < 0.01, * * P < 0.001和 * * * P < 0.0001.
Construction of PANoptosis diagnostic models in IPF
Three machine learning algorithms, specifically generalized linear model (GLM), random forest (RF), and support vector machine (SVM) were employed to develop diagnostic models for PANoptosis in IPF. The combined datasets were partitioned randomly into training and validation sets at a 5:5 ratio. The area under ROC curves were used to evaluate the performance of the models. The training set yielded AUC values of 100%, 81.84%, and 84.03% for RF, SVM, and GLM, respectively (Fig. 9A). In the validation set, the AUC values for RF, SVM, and GLM were 74.99%, 72.05%, and 73.39%, respectively (Fig. 9B). The results indicated that the machine learning models of RF, SVM, and GLM performed well in terms of diagnostic accuracy, with RF showing the highest performance.
Potential therapeutic drugs for IPF patients based on PDEGs
Arsenic trioxide and dinoprostone were obtained from the CTD database by searching for IPF and 9 PDEGs, providing new choices for the clinical treatment of IPF patients in the future (Table S6).
Disscussion
PANoptosis was a complex and regulated form of programmed cell death that incorporated pyroptosis, apoptosis, and necroptosis8. Studies had identified a strong correlation between PANoptosis and various human diseases, such as neurodegenerative disorders, cancer, infectious diseases, and inflammatory conditions25,26,27,28,29,30. It was currently hypothesized that the suppression of myofibroblast apoptosis played a significant role in the development of IPF. Aging alveolar epithelial cells released substantial quantities of fibrogenic growth factors and inflammatory mediators, resulting in the mobilization and stimulation of myofibroblasts, which subsequently generated an overabundance of extracellular matrix, intensifying epithelial damage and myofibroblast activation2,3. Dysfunction in the clearance of apoptotic cells in the lungs triggered inflammation and fibrosis. Considering the diverse impact of PANoptosis in IPF, directing efforts towards either encouraging or impeding this process might present a viable therapeutic approach.
This study utilized differential gene expression analysis, WGCNA, and multiple machine learning algorithms to identify diagnostic biomarkers linked to PANoptosis by analyzing transcriptomic data of IPF. Furthermore, consensus clustering analysis was conducted using PDEGs to differentiate between two PANoptosis subtypes of IPF. Additionally, differences in immune microenvironment and pathway expression between the subtypes were analyzed. Arsenic trioxide and dinoprostone were identified as potential therapeutic compounds for IPF through the CTD database. Subsequently, three machine learning diagnostic models were developed using PDEGs to assist in the diagnosis and treatment of IPF patients, offering valuable insights for clinical management.
This research pinpointed three potential diagnostic biomarkers linked to PANoptosis in IPF: MMP9, FCMR, and NIBAN3. The process of lung injury was mediated by MMP2 and MMP9, which promoted lung pyroptosis31. Experimental studies demonstrated that MMP9 was involved in the development of fibrosis, and increased levels of MMP9 in the blood indicated a worse prognosis for those with IPF32. Currently, the monoclonal antibody andecaliximab, which targeted human MMP9, was being used in clinical settings to exert anti-fibrotic effects in the treatment of IPF32. NIBAN3, primarily expressed by B cells, functioned as a novel regulator of B cell receptor (BCR) signal transduction. B cell aggregates located proximal to active fibrotic areas within lung tissue were identified in a murine model of bleomycin-induced pulmonary fibrosis, underscoring the pivotal function of B cells as effector cells in the pathogenesis and advancement of IPF33. Interestingly, marked changes in BCR signaling pathways were elicited in IPF patients receiving nintedanib therapy34. These results proposed that the manipulation of NIBAN3 to regulate B cells and BCR signaling pathways could potentially offer a promising therapeutic approach for IPF. Fas apoptotic inhibitory molecule 3 (FCMR) played a crucial role in safeguarding cells from apoptosis triggered by FAS, TNF, and FADD within the immune system35. Research indicated that FCMR was indispensable for the differentiation and sustenance of B cells, as well as for the inhibition of autoreactive B cells and protection against antigenic threats. B cells lacking FCMR exhibited heightened susceptibility to apoptosis induced by BCR signal transduction36. Moreover, studies had shown that FCMR played a role in modulating the equilibrium between apoptosis and non-apoptotic receptor signal transduction by facilitating RIP1 ubiquitination37. In mice with asymptomatic mycobacterium tuberculosis infection, FCRM was highly expressed in the perivascular and peribronchial regions38. The anti-apoptotic function of FCRM opened a new therapeutic prospect for the treatment of IPF.
