Abstract
To compare the efficacy and advantages of mini percutaneous nephrostomy (MPCN), micropercutaneous nephrostomy (MicroPCN), and retrograde ureteric stenting (RUS) in the treatment of acute upper urinary tract calculi (UUTC) obstruction with hydronephrosis and infection, and verify the safety and indications of clinical application of micropercutaneous nephrostomy. Clinical-epidemiological data of patients with acute upper urinary tract calculi obstruction and infection treated in Ningbo No.2 hospital were retrospectively collected from May 2019 to May 2023. 64 patients (20 patients in MPCN group, 13 patients in MicroPCN group, and 31 patients in RUS group) were eligible for analysis based on inclusion and exclusion criteria. P value < 0.05 was considered statistically significant. There were no significant differences in peri-intervention temperature, multiple infection indicators and complications among the three groups. The nutritional status and peri-intervention coagulation function of patients in MicroPCN and RUS groups were poor, the CRP and proportion of using carbapenem advanced antibiotics were higher. The length of hospital stay and the length of hospital stay after the intervention in MPCN and MicroPCN groups were longer, the length and width of calculi were larger, and the degree of hydronephrosis was heavier. Patients in the MicroPCN group had the worst general condition, the lowest hemoglobin before intervention, the longest withdrawal time of vasoactive drugs. MPCN, MicroPCN, and RUS are safe and effective in relieving acute upper urinary tract calculi obstruction complicated with infection. MicroPCN has more advantages for patients with critical illness or complex obstruction urinary lithiasis.
Similar content being viewed by others
Introduction
Upper urinary tract calculi (UUTC) are a common cause of upper urinary tract obstruction with hydronephrosis and infection. The pathogenesis of UUTC is that bacteria accumulate in the collection system during obstruction, and then hydronephrosis aggravates intrarenal pressure1, which leads to bacteria entering the blood and causing bacteremia, resulting in the release of inflammatory factors and systemic inflammatory response syndrome, of which 10% progress to sepsis2. Patients with diabetes mellitus (OR = 3.591, p = 0.0098)2 and an abnormal immune system are more likely to progress to septic shock3, and the mortality rate is as high as 20–40%4,5. Early diagnosis and treatment of urogenic sepsis is essential. Emergency surgical urine drainage is the key to avoiding serious complications and even death. There are reports suggesting that the mortality of patients with calculi related sepsis without surgical drainage is twice that of patients treated with percutaneous nephrostomy (PCN) or RUS6. Previous conventional surgical methods include PCN and RUS, both of which have their own advantages and limitations7,8. With the development of technology, minimally invasive treatment of infection has become a hot field, and drainage catheters have also been continuously improved to meet the demand. Currently, the commonly used drainage catheters in clinical practice are 5–14 F pigtail catheters and 12–24 F balloon retention catheters, which are widely used for indwelling drainage catheters after percutaneous nephrolithotomy (PCNL)9. In this study, MPCN indwelled 12–18 F balloon retention catheters, MicroPCN indwelled 6–8 F pigtail catheters, the latter avoided the multiple expansion of the expander, which is simpler and less traumatic (Table 1). In recent years, although many urologists have tried to use thinner drainage catheters to control infection, there has been a lack of relevant literature support. This study aims to verify the efficacy and safety of MPCN and MicroPCN.
Patients and methods
Statement of ethics
This study solely used available summary data that was approved for human experimentation by Ningbo Second Hospital Medical Ethics Committee. Approved by Ningbo Second Hospital Medical Ethics Committee, informed consent is exempted. All methods were performed in accordance with the relevant guidelines and regulations.
Inclusion and exclusion criteria
Inclusion criteria: (I) imaging confirmed obstructive urolithiasis, (II) maximum temperature (Tmax) ≥ 38 °C; and/or total white cell (TWC) count ≥ 12 000/µl within 24 h before intervention. Exclusion criteria: (I) non-infectious fever and elevated total white cell count, (II) incomplete data.
