Abstract
White blood cell (WBC) ratios are used as diagnostic markers for various inflammatory or tumor diseases as well as stress in a broad range of species. The aim of this work was to provide data on five WBC ratios (neutrophil-to-lymphocyte ratio [NLR], band neutrophil-to-lymphocyte ratio [BLR], band neutrophil-to-neutrophil-to-lymphocyte ratio [BNLR], band neutrophil-to-neutrophil ratio [BNR] and lymphocyte-to-monocyte ratio [LMR]) in South American camelids (SAC) and characterize their association with demographic and important diagnostic parameters. Medical records of 307 SAC (275 alpacas, 32 llamas) that were presented at a veterinary teaching hospital were evaluated retrospectively. WBC ratios were calculated based on hematologic results of the initial blood samples. The influence of species, sex, age, body condition score, WBC count, and anemia on those ratios was investigated using descriptive statistics and generalized linear models. NLR, BLR and LMR were found to be significantly influenced by age and WBC count. Associations of individual WBC ratios with species, nutritional status or an anemic condition could be detected. NLR was 4.32; 2.31–7.81 (median; IQR), BLR 0.24; 0.07–0.87, BNLR 3.66 × 10–3; 1.17 × 10–3 − 14.20 × 10–3, BNR 0.06; 0.02–0.15 and LMR was 7; 3.54–14.67. Our data might serve as a basis for further studies on WBC ratios in SAC. The animals in this study showed a variety of underlying diseases. It should hence be noted that these values are orientation values and provide a representative overview of conditions in a clinic, but are not suitable as reference values for healthy animals.
Similar content being viewed by others
Introduction
Clinical pathology plays an important role in the veterinary care of South American camelids (SAC, alpacas and llamas). Due to the stoic character of these animals, clinical symptoms of disease like emaciation and anemia often remain undetected for a long time1,2,3. A large number of the animals presented at the clinic exhibit a reduced Body Condition Score (BCS) and a decreased packed cell volume (PCV), which is usually closely associated with each other2. The BCS is not only associated with the red blood count, but also with the WBC (white blood cell) differential. In llamas, we observed increased neutrophils and decreased lymphocytes in animals with low BCS2. Based on conventional WBC differential data, further hematologic parameters can easily be calculated: the neutrophil-to-lymphocyte ratio (NLR), which is used as a prognostic marker for tumor diseases4, or inflammatory conditions such as chronic enteropathies5 or mastitis6 in other species. The neutrophil-to-lymphocyte ratio was already mentioned for both alpacas7,8,9 and llamas10,11,12. Further information on the characteristics of these parameters is, however, lacking in those publications, which are limited to reference intervals of a few animals or individual case reports. Nonetheless, NLR may also be of particular interest for SAC, as it has also potential not to be used only as a marker for disease, but also as a stress marker, which was already studied in other species13,14,15,16. Many factors like climate or social isolation can lead to stress in SAC17,18. Although there is little data on SAC compared to other species, stress is assumed to be a factor contributing to the development of gastric ulcers, which are commonly observed in camelid medicine19,20.
In addition to the NLR, other WBC ratios like lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), band neutrophil-to-lymphocyte ratio (BLR), or band neutrophil-to-neutrophil-to-lymphocyte ratio (BNLR) have been reported as prognostic markers for different inflammatory or tumor diseases in veterinary medicine21,22,23,24. However, to date, information on these parameters for SAC is completely absent in the literature.
In the present retrospective study, we therefore characterized five different WBC ratios in 307 SACs presented at our clinic. In addition to the ratios described so far (NLR, LMR, BLR, BNLR), we added the ratio of band neutrophils-to-neutrophils (BNR), which to our knowledge has not been described in the literature but may indicate the age progression of circulating neutrophils independently of lymphocytes. A higher proportion of young neutrophils (band neutrophils, metamyelocytes, myelocytes), preliminary stages of segmented neutrophils25, would increase the BNR.
Possible influences of species, sex, age, body condition score, WBC count, and anemia on each of those leukocyte ratios were tested using generalized linear models.
Methods
Data collection
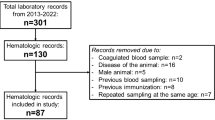
For the retrospective data analysis, the findings of a total of 307 SACs were compiled from the electronic patient files and from the laboratory information system of the Clinic for Swine and Small Ruminants, Forensic Medicine and Ambulatory Service, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany. The study was approved by the Ethics Committee of the University of Veterinary Medicine Hannover (approval code: TiHo-REC_14_10_24), all methods were performed in accordance with the relevant guidelines and regulations.