Inflammation and changes in the immune microenvironment were key factors in the pathogenesis of IPF. The activation of immune responses destroyed both the airways and the parenchyma, followed by collagen and fiber accumulation, resulting in fibrosis in the lungs39. Our study identified PDEGs that delineated two distinct subtypes of IPF: Cluster 1, characterized by adaptive immune response cell features, and Cluster 2, characterized by innate immune response cell features. Yin et al. had shown that IPF patients with high infiltration of innate immune cells had worse prognosis40. This was consistent with our findings, which indicated Cluster 1 demonstrated increased survival and sensitivity to immune checkpoints, implying potential enhancement in the effectiveness of immunotherapeutic treatments for these individuals. There were potential mechanisms of interaction between IPF and lung cancer in terms of epidemiology, mechanisms, and genetics41, so we investigated the effect of immune checkpoints on IPF. Prior preclinical studies had demonstrated that immune checkpoint inhibition could mitigate pulmonary fibrosis progression42. Therefore, our study revealed that different subtypes based on PDEGs showed distinct sensitivity to immune checkpoints, providing a new strategy for future clinical treatment of IPF patients.
Cluster 1 showed a significant enrichment in pathways associated with DNA damage and repair mechanisms. Multiple DNA damage events occurred during the development and progression of IPF, including macrophage polarization, excessive fibroblast proliferation, epithelial-mesenchymal transition, and compromised epithelial cell function43,44. After DNA damage occurred, the activation of DNA repair pathways through a sequence of signaling events was essential for the preservation of genomic integrity. Furthermore, the increased levels of oxidative DNA damage resulting from inflammatory reactions, as well as the apoptosis of epithelial cells and other cell types in IPF, emphasized the crucial function of DNA repair in safeguarding genomic stability and inhibiting fibrosis43,44. Conversely, Cluster 2 demonstrated prominent enrichment in pathways related to lipid cholesterol metabolism and vascular remodeling. In addition to inducing endoplasmic reticulum stress, lipids induced cell apoptosis and enhanced the expression of profibrotic biomarkers, which all contributed to the onset and progression of IPF45. High cholesterol levels in the alveolar cells led to pulmonary fibrosis. However, low cholesterol level might adversely affect the treatment outcome in patients with acute exacerbations of IPF, which might be associated with malnutrition46. The immune cells released inflammatory mediators and cytokines to bind to cytokines receptors on vascular endothelial cells, smooth muscle cells, and fibroblasts, leading to an inflammatory response47. Perivascular inflammation led to vasoconstriction and vascular remodeling. Endothelial dysfunction and vascular remodeling stimulated fibrogenesis in IPF48. The combined effect of these factors led to the occurrence of IPF. The identification of the two subtypes will help us better understand the pathogenesis of IPF, and guide early prevention of risk factors and early detection.