Data analysis
The demographic and clinical characteristics of patients included age, gender, Barthel, nutritional risk screening (NRS), Charlson, number of days of hospital stay, duration of surgery, diabetes mellitus, length and width of the calculus, laterally the obstruction, positions of the calculus, degree of hydronephrosis, urine routine, blood / urine bacterial culture, type of antibiotics used, prognosis, complications after the intervention (lumen blockage, bleeding, intervention failure, sepsis after intervention, ureter / peripheral organ injury).
The peri-intervention parameters and outcome measures included: (I) number of days postintervention for a fever to subside, (II) number of days of hospital stay postintervention, (III) number of days for total white cells to normalize after the intervention, (IV) number of days for Serum creatinine to normalized or reduce by more than 20%, (V) number of days postintervention to wean off the vasoactive agent, (VI) serum creatinine levels at follow-up. Peri-intervention parameters included Tmax, number of analgesics used, number of ICU admissions, lowest MAP, CRP, TWC, serum creatinine, hemoglobin, respiratory /cardiovascular/nervous system/live/coagulation/renal/total SOFA score, sepsis, and septic shock.
Interventions
Mini Percutaneous Nephrostomy (MPCN): the patient takes the prone position and local anesthesia, locates the intersection of the 11th or 12th costal margin and the posterior axillary line on the affected side as the puncture point under the guidance of ultrasound, uses an 18G puncture needle to puncture to the renal pelvis or calyces, and after the needle core has urine led out, places the guide wire, cuts the skin incision (about 5 mm) from the puncture position, pulls out the puncture needle, expands to 12–16 F, places the outer sheath, and then takes out the inner core, retains a 12–16 F drainage catheter along the denuded sheath and fixes it.
Micropercutaneous nephrostomy (MicroPCN): the body position, anesthesia method, positioning, and method of puncture are the same as those of MPCN. The guide wire was inserted after a successful puncture, then the puncture needle needed to pulled out, and then the fascial expansion sheath was used along the guide wire for single expansion. At last, the 6/8 F pigtail catheter was placed with a depth of 15–20 cm and fixed. (Fig. 1).
Retrograde ureteral stenting (RUS): the patient took the lithotomy position and was under local anesthesia. A 22 F wolf cystoscope or an 8–9.8 F wolf rigid ureteroscope were used under direct vision. A 5–7 f ureteral stent was placed after the guide wires inserted retrogradely into the ureter at last.
Statistical analysis
IBM SPSS Statistics ver. 26.0 was used for statistical analysis. Patients’ demographics, baseline clinical characteristics, characteristics of ureteral obstructions, peri-intervention parameters, and outcome measures were summarized descriptively. The numerical variables were expressed as mean and standard deviation (SD), and analysis of Kruskal-Wallis was used to compare groups. The number and percentage of subjects in each group were reported for categorical variables, and the Pearson Chi-square test was used to compare groups. A p value < 0.05 was considered statistically significant.
Results
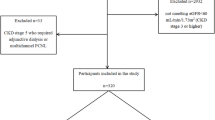
From May 2019 to May 2023, 31 patients underwent MPCN, 18 patients underwent MicroPCN, and 65 patients underwent RUS. According to the inclusion and exclusion criteria, a total of 64 patients (20 patients in the MPCN group, 13 patients in the MicroPCN group, and 31 patients in the RUS group) were eligible for analysis (Fig. 2).