Data were collected from 275 alpacas (median age: 1586 days; IQR: 546–2636 days) and 32 llamas (median age: 1731 days; IQR: 704–3917 days) that were presented at the clinic between May 2019 and January 2023. As part of the clinical routine, a standardized clinical examination was carried out on admission of the animals, and a blood sample (EDTA Monovette 9 mL K3E, Sarstedt AG & Co. KG, Nümbrecht, Germany) was taken from the jugular vein of the animal and processed in the clinic’s own laboratory. Details of the hematologic examination were already described in a previous study2.
The following data were used in this study:
Clinical data
-
Species: alpaca (A) or llama (L).
-
Sex: male (m) or female (f).
-
Age [days]: the age was calculated by subtracting the date of birth from the date of examination.
-
Season: the month of admission of the animal to the clinic.
-
BCS: BCS was determined by palpation of the lumbar spine and scored in 0.5 increments on a scale from 1 (emaciated) to 5 (obese)26.
Hematologic data
-
PCV [l/l]: PCV was determined by centrifugation in microhematocrit capillary2.
-
WBC count [109/l]: WBC count was determined microscopically using a Neubauer counting chamber; if normoblasts (nucleated red blood cells) were found, the WBC count was corrected mathematically2.
-
WBC ratios [dimensionless parameters]: WBC ratios were calculated from the results of the microscopic WBC differentiation. Therefore, 200 WBC from each stained blood smear were differentiated by trained laboratory personnel2.
-
NLR = (segmented neutrophils [%] + band neutrophils [%] + metamyelocytes [%] + myelocytes [%]) / lymphocytes [%].
-
BLR = (band neutrophils [%] + metamyelocytes [%] + myelocytes [%]) / lymphocytes [%].
-
BNLR = [(band neutrophils [%] + metamyelocytes [%] + myelocytes [%]) / segmented neutrophils [%]] / lymphocytes [%].
-
BNR = (band neutrophils [%] + metamyelocytes [%] + myelocytes [%]) / (segmented neutrophils [%] + band neutrophils [%] + metamyelocytes [%] + myelocytes [%])
-
Further categorical data were also created from some of the clinical and laboratory diagnostic findings:
-
BCS-category: the animals were divided into two groups according to their nutritional status: lean-yes (BCS < 3) and lean-no (BCS ≥ 3).
-
Anemia-category: the animals were divided into two groups according to their PCV: anemia-yes (PCV ≤ 25 L/L) and anemia-no (PCV > 25 L/L).
-
WBC-category: the animals were divided into three groups according to their WBC count: WBC-decreased (leukopenia), WBC-normal (WBC count within the reference interval), WBC-increased (leukocytosis). The classification into these groups was made according to the reference intervals of Hengrave-Burri et al.27.
-
Normoblasts-category: the animals were divided into two groups according to the occurrence of normoblasts in the peripheral blood: normoblasts-yes and normoblasts-no.
Only those animals for which all the aforementioned parameters were available were included in the study.
Statistical analysis
Descriptive statistics
The WBC ratios were characterized using descriptive statistics in SAS (SAS Enterprise Guide 7.1) [mean (M), standard deviation (SD), median (Mdn), minimum (Min), maximum (Max), lower quartile (Q1), upper quartile (Q3)].
Generalized linear modeling
Modeling was conducted applying the software R28. The five leukocyte ratios (NLR, BLR, BNLR, BNR, and LMR) were examined as dependent variables using generalized linear models with Gaussian assumption for effects of several fixed factors in each two basic models. To achieve normality, NLR values were log transformed. Both models included species, sex, and age as important demographic factors as well as the month of sample collection (season). Model 1 also included BCS, WBC count, and PCV. Model 2 additionally included BCS-category, WBC-category, Anemia-category, and Normoblasts-category. Models were calculated using the “lme4” package29. An automated model selection approach was utilized to choose the most relevant combinations of fixed factors from the two basic models. The model selection process involved calculating all possible combinations of variables within each basic model using the “dredge()” function from the R package “MuMIn”30. The optimal combination of factors from a set of multiple candidate models was determined using the Akaike Information Criterion (AIC), following the methodology outlined by Burnham and Anderson31. To address small sample sizes, the corrected AIC (AICc) method recommended by Hurvich and Tsai32 was employed. Models with the highest statistical support and those with similarly low AICc values (∆AICc < 2) were considered for interpretation. Post hoc tests, specifically Tukey tests, were conducted to examine the significant effects of factors with more than two categories. These tests were performed using the “multcomp” package33. P- values less than 0.05 were considered as statistical significant.