Utilizing the CTD database, we identified the PDEGs as promising targets for arsenic trioxide (ATO) and dinoprostone in the management of IPF. As a safe and effective anti-tumor drug, ATO had been found to counteract the pro-fibrotic activity in IPF, and prevent the proliferation and migration of lung fibroblasts49,50,51. By inducing necroptosis and ferroptosis, ATO promoted prophylactic and therapeutic immune responses against solid tumors52. The combination of XIAP (inhibitor of apoptosis protein) and ATO could be an attractive strategy for combating apoptosis-resistant cancer in clinical trials53. Through NF-κB and MAPK signaling pathways, ATO enhanced expression of BCL-2 in bronchial epithelial cells during apoptosis54. Maybe ATO would be more beneficial to treat IPF with a therapeutic strategy that directly targets PANoptosis pathways. Recent research had shown that the natural prostaglandin dinoprostone, also known as prostaglandin E2 (PGE2), in conjunction with roflumilast, effectively suppressed the pro-fibrotic effects of the extracellular matrix on lung fibroblasts. In addition to inhibiting proliferation and collagen synthesis, PGE2 modulates the conversion of fibroblasts to myofibroblasts55. Studies had shown that the COX-2/PGE2 system was involved in human cardiac fibroblast apoptosis, a process that played a key role in the normal physiological function of the heart56. PGE2 might be involved in the regulation of fibrogenic processes through various apoptotic pathways. Our results were consistent with prior studies, validating the robustness of our findings. Hence, the evaluation of the therapeutic efficacy of these two compounds in IPF patients merits clinical investigation.
In the study, we analyzed blood transcriptomic data of IPF from the GEO database, leading to the identification of three potential biomarkers. However, there were notable limitations to our research. Firstly, the datasets utilized in this study were predominantly derived from European and American populations, which raised concerns about the generalizability of our findings to other global populations. This limitation emphasized the necessity for broader demographic representation in future studies. Furthermore, our results were based solely on extensive bioinformatics analyses, lacking validation through molecular experiments and population cohorts. Addressing these gaps will be a primary focus of our subsequent research endeavors.
Conclusion
This study aimed to investigate the relationship between PANoptosis and IPF, identifying two molecular subtypes of IPF and elucidating their molecular characteristics by utilizing peripheral blood expression profile. Additionally, potential biomarkers with clinical diagnostic and therapeutic implications were identified. Machine learning diagnostic models were developed to explore the impact of PANoptosis-related gene modifications on IPF.
Data availability
The datasets generated during the current study are available in the GEO database (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE28042, GSE33566, GSE93606, and GSE132607. All data analysed during this study are included in this published article and its supplementary information files.
References
Spagnolo, P. et al. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol. Ther. 222, 107798. https://doi.org/10.1016/j.pharmthera.2020.107798 (2021).
Richeldi, L., Collard, H. R. & Jones, M. G. Idiopathic pulmonary fibrosis. Lancet (London England). 389, 1941–1952. https://doi.org/10.1016/s0140-6736(17)30866-8 (2017).
Moss, B. J., Ryter, S. W. & Rosas, I. O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 17, 515–546. https://doi.org/10.1146/annurev-pathol-042320-030240 (2022).
Podolanczuk, A. J. et al. Idiopathic pulmonary fibrosis: state of the art for 2023. Eur. Respir J. 61https://doi.org/10.1183/13993003.00957-2022 (2023).
Kreuter, M., Bonella, F., Wijsenbeek, M., Maher, T. M. & Spagnolo, P. Pharmacological Treatment of Idiopathic Pulmonary Fibrosis: Current Approaches, Unsolved Issues, and Future Perspectives. BioMed research international 329481 (2015). (2015). https://doi.org/10.1155/2015/329481
Tower, J. Programmed cell death in aging. Ageing Res. Rev. 23, 90–100. https://doi.org/10.1016/j.arr.2015.04.002 (2015).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell. 116, 205–219. https://doi.org/10.1016/s0092-8674(04)00046-7 (2004).
Zhu, P. et al. Advances in mechanism and regulation of PANoptosis: prospects in disease treatment. Front. Immunol. 14, 1120034. https://doi.org/10.3389/fimmu.2023.1120034 (2023).
Chen, S., Jiang, J., Li, T., Huang, L. & PANoptosis Mechanism and role in Pulmonary diseases. Int. J. Mol. Sci. 24https://doi.org/10.3390/ijms242015343 (2023).