Patients’ demographic, baseline clinical characteristics, and characteristics of ureteral obstructions (Table 1)
The mean age of the patients was 57.6 (± 12.7) years old, and women were more common (57.8%). Among them, 12 patients (18.8%) were complicated with diabetes mellitus, and calculi were most common in the left side (56.3%), ureter (76.6%), and upper segment (60.9%)10. There were significant differences among the three groups in the following aspects (P < 0.05): (I) Nutritional Risk Screening (0.1 ± 0.4 in MPCN vs. 1.0 ± 2.0 in MicroPCN vs. 1.0 ± 1.2 in RUS), (II) Number of days of hospital stay (15.5 ± 6.5 days in MPCN vs. 14.9 ± 7.0 days in MicroPCN vs. 10.7 ± 7.3 days in RUS), (III) Length of the calculus (16.4 ± 18.1 mm in MPCN vs. 21.1 ± 16.1 mm in MicroPCN vs. 8.6 ± 5.5 mm in RUS), (IV) Width of the calculus (12.7 ± 14.4 mm in MPCN vs. 14.0 ± 10.1 mm in MicroPCN vs. 6.2 ± 3.0 mm in RUS), (V) Degree of hydronephrosis (13 patients with mild in MPCN vs. 5 patients with mild in MicroPCN vs. 28 patients with mild in RUS), (VI) Type of antibiotic use (1 patients with carbapenem in MPCN vs. 7 patients with carbapenem in MicroPCN vs. 11 patients with carbapenem in RUS), (VII) Prognosis (0 patients with death in MPCN vs. 3 patients with death in MicroPCN vs. 0 patients with death in RUS). The nutritional status of patients in the MicroPCN and RUS groups was poor, and the proportion of patients using carbapenem advanced antibiotics was higher. The length of hospital stay was longer, the length and width of calculi were larger, and the degree of hydronephrosis was heavier in the MPCN and MicroPCN groups. The mortality rate of patients in the MicroPCN group was the highest.
Peri-intervention parameters
We included the latest data within 24 h before the intervention, the data within 24 / 48 h after the intervention, the other outcome measures, and follow-up creatinine for analysis (the normal data for the number of days of hospital stay postintervention, the number of days for total white cells to normalize after the intervention, the number of days for serum creatinine to normalize or reduce by more than 20%, the number of days postintervention to wean off the Vasoactive agent, and 3% of unrecorded data were replaced by the mean value) (Table 2).
24 h preintervention parameters and outcome measures
The mean Tmax of the three groups of patients before the intervention was 38.6 (± 1.0) °C, 34.4% of patients used analgesics, and the ICU occupancy rate was 12.5%. According to the sepsis 3.0 standard11, 32 patients had sepsis, and 12 patients had septic shock. There were significant differences among the following indicators (P < 0.05): (I) Lowest MAP (90.5 ± 13.0 mmHg in MPCN vs. 78.3 ± 15.0 mmHg in MicroPCN vs. 75.4 ± 13.3 mmHg in RUS), (II) CRP (76.2 ± 61.2 mg/l in MPCN vs. 161.1 ± 73.8 mg/l in MicroPCN vs. 133.6 ± 96.2 mg/l in RUS), (III) Hemoglobin (125.1 ± 23.5 g/l in MPCN vs. 104.5 ± 21.6 g/l in MicroPCN vs. 114.7 ± 22.7 g/l in RUS), (IV) Coagulation function (0.0 ± 0.0 in MPCN vs. 0.6 ± 0.9 in MicroPCN vs. 0.5 ± 0.8 in RUS). Patients in the MicroPCN and RUS groups had higher CRP, lower MAP, and poorer coagulation function before intervention. The MicroPCN group had the lowest hemoglobin before intervention.
Post-intervention parameters and outcome measures
The mean Tmax of the three groups of patients was 37.5 ± 0.8 °C within 24 h postintervention and 37.4 ± 0.7 °C within 48 h postintervention, 39.1% of the patients used analgesics, the ICU occupancy rate was 10.9%, 29 patients had sepsis, and 12 patients had septic shock. There were significant differences in the following indicators among the groups (P < 0.05): (I) Lowest MAP (91.0 ± 13.0 mmHg in MPCN vs. 80.9 ± 13.1 mmHg in MicroPCN vs. 78.6 ± 11.6 mmHg in RUS), (II) Coagulation function (0.0 ± 0.2 in MPCN vs. 0.5 ± 1.1 in MicroPCN vs. 0.7 ± 1.0 in RUS), (III) Number of days postintervention to wean off the Vasoactive agent (2.1 ± 0.2 day in MPCN vs. 2.8 ± 1.6 day in MicroPCN vs. 1.8 ± 0.4 in RUS), (IV) Number of days of hospital stay postintervention (12.2 ± 4.5 days in MPCN vs. 12.7 ± 6.3 days in MicroPCN vs. 7.9 ± 5.6 days in RUS). The patients in the MicroPCN and RUS groups had the lower lowest MAP and worse coagulation function within 24 h postintervention. Patients in the MicroPCN group discontinued vasoactive drugs for the longest time. The number of days of hospital stay postintervention was longer in the MPCN and MicroPCN groups.