Results
A detailed overview of the results of the descriptive statistics can be found in Supplementary Table S1. The results of the generalized linear models are displayed in Supplementary Table S2. Figure 1 provides a simplified schematic illustration of the modeling results, represented by the results of the models with lowest AICc values (best models). Relevant findings for each ratio will be explained in the following paragraphs.
Neutrophil-to-lymphocyte ratio (NLR)
The median NLR of the investigated animals was 4.32 (Mdn; IQR: 2.31–7.81) and revealed a broad range with values up to 99. Significant effects on NLR were found for age, WBC count, WBC-category, Anemia-category, and PCV. Animals with leukocytosis had a higher NLR of 9.61 (Mdn; IQR: 6.45–18.8) than animals with a leukocyte count in the reference interval (Mdn: 3.84; IQR: 2.51–7.10) or animals with leukopenia (Mdn: 2.84; IQR: 1.76–5.07). Animals with anemia revealed a higher NLR (Mdn: 5.20; IQR: 2.69–9.03) than animals without anemia (Mdn: 4.09; IQR: 2.18–7.10). Increasing age as well as decreasing PCV led to increasing NLR. Although the occurrence of normoblast was included in the best model of Model2, modeling did not reveal any significant effects on NLR. Species, sex, season, BCS, and BCS-category were not included in the models with lowest AICc values and therefore probably had no particular influence on the NLR.
Band neutrophil-to-lymphocyte ratio (BLR)
The BLR of the animals revealed a median of 0.24 (IQR: 0.07–0.87) and ranged up to 11. Species, age, BCS-category, WBC count, WBC-category led to significant differences in this parameter. Alpacas had lower BLR (Mdn: 0.23; IQR: 0.07–0.84) than llamas (Mdn: 0.28; IQR: 0.09–1.47), lean animals had higher BLRs (Mdn: 0.41; IQR: 0.12–1.15) than non-lean animals (Mdn: 0.15; IQR: 0.05–0.53). Animals with leukocytosis had higher BLRs (Mdn: 0.55; IQR: 0.20–1.76) than animals with a leukocyte count in the reference interval (Mdn: 0.19; IQR: 0.05–0.72) or animals with leukopenia (Mdn: 0.25; IQR: 0.05–0.79). Sex, season, BCS, PCV, Anemia-category, and Normoblasts-category were not included in the best models.
Band neutrophil-to-neutrophil-to-lymphocyte ratio (BNLR)
The median BNLR was 3.66 × 10–3 (IQR: 1.17 × 10–3 − 14.20 × 10–3); this WBC ratio had a wide range of up to 185.19 × 10–3.
None of the investigated parameters were included in the best model.
Band neutrophil-to-neutrophil ratio (BNR)
The BNR was 0.06 (Mdn; IQR: 0.02–0.15), with the highest value in a single animal of 0.87. Significant differences in the BNR were shown for the parameter BCS-category. Investigations of BCS-category revealed a statistical trend; lean animals had higher values (Mdn: 0.08; IQR: 0.02–0.23) than non-lean animals (Mdn: 0.04; IQR: 0.01–0.11). Although the WBC count was included in the best model of Model 1, no significant effect on BNR was detected. The parameters species, sex, age, season, BCS, WBC-category, Anemia-category, PCV, and Normoblasts-category were not included in the best models.
Lymphocyte-to-monocyte ratio (LMR)
The LMR had a median of 7 (IQR: 3.54–14.67) and a maximum of 97. As there can be no 0 values in the LMR, there is always a lower limit above 0. The lowest value recorded in an animal was 0.13. Significant influences were observed for the parameters age, WBC count, and WBC-category. The investigation of sex and BCS-category revealed statistical trends. LMR decreased with age of the animals. Leukocytosis was associated with lower LMR (Mdn: 4; IQR: 1.8–7.33), whereas animals with a leukocyte count in the reference interval (Mdn: 8; IQR: 4–17) or animals with leukopenia (Mdn: 8.25; IQR: 3.67-15) revealed higher LMR. Although there was a statistical trend concerning sex, both males and females revealed a median of 7 in the LMR. However, the mean in females (12.51) was slightly higher than in males (10.25). Furthermore, lean animals had lower ratios (Mdn: 5.71; IQR: 3.11–12.63) than non-lean animals (Mdn: 8.2; IQR: 4–15).