Cui, Y. et al. MiR-29a-3p improves Acute Lung Injury by reducing alveolar epithelial cell PANoptosis. Aging Dis. 13, 899–909. https://doi.org/10.14336/ad.2021.1023 (2022).
He, Y. Q. et al. Ursodeoxycholic acid alleviates sepsis-induced lung injury by blocking PANoptosis via STING pathway. Int. Immunopharmacol. 125, 111161. https://doi.org/10.1016/j.intimp.2023.111161 (2023).
Messaoud-Nacer, Y. et al. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell. Death Dis. 13, 269. https://doi.org/10.1038/s41419-022-04664-5 (2022).
Jäger, B. et al. The NLRP3-Inflammasome-Caspase-1 pathway is upregulated in idiopathic pulmonary fibrosis and Acute exacerbations and is inducible by apoptotic A549 cells. Front. Immunol. 12, 642855. https://doi.org/10.3389/fimmu.2021.642855 (2021).
Wang, L. et al. Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases. Cell. Death Dis. 14, 851. https://doi.org/10.1038/s41419-023-06370-2 (2023).
Greener, J. G., Kandathil, S. M., Moffat, L. & Jones, D. T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell. Biol. 23, 40–55. https://doi.org/10.1038/s41580-021-00407-0 (2022).
Pan, J. et al. Unsupervised machine learning identifies predictive progression markers of IPF. Eur. Radiol. 33, 925–935. https://doi.org/10.1007/s00330-022-09101-x (2023).
Furukawa, T. et al. A comprehensible machine learning tool to differentially diagnose idiopathic pulmonary fibrosis from other chronic interstitial lung diseases. Respirology. 27, 739–746. https://doi.org/10.1111/resp.14310 (2022).
Oldham, J. M. et al. Proteomic biomarkers of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir Crit. Care Med. 209, 1111–1120. https://doi.org/10.1164/rccm.202301-0117OC (2024).
Huang, Y. et al. Machine learning of plasma proteomics classifies diagnosis of interstitial lung disease. Am. J. Respir Crit. Care Med. 210, 444–454. https://doi.org/10.1164/rccm.202309-1692OC (2024).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9, 559. https://doi.org/10.1186/1471-2105-9-559 (2008).
Dong, J. & Horvath, S. Understanding network concepts in modules. BMC Syst. Biol. 1, 24. https://doi.org/10.1186/1752-0509-1-24 (2007).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–d592. https://doi.org/10.1093/nar/gkac963 (2023).
Charoentong, P. et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell. Rep. 18, 248–262. https://doi.org/10.1016/j.celrep.2016.12.019 (2017).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7. https://doi.org/10.1186/1471-2105-14-7 (2013).
Pan, H., Pan, J., Li, P. & Gao, J. Characterization of PANoptosis patterns predicts survival and immunotherapy response in gastric cancer. Clin. Immunol. (Orlando Fla). 238, 109019. https://doi.org/10.1016/j.clim.2022.109019 (2022).
Lin, J. F. et al. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal. Transduct. Target. Therapy. 7, 54. https://doi.org/10.1038/s41392-022-00889-0 (2022).
Yi, X. et al. Construction of PANoptosis signature: novel target discovery for prostate cancer immunotherapy. Mol. Therapy Nucleic Acids. 33, 376–390. https://doi.org/10.1016/j.omtn.2023.07.010 (2023).
Zhang, E. & Dai, F. Diagnostic model for Alzheimer’s Disease based on PANoptosis-Related genes. J. Alzheimer’s Disease: JAD. 97, 813–828. https://doi.org/10.3233/jad-231103 (2024).
Jiang, W., Deng, Z., Dai, X., Zhao, W. & PANoptosis A new insight into oral infectious diseases. Front. Immunol. 12, 789610. https://doi.org/10.3389/fimmu.2021.789610 (2021).