All patients were followed-up for 1 month to 2 years. At the time of follow-up review, 23 / 37 patients (62%) with elevated serum creatinine before intervention had returned to normal, 58 / 64 patients (90%) had significantly improved renal function, and 6 patients had renal dysfunction due to renal atrophy after the removal of obstructive factors, subsequently following up in the nephrology department long-term.
Discussion
As the main clinical manifestation of obstruction and infection in urinary lithiasis, fever is considered a surgical indication for emergency urinary drainage12,13. Previously, it was believed that PCN and RUS were both effective drainage methods14, with their own unique advantages. The choice of drainage method mostly depends on the habits of the surgeon in charge and the characteristics of the lithiasis15. According to the literature, there was no significant difference between the two groups in temperature recovery time, creatinine recovery time, postintervention pain, postintervention stent / catheter displacement, postintervention urosepsis, bleeding, or other complications15,16, which is consistent with the results of this study. Patients in MPCN, MicroPCN, and RUS did not show significant differences in temperature, multiple infection indicators, or complications.
Compared with PCN, RUS decompresses through the natural lumen of the human body and has less trauma, lower surgical difficulty, a simpler operation, and a shorter hospital stay14, making it the preferred treatment for the treatment of incomplete acute upper urinary tract calculi obstruction. In addition, for patients with urinary calculi complicated by ureteral stenosis, RUS can dilate the ureter, which is helpful for subsequent lithotripsy treatment. However, the ureteral stent prevents the complete closure of the ureterovesical junction (UVJ), resulting in urine reflux causing pain and discomfort in the lower back of patients, which often requires drug analgesic treatment17, and may increase the intrarenal pressure and aggravate infection, finally increasing the risk of bacterial colonization. Indwelling a drainage catheter after PCN can help quickly reduce the pressure of the renal pelvis, protect the kidney, relieve infection faster, and have better safety and efficacy for patients with septic shock18. It can also effectively avoid hematuria symptoms caused by active friction due to the presence of a ureteral stent, and the incidence of hematuria is much lower than that of RUS16. In addition, although there is no obvious difference in the success rate of surgery between the two (PCN vs. RUS = 99% vs. 98%)19,20, RUS sometimes failed to catheterize due to complete obstruction of calculi, ureteral stricture, inability to identify the ureteral orifice, and other21 reasons, and finally needed PCN treatment, which is consistent with the results of this study and increases the economic and psychological burden of patients. The failure rate of RUS in actual clinical work is higher. In this study, two patients with intraintervention RUS failure were immediately changed to MPCN or MicroPCN, which was not reflected in the record. Therefore, some experts recommend PCN, which has fewer contraindications and can be performed under local anesthesia at the bedside. Although it may be exposed to radiation, it has higher efficiency in controlling septic shock and lower rates of hematuria and catheter failure16.
With the rapid development of the concept of minimally invasive surgery, Kaijun Wu first proposed the concept of mini-percutaneous nephrolithotomy (MPCNL) in 199322, and Helal first officially reported the use of MPCNL for clinical use in 199723, which established 16–18 F percutaneous renal microchannels, and its safety and effectiveness were confirmed in many subsequent reports24. Subsequently, learning how to use thinner channels more safely and effectively has become a trend. Desai proposed the concept of microperc in 2011, and its channel is only 4.8 F25. Adhering to the concept of minimally invasive surgery, MPCN and MicroPCN came into being. Although many urologists have used 5–16 F microchannels to treat calculi obstruction with infection, the safety of such microchannels has yet to be verified.