Simplified schematic presentation of the generalized linear model results of WBC ratios of 307 South American camelids (275 alpacas and 32 llamas) that were presented to a veterinary teaching hospital. Since some parameters are based on more than one single p-value, the presentation has been schematized and symbols have been used for better readability. The detailed statistical results can be found in Supplementary Table S2. NLR: neutrophil-to-lymphocyte ratio, BLR: band neutrophil-to-lymphocyte ratio, BNLR: band neutrophil-to-neutrophil-to-lymphocyte ratio, BNR: band neutrophil-to-neutrophil ratio, LMR: lymphocyte-to-monocyte ratio, A: alpaca, L: llama, m: male, f: female, n.i.: not included in the model, n.s.: not significant (p ≥ 0.05), s.d.: significant difference (p < 0.05), +: the WBC ratio increases with increasing independent variable, -: the WBC ratio decreases with increasing independent variable. Brackets indicate a statistical trend (0.1 > p > 0.05). The number of animals included in the LMR was only n = 282, as no LMR could be determined in the animals in which no monocytes were differentiated in the blood smear.
Discussion
In our study, we presented data on different WBC ratios from a large number of hospitalized SAC in relation to species, sex, age, season, BCS, WBC count, and PCV for the first time. The NLR, BLR, and LMR were found to be significantly influenced by the age of the animal and the WBC count. Furthermore, an association of individual WBC ratios with species, nutritional status, or an anemic condition could be detected. Although the median age of the llamas in the population we studied was higher than that of the alpacas, this difference turned not out to be statistically significant (p = 0.15; Mann-Whitney U test), we therefore assume that this did not bias the findings concerning species and age. The influence of species, sex, age, and season on neutrophils and lymphocytes in SAC has been previously described27,34. Hengrave Burri and colleagues found higher numbers of each of the segmented and band neutrophils as well as lymphocytes in llamas than alpacas. They found no difference between male and female animals in either species, but reported that crias of both species had significantly more lymphocytes than adult animals27. However, Husakova and colleagues found lower numbers of both lymphocytes and neutrophils in alpaca crias than in adult alpacas34. They also found lower lymphocyte counts in males than in females and lower numbers of neutrophils and monocytes in winter than in summer34. In our evaluation, we did not find any influence of the month of sampling on any of the WBC ratios. The findings concerning lymphocytes in the study by Husakova et al.34 is reflected in a trend to lower LMR in males than females in our population. The fact that NLR increases with age of the animal was also observed in another population where a retrospective examination of hematologic findings in healthy female alpacas from an experimental herd showed a moderate correlation between NLR and age (rs=0.59; p < 0.001)35. An increase in neutrophils could perhaps be a compensation for a decrease in neutrophil activity with age. Wenisch and colleagues found a reduced phagocytic ability and bactericidal activity of neutrophils in elderly humans36. However, the extent to which this can be applied to SAC remains unclear. A higher BLR in llamas in this study compared to alpacas is consistent with the findings of Hengrave Burri et al.27 who found that band neutrophils were significantly higher in llamas than in alpacas. The observation by Husakova et al.34 that female alpacas had higher lymphocyte counts than males is also reflected in the slightly higher LMR of females in our study. The aforementioned associations of BCS with neutrophils and lymphocytes2 are also reflected in the NLR, BLR, and LMR, although it should be noted that part of the population examined in the present study was already included in the previous study2.
Already in 1983 Montes et al. specified the NLR for 30 Chilean alpacas of both sexes as 0.73 ± 0.26 with a range of 0.2–1.19. Hajduk examined 29 alpacas of both sexes that were imported to Australia and gave a reference interval for the NLR of 0.5–2.9 in 19927. The NLR of 89 adult llamas was given as 1.54 without further specification whether this value represented the mean or median by Fowler as well as Foster et al.12,37. When compared with the NLRs obtained in the present study, it is noticeable that those previously reported NLRs were lower, which may be explained by the fact that those values were obtained from healthy animals, whereas the ratios of the present study were obtained from hospitalized animals. In a study of systemic inflammation in alpacas, six healthy castrated animals were treated with LPS and compared with a control group of another six healthy castrated individuals at various time points8. The NLRs of the control group were higher than those previously reported values and similar to our results, ranging from 3.06 to 4.62, while the NLRs of the LPS group increased to 9.61 after 12 h and reached a maximum of 13.67 after 24 h. Those findings by Passler et al.8 illustrate that NLR is a suitable indicator for inflammation in alpacas. However, when interpreting the NLR, it is important to keep in mind that NLR is not a specific parameter. In addition to inflammation, stress, for example transport stress, can lead to an increase in neutrophils and a decrease in lymphocytes38, which also results in an increased NLR, which has been shown in other species like cattle39 or dogs13.