Karki, R. et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 7, eabo6294. https://doi.org/10.1126/sciimmunol.abo6294 (2022).
Zhou, P., Song, N. C., Zheng, Z. K., Li, Y. Q. & Li, J. S. MMP2 and MMP9 contribute to lung ischemia-reperfusion injury via promoting pyroptosis in mice. BMC Pulm Med. 22, 230. https://doi.org/10.1186/s12890-022-02018-7 (2022).
Espindola, M. S. et al. Differential responses to Targeting Matrix Metalloproteinase 9 in idiopathic pulmonary fibrosis. Am. J. Respir Crit. Care Med. 203, 458–470. https://doi.org/10.1164/rccm.201910-1977OC (2021).
Prêle, C. M. et al. Plasma cell but not CD20-mediated B-cell depletion protects from bleomycin-induced lung fibrosis. Eur. Respir. J. 60https://doi.org/10.1183/13993003.01469-2021 (2022).
Neys, S. F. H. et al. Aberrant B cell receptor signaling in Naïve B cells from patients with idiopathic pulmonary fibrosis. Cells. 10https://doi.org/10.3390/cells10061321 (2021).
Kubagawa, H. et al. Nomenclature of Toso, Fas apoptosis inhibitory molecule 3, and IgM FcR. J. Immunol. (Baltimore Md. : 1950). 194, 4055–4057. https://doi.org/10.4049/jimmunol.1500222 (2015).
Choi, S. C. et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. Journal of immunology (Baltimore, Md.: 190, 987–996 (2013). (1950). https://doi.org/10.4049/jimmunol.1202227
Nguyen, X. H. et al. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. 118, 598–608. https://doi.org/10.1182/blood-2010-10-313643 (2011).
Koyuncu, D. et al. B cells in perivascular and peribronchiolar granuloma-associated lymphoid tissue and B-cell signatures identify asymptomatic Mycobacterium tuberculosis lung infection in Diversity Outbred mice. Infect. Immun. 92, e0026323. https://doi.org/10.1128/iai.00263-23 (2024).
Xu, F. et al. The transition from normal lung anatomy to minimal and established fibrosis in idiopathic pulmonary fibrosis (IPF). EBioMedicine. 66, 103325. https://doi.org/10.1016/j.ebiom.2021.103325 (2021).
Yin, Y. Q. et al. Construction of prediction model of inflammation related genes in idiopathic pulmonary fibrosis and its correlation with immune microenvironment. Front. Immunol. 13, 1010345. https://doi.org/10.3389/fimmu.2022.1010345 (2022).
Samarelli, A. V. et al. Molecular mechanisms and Cellular Contribution from Lung Fibrosis to Lung Cancer Development. Int. J. Mol. Sci. 22https://doi.org/10.3390/ijms222212179 (2021).
Tan, J., Xue, Q., Hu, X. & Yang, J. Inhibitor of PD-1/PD-L1: a new approach may be beneficial for the treatment of idiopathic pulmonary fibrosis. J. Transl Med. 22, 95. https://doi.org/10.1186/s12967-024-04884-7 (2024).
Wolters, P. J., Collard, H. R. & Jones, K. D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 9, 157–179. https://doi.org/10.1146/annurev-pathol-012513-104706 (2014).
Cheng, P., Li, S. & Chen, H. Macrophages in Lung Injury, Repair, and fibrosis. Cells. 10https://doi.org/10.3390/cells10020436 (2021).
Chen, R. & Dai, J. Lipid metabolism in idiopathic pulmonary fibrosis: from pathogenesis to therapy. J. Mol. Med. (Berl). 101, 905–915. https://doi.org/10.1007/s00109-023-02336-1 (2023).
Hachisu, Y. et al. Possible serological markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Med. (Kaunas). 55. https://doi.org/10.3390/medicina55050132 (2019).