In addition to the advantages of traditional PCN, MPCN has the characteristics of being more minimally invasive and having less surgical risk, and there were no complications such as massive bleeding, drainage catheter blockage, or poor drainage aggravating infection in this study. Only one patient with a pleural injury improved after conservative treatment. MPCN is safe and effective. MicroPCN avoids multiple expansions of the renal puncture set, is more minimally invasive, and is more suitable for critically ill patients. In addition, two patients in this study used dual-channel MicroPCN to control infection, which was successfully completed and had a significant effect. MicroPCN also has more advantages for complex calculi requiring multi-channel obstruction relief. In the MicroPCN group, one patient with postintervention sepsis caused by lumen blockage had smooth drainage after low-pressure flushing, and his temperature returned to normal on the third day. After long-term clinical verification, we prefer MicroPCN with mature technology for critically ill patients. Therefore, due to our selection bias, patients in the MicroPCN group in this study had the worst general condition, the lowest hemoglobin value before the intervention, the highest ICU occupancy rate before the intervention (3/13, 23%), the highest mortality rate after the intervention (3/13, 23%), and the longest number of days to wean off the vasoactive agent. After early intervention with MicroPCN, the body temperature, total white blood cell count, and CRP of these three deceased patients improved to varying degrees, and no serious complications occurred. One patient eventually died of cerebral hemorrhage, one died of acute heart failure, and one was discharged automatically due to economic problems. Although the hemoglobin value before the intervention of MicroPCN was the lowest, there was no significant difference in the hemoglobin value of the three groups 24 h after the intervention (P = 0.219). Among them, 7 patients (35%) in the MPCN group had a postintervention hemoglobin reduction of more than 10 g/l, and only 1 patient (7%) in the MicroPCN group had less intraintervention blood loss. Our center has achieved remarkable maturity in MicroPCN technology, ensuring minimal trauma and precise therapeutic outcomes. Consequently, we persist in utilizing this advanced technology in the clinical management of related conditions. Nevertheless, MicroPCN remains underutilized in other regions of China. Our goal is to actively promote MicroPCN nationwide, ensuring that patients with related disorders have access to this superior treatment option. In summary, MicroPCN is minimally invasive and effective, and we recommend it for critically ill or complex calculi patients (Table 3).
Limitations
This is a retrospective study. The baseline characteristics of the three interventions are essentially different. Patients’ parameters were not recorded consistently before and after the intervention. Therefore, we chose to use the highest or lowest parameters recorded within 24 h before and 24/48 hours after the intervention, which resulted in about 3% of the data without corresponding records, and the mean was used instead. Therefore, prospectively developing a randomized controlled cohort with a larger amount of data and a good match will help reduce the potentially useful data that may be lost. On the one hand, the decision to take intervention measures is based on the habits and preferences of urologists. Therefore, there is an inherent selection bias. Our study does not include data and analysis of calculi composition or secondary treatment of calculi.
Conclusion
MPCN, MicroPCN, and RUS had no significant difference in temperature, infection indicators, or complications and were safe and effective in relieving acute upper urinary tract calculi obstruction complicated with infection. MicroPCN avoids multiple expansions of the renal puncture set with small trauma and a simple operation. It has more advantages for patients with poor general conditions, critical conditions, many comorbidities, poor coagulation function, and who need multi-channel obstruction relief and bedside intervention.
Data availability
The raw data has been uploaded in supplementary file. The datasets used and analysed during the current study available from the corresponding author on reasonable request.Corresponding author: Zhebin Gao E-mail: [email protected].