The NLR is mentioned in other papers on hematology in SAC, but without giving specific values10,11,40,41. According to Fowler and Zinkl10 and van Houten et al.11, the NLR in SAC is similar to that of humans, dogs, cats, and horses. Nonetheless, it should be noted that stress-induced neutrophilia can be particularly pronounced in SAC, and according to Bildfell et al.40 there can also be a significant stress-induced increase in band neutrophils. This potential for high NLR is also evident in our data: a quarter of the animals in our study had an NLR > 7.81. According to Zahorec42, in a graduation for NLR in humans, values above 7 indicate inflammation or stress. Whether this classification is also suitable for SAC should be investigated in further studies on NLR. Hickman reported that NLR was significantly higher in mice with chronic stress compared to mice without stress14. The NLR was further reported to be a better parameter to detect chronic stress than measurement of cortisol15. If this also applies to SAC, the NLR would be an easy and inexpensive parameter to measure or indicate stress. Furthermore, there may be an association between NLR and the severity of gastric ulcers; data from human medicine showed that patients with peptic ulcer perforation had significantly higher NLR than both patients with peptic ulcer disease and patients in the control group43.
The age-related decline observed for LMR was also observed in the healthy population of female alpacas mentioned above with a weak association between age and LMR (rs=-0.31; p < 0.01)35. To the best of our knowledge, no other study on LMR or BLR, BNLR or BNR in SAC can currently be found in the literature. In both human44,45 and veterinary medicine21, LMR serves as a prognostic marker for various tumor diseases, and could therefore also be useful for SAC. Since monocytosis can also be part of a stress leukogram in some species46, a decrease in LMR could possibly indicate stress in addition to an increase in NLR. When interpreting the LMR, it must be considered that only a few monocytes are detected in the blood smear when evaluating 200 WBCs, and one misclassified cell can result in a large difference.
BNLR and BLR were investigated in canine inflammatory response syndrome by Pierini et al.24. In contrast to NLR, which was significantly lower in septic dogs than in aseptic dogs, no associations were found for BNLR and BLR. However, Gori et al. found significantly higher values for BLR and BNLR in cats with feline systemic inflammatory response syndrome and in cats with sepsis than in healthy cats23. The significance of these BNLR, BLR, and BNR for SAC should be investigated in further studies.
Limitations
As this was a retrospective study on hospitalized animals, the given ratios are not intended to serve as reference values, but only for orientation. It should be noted that the animals presented to the clinic suffered from one or more pathological conditions, and therefore they do not reflect the condition of healthy animals. As the evaluation is based on a large data set over several years, including a large proportion of SAC presented to our clinic, we believe that the results reflect a representative overview of the conditions in diseased SAC. The diagnoses of the animals were not included in the evaluation, as the available data would not be sufficient to compare different diagnoses. Implementing single diagnoses in the evaluation would further have resulted in too many variables, so we focused on alterations in the standardized hematologic parameters and BCS. The BCS in SAC by palpation of the lumbar spine as a clinical parameter was shown to give an objective estimation of the nutritional status, even when it is assessed by different examiners47. Common reasons for presentation of SAC in our clinic are nonspecific weakness and anorexia, acute abdominal pain, dyspnea and diarrhea. A detailed description of the type and frequency of pathological findings in the SAC presented in our clinic was recently published elsewhere by Neubert and colleagues48. The most frequent changes were pathological alterations of the gastrointestinal tract (48.7%), the liver (31.2%) and the respiratory tract (20.8%)48. However it should be noted that the incidence of individual diseases varies according to age and sex48, and a bias in these parameters cannot be ruled out on the basis of our data. For a few of the animals admitted to the clinic, the exact date of birth was unknown and was therefore estimated. The age in days was an approximation for these animals. No differences between intact and neutered were investigated in the male animals. As the platelets had not been checked manually and were not included in the data set, no statement could be made about the PLR in this study.