Guihaire, J. et al. Immunomodulation Therapy using Tolerogenic macrophages in a Rodent Model of Pulmonary Hypertension. Stem Cells Dev. 30, 515–525. https://doi.org/10.1089/scd.2021.0007 (2021).
Yanagihara, T. et al. Vascular-parenchymal cross-talk promotes Lung Fibrosis through BMPR2 Signaling. Am. J. Respir Crit. Care Med. 207, 1498–1514. https://doi.org/10.1164/rccm.202109-2174OC (2023).
Joannes, A. et al. Arsenic trioxide inhibits the functions of lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Toxicol. Appl. Pharmcol. 441, 115972. https://doi.org/10.1016/j.taap.2022.115972 (2022).
Luo, F. et al. Arsenic trioxide inhibits transforming growth factor-β1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir. Res. 15, 51. https://doi.org/10.1186/1465-9921-15-51 (2014).
Zhong, L. et al. Arsenic trioxide inhibits the differentiation of fibroblasts to myofibroblasts through nuclear factor erythroid 2-like 2 (NFE2L2) protein and the Smad2/3 pathway. J. Cell. Physiol. 234, 2606–2617. https://doi.org/10.1002/jcp.27073 (2019).
Chen, J. et al. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell. Mol. Immunol. 20, 51–64. https://doi.org/10.1038/s41423-022-00956-0 (2023).
Shi, L. et al. Clinically used drug arsenic trioxide targets XIAP and overcomes apoptosis resistance in an organoid-based preclinical cancer model. Chem. Sci. 15, 8311–8322. https://doi.org/10.1039/d4sc01294a (2024).
Tang, J. et al. Arsenic trioxide induces expression of BCL-2 expression via NF-κB and p38 MAPK signaling pathways in BEAS-2B cells during apoptosis. Ecotoxicol. Environ. Saf. 222, 112531. https://doi.org/10.1016/j.ecoenv.2021.112531 (2021).
Moshkovitz, N., Shochet, E., Shitrit, D. & G. & Prostaglandin E2 (PGE2) and Roflumilast involvement in IPF Progression. Int. J. Mol. Sci. 24https://doi.org/10.3390/ijms241512393 (2023).
Yang, C. C., Hsiao, L. D., Shih, Y. F., Su, M. H. & Yang, C. M. Sphingosine 1-Phosphate-upregulated COX-2/PGE(2) System contributes to Human Cardiac Fibroblast apoptosis: involvement of MMP-9-Dependent transactivation of EGFR Cascade. Oxid. Med. Cell. Longev. 2022 (7664290). https://doi.org/10.1155/2022/7664290 (2022).
Funding
This project was supported by grants from the Shanxi Province Applied Basic Research Project (No. 202303021222360), Shanxi Provincial Research Foundation for Basic Research (No. 202103021223011), Shanxi Provincial Research Foundation for Basic Research (No. 202103021223425), the special fund for Science and Technology Innovation Teams of Shanxi Province (No. 202304051001045) and Shanxi Graduate Education Innovation Plan Project (No. 2023KY357).
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important content. Li Wu and Qi Wang developed the methodology and acquired the related data. Conception and design of the study: Zhiqiang Chen and Wanjin Guo. Acquisition of data: Rui Xu and Kaixin Bi. Analysis and interpretation of data: Jing Li, Yabing Liu and Jia Wang. Drafting the article: Li Wu, Yang Liu and Yifan Zhang. Revising the article critically: Qi Wang and Zhiqiang Chen. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Medical writing, editorial, and other assistance
We gratefully acknowledge contributions from the GEO, GeneCards and the Comparative Toxicogenomics Databases.
Disclosures
All named authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with ethics guidelines
This article does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, L., Liu, Y., Zhang, Y. et al. Identification of PANoptosis-related genes for idiopathic pulmonary fibrosis by machine learning and molecular subtype analysis. Sci Rep 14, 24068 (2024). https://doi.org/10.1038/s41598-024-76263-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76263-7