Conclusion and outlook
The results of this study show that the WBC ratios in hospitalized SAC can have wide ranges. Based on our results, it can be assumed that the WBC ratios vary depending on the species, sex, age, BCS, WBC count, and PCV of the animals. In analogy to other species, increased NLR and decreased LMR could be an indicator of stress in SAC and, like BNLR, BLR, and BNR, could be used as prognostic parameters for various diseases.
The fact that WBC ratios can be very easily determined from an existing WBC differential at no additional cost means that there is great potential for their use as diagnostic markers in everyday veterinary practice. Further studies are needed to investigate the impact of NLR on different disease patterns in SAC.
Data availability
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Abbreviations
- A:
-
Alpaca
- AIC:
-
Akaike Information Criterion
- BCS:
-
Body Condition Score
- BLR:
-
Band neutrophil-to-lymphocyte ratio
- BNLR:
-
Band neutrophil-to-neutrophil-to-lymphocyte ratio
- BNR:
-
Band neutrophil-to-neutrophil ratio
- f:
-
Female
- IQR:
-
Interquartile range
- L:
-
Llama
- LMR:
-
Lymphocyte-to-monocyte ratio
- m:
-
Male
- Max:
-
Maximum
- Mdn:
-
Median
- Min:
-
Minimum
- n.i.:
-
Not included
- NLR:
-
Neutrophil-to-lymphocyte ratio
- n.s.:
-
Not significant
- PCV:
-
Packed cell volume
- PLR:
-
Platelet-to-lymphocyte ratio
- Q1:
-
Lower quartile
- Q3:
-
Upper quartile
- s.d.:
-
significant difference
- SAC:
-
South American camelid
- SD:
-
Standard deviation
- WBC:
-
White Blood Cell
References
Van Saun, R. J. Nutritional requirements and assessing nutritional status in camelids. Vet. Clin. North. Am. Food Anim. Pract. 25, 265–279. https://doi.org/10.1016/j.cvfa.2009.03.003 (2009).
Wagener, M. G., Neubert, S., Punsmann, T. M., Wiegand, S. B. & Ganter, M. Relationships between body condition score (BCS), FAMACHA©-Score and haematological parameters in alpacas (Vicugna pacos), and Llamas (Lama glama) presented at the veterinary clinic. Animals 11, 2517. https://doi.org/10.3390/ani11092517 (2021).
Storey, B. E. et al. Validation of the FAMACHA© system in South American camelids. Vet. Parasitol. 243, 85–91. https://doi.org/10.1016/j.vetpar.2017.06.004 (2017).
Faria, S. S. et al. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 10, 702. https://doi.org/10.3332/ecancer.2016.702 (2016).
Becher, A., Suchodolski, J. S., Heilmann, J. M. & Steiner & Blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy. J. Vet. Diagn. Invest. 33, 516–527. https://doi.org/10.1177/1040638721992057 (2021).
Guan, R. W., Wang, D. M., Wang, B. B., Jiang, L. Y. & Liu, J. X. Prognostic potential of pre-partum blood biochemical and immune variables for postpartum mastitis risk in dairy cows. BMC Vet. Res. 16, 136. https://doi.org/10.1186/s12917-020-02314-6 (2020).
Hajduk, P. Haematological reference values for alpacas. Aust Vet. J. 69, 89–90. https://doi.org/10.1111/j.1751-0813.1992.tb15558.x (1992).
Passler, T. et al. Evaluation of methods to improve the diagnosis of systemic inflammation in alpacas. J. Vet. Intern. Med. 27, 970–976. https://doi.org/10.1111/jvim.12102 (2013).
Montes, G., Stutzin, M., Correa, J. & Glade, A. Estudio hematológico de proteínas totales y fibrinógeno en alpacas (Lama pacos) de la provincia de parinacota, Chile. Arch. Med. Vet. 15, 37–41 (1983).
Fowler, M. & Zinkl, J. Reference ranges for hematologic and serum biochemical values in llamas (Lama glama). Am. J. Vet. Res. 50, 2049–2053 (1989).
Van Houten, D., Weiser, M., Johnson, L. & Garry, F. Reference hematologic values and morphologic features of blood cells in healthy adult llamas. Am. J. Vet. Res. 53, 1773–1775 (1992).
Foster, A., Bidewell, C., Barnett, J. & Sayers, R. Haematology and biochemistry in alpacas and llamas. Pract. 31, 276–281. https://doi.org/10.1136/inpract.31.6.276 (2009).
Herbel, J., Aurich, J., Gautier, C., Melchert, M. & Aurich, C. Stress response of beagle dogs to repeated short-distance road transport. Animals. 10, 2114. https://doi.org/10.3390/ani10112114 (2020).
Hickman, D. L. Evaluation of the neutrophil: lymphocyte ratio as an indicator of chronic distress in the laboratory mouse. Lab. Anim. (NY) 46, 303–307. https://doi.org/10.1038/laban.1298 (2017).
Swan, M. P. & Hickman, D. L. Evaluation of the neutrophil-lymphocyte ratio as a measure of distress in rats. Lab. Anim. (NY). 43, 276–282. https://doi.org/10.1038/laban.529 (2014).
Minka, N. S., Ayo, J. O., Sackey, A. K. B. & Adelaiye, A. B. Assessment and scoring of stresses imposed on goats during handling, loading, road transportation and unloading, and the effect of pretreatment with ascorbic acid. Livest. Sci. 125, 275–282. https://doi.org/10.1016/j.livsci.2009.05.006 (2009).
Kapustka, J. et al. The influence of selected factors on wool cortisol concentration in alpacas (Vicugna pacos). Gen. Comp. Endocrinol. 350, 114474. https://doi.org/10.1016/j.ygcen.2024.114474 (2024).
Pollard, J. & Littlejohn, R. Effects of social isolation and restraint on heart rate and behaviour of alpacas. Appl. Anim. Behav. Sci. 45, 165–174. https://doi.org/10.1016/0168-1591(95)00588-J (1995).
Smith, B. B., Pearson, E. G. & Timm, K. I. Third compartment ulcers in the llama. Vet. Clin. North. Am. Food Anim. Pract. 10, 319–330. https://doi.org/10.1016/s0749-0720(15)30565-x (1994).
Hund, A. & Wittek, T. Abomasal and Third Compartment Ulcers in ruminants and South American Camelids. Vet. Clin. North. Am. Food Anim. Pract. 34, 35–54. https://doi.org/10.1016/j.cvfa.2017.10.003 (2018).
Marconato, L. et al. Peripheral blood lymphocyte/monocyte ratio as a useful prognostic factor in dogs with diffuse large B-cell lymphoma receiving chemoimmunotherapy. Vet. J. 206, 226–230. https://doi.org/10.1016/j.tvjl.2015.07.009 (2015).
Neumann, S. Neutrophil-to‐lymphocyte and platelet‐to‐lymphocyte ratios in dogs and cats with acute pancreatitis. Vet. Clin. Pathol. 50, 45–51. https://doi.org/10.1111/vcp.12979 (2021).
Gori, E., Pierini, A., Lippi, I., Lubas, G. & Marchetti, V. Leukocytes ratios in feline systemic inflammatory response syndrome and sepsis: A retrospective analysis of 209 cases. Animals. 11, 1644. https://doi.org/10.3390/ani11061644 (2021).
Pierini, A. et al. Neutrophil-to-lymphocyte ratio, nucleated red blood cells and erythrocyte abnormalities in canine systemic inflammatory response syndrome. Res. Vet. Sci. 126, 150–154. https://doi.org/10.1016/j.rvsc.2019.08.028 (2019).
Tak, T., Tesselaar, K., Pillay, J., Borghans, J. A. M. & Koenderman, L. What’s your age again? Determination of human neutrophil half-lives revisited. J. Leukoc. Biol. 94, 595–601. https://doi.org/10.1189/jlb.1112571 (2013).
Wagener, M. G. & Ganter, M. Body condition scoring in South American camelids. Prakt Tierarzt 101, 684–696. https://doi.org/10.2376/0032-681X-2020 (2020).
Hengrave Burri, I., Tschudi, P., Martig, J., Liesegang, A. & Meylan, M. Neuweltkameliden in Der Schweiz. II. Referenzwerte für hämatologische und blutchemische parameter. Schweiz. Arch. Tierheilkd 147, 335–343. https://doi.org/10.1024/0036-7281.147.8.335 (2005).
Team, R. C. R: A language and environment for statistical computing. R Foundation Stat. Comput. (2021).
Bates, D. et al. Package ‘lme4.’ (2009).
Barton, K. & Mu-MIn Multi-model inference. R Package Version 0.12.2/r18. Issue 1. http://R-Forge.R-project.org/projects/mumin/
Burnham, K. & Anderson, D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002).
Hurvich, C. M. & TsaiI, C. L. Regression and time series model selection in small samples. Biometrika. 76, 297–307. https://doi.org/10.1093/biomet/76.2.297 (1989).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in General Parametric models. Biom J. 50, 346–363. https://doi.org/10.1002/bimj.200810425 (2008).
Husakova, T., Pavlata, L., Pechova, A., Tichy, L. & Hauptmanova, K. The influence of sex, age and season on the haematological profile of alpacas (Vicugna pacos) in Central Europe. Vet. Med. (Praha) 60, 407–414. https://doi.org/10.17221/8415-VETMED (2015).
Wagener, M. G., Kornblum, M., Kiene, F., Ganter, M. & Teichmann, U. Hematologic parameters in female alpacas during age progression: A retrospective study. Sci. Rep. 14, 492. https://doi.org/10.1038/s41598-023-50572-9 (2024).
Wenisch, C., Patruta, S., Daxböck, F., Krause, R. & Hörl, W. Effect of age on human neutrophil function. J. Leukoc. Biol. 67, 40–45. https://doi.org/10.1002/jlb.67.1.40 (2000).
Fowler, M. E. & Bravo, P. W. Hemic and lymphatic systems. Medicine and Surgery of Camelids 407–422. https://doi.org/10.1002/9781118785706.ch15 (Blackwell Publishing Ltd, (2010).
Carroll, J. A. & Forsberg, N. E. Influence of stress and Nutrition on cattle immunity. Vet. Clin. North. Am. Food Anim. Pract. 23, 105–149. https://doi.org/10.1016/j.cvfa.2007.01.003 (2007).
Deters, E. L. & Hansen, S. L. Long-distance transit alters liver and skeletal muscle physiology of beef cattle. Animal 16, 100555. https://doi.org/10.1016/j.animal.2022.100555 (2022).
Bildfell, R. J., Löhr, C. V. & Tornquist, S. J. Diagnostic sampling and gross pathology of new world camelids. Vet. Clin. North. Am. Food Anim. Pract. 28, 577–591. https://doi.org/10.1016/j.cvfa.2012.07.001 (2012).
Tornquist, S. J. Clinical pathology of llamas and alpacas. Vet. Clin. North. Am. Food Anim. Pract. 25, 311–322. https://doi.org/10.1016/j.cvfa.2009.03.004 (2009).
Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 122, 474–488. https://doi.org/10.4149/BLL_2021_078 (2021).
Tanrikulu, Y. et al. Is the neutrophil-to-lymphocyte ratio a potential diagnostic marker for peptic ulcer perforation? A retrospective cohort study. Am. J. Emerg. Med. 34, 403–406. https://doi.org/10.1016/j.ajem.2015.11.009 (2016).
Nishijima, T. F., Muss, H. B., Shachar, S. S., Tamura, K. & Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 41, 971–978. https://doi.org/10.1016/j.ctrv.2015.10.003 (2015).
Gu, L. et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 7, 31926. https://doi.org/10.18632/oncotarget.7876 (2016).
Roland, L., Drillich, M. & Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Invest. 26, 592–598. https://doi.org/10.1177/1040638714546490 (2014).
Wagener, M. G. et al. The influence of different examiners on the body Condition score (BCS) in south American camelids—experiences from a mixed llama and alpaca herd. Front. Vet. Sci. 10, 1126399. https://doi.org/10.3389/fvets.2023.1126399 (2023).
Neubert, S. et al. Pathological findings in south American camelids presented at a farm animal clinic in Northern Germany (2005–2021). Vet. Res. Commun. https://doi.org/10.1007/s11259-024-10369-1 (2024).
Acknowledgements
The authors gratefully acknowledge the help of Frances Sherwood-Brock, English Editorial Office, University of Veterinary Medicine Hannover, Foundation for proofreading the manuscript to ensure correct English. We acknowledge financial support by the Open Access Publication Fund of the University of Veterinary Medicine Hannover, Foundation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MGW wrote the manuscript. Data were analyzed by MGW, FK, and MK. The study was designed by MGW and FK and supervised by MG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The study was approved by the Ethics Committee of the University of Veterinary Medicine Hannover (approval code: TiHo-REC_14_10_24), all methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagener, M.G., Kornblum, M., Ganter, M. et al. Characterization of white blood cell ratios in South American camelids presented at a veterinary teaching hospital. Sci Rep 14, 26031 (2024). https://doi.org/10.1038/s41598-024-76985-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76985-8