Abbreviations
- MPCN:
-
Mini Percutaneous Nephrostomy
- PCN:
-
Percutaneous Nephrostomy
- PCNL:
-
Percutaneous Nephrolithotomy
- MPCNL:
-
Mini-Percutaneous Nephrolithotomy
- MicroPCN:
-
Micropercutaneous Nephrostomy
- RUS:
-
Retrograde Ureteric Stenting
- UUTC:
-
Upper Urinary Tract Calculi
- CRP:
-
C-reactive Protein
- OR:
-
Odds Ratio
- TWC:
-
Total White Cell
- NRS:
-
Nutritional Risk Screening
- MAP:
-
Mean Arterial Pressure
- ICU:
-
Intensive Care Unit
- SOFA:
-
Sequential Organ Failure Assessment
- UVJ:
-
Ureterovesical Junction
References
Liang, X. et al. Risk factors and outcomes of urosepsis in patients with calculous pyonephrosis receiving surgical intervention: a single-center retrospective study. BMC Anesthesiol. 19 (1), 61 (2019).
Yamamichi, F., Shigemura, K., Kitagawa, K. & Fujisawa, M. Comparison between non-septic and septic cases in stone-related obstructive acute pyelonephritis and risk factors for septic shock: a multi-center retrospective study. J. Infect. Chemother.24 (11), 902–906 (2018).
Nicolle, L. E. & AMMI Canada Guidelines Committee*. Complicated urinary tract infection in adults. Can. J. Infect. Dis. Med. Microbiol.16 (6), 349–360 (2005).
Reyner, K., Heffner, A. C. & Karvetski, C. H. Urinary obstruction is an important complicating factor in patients with septic shock due to urinary infection. Am. J. Emerg. Med.34 (4), 694–696 (2016).
Yamamichi, F. et al. Shock due to urosepsis: A multicentre study. Can. Urol. Assoc. J.11(3–4), E105–E109 (2017 ).
Borofsky, M. S. et al. Surgical decompression is associated with decreased mortality in patients with sepsis and ureteral calculi. J. Urol.189 (3), 946–951 (2013).
Vahlensieck, W., Friess, D., Fabry, W., Waidelich, R. & Bschleipfer, T. Long-term results after acute therapy of obstructive pyelonephritis. Urol. Int.94 (4), 436–441 (2015).
He, Y. et al. Computed tomography angiography with 3D reconstruction in diagnosis of hydronephrosis cause by aberrant renal vessel: a case report and mini review. J. Xray Sci. Technol.26 (1), 125–131 (2018).
Paul, E. M., Marcovich, R., Lee, B. R. & Smith, A. D. Choosing the ideal nephrostomy tube. BJU Int.92 (7), 672–677 (2003).
St Lezin, M., Hofmann, R. & Stoller, M. L. Pyonephrosis: diagnosis and treatment. Br. J. Urol.70 (4), 360–363 (1992).
Singer, M. et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 315 (8), 801–810 (2016).
Brown, P. D. Management of urinary tract infections associated with nephrolithiasis. Curr. Infect. Dis. Rep.12 (6), 450–454 (2010).
Preminger, G. M. et al. 2007 guideline for the management of ureteral calculi. J. Urol.178 (6), 2418–2434 (2007).
Zul Khairul Azwadi, I., Norhayati, M. N. & Abdullah, M. S. Percutaneous nephrostomy versus retrograde ureteral stenting for acute upper obstructive uropathy: a systematic review and meta-analysis. Sci. Rep.11 (1), 6613 (2021).
Pearle, M. S. et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J. Urol.160 (4), 1260–1264 (1998).
Gang Wu, X. et al. Meta-analysis of perioperative results and safety of percutaneous nephrostomy and retrogradeureteral stenting in the treatment of acute obstructive upper urinary tract infection. Chin. J. Urol.44 (2), 128–133 (2023).
Mokhmalji, H. et al. Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: a prospective, randomized clinical trial. J. Urol.165 (4), 1088–1092 (2001).
Shiyong Qi, Q. et al. The comparison of two surgical decompressions for patients with upper urinary tract calculi and sepsis. Chin. J. Urol.41 (4), 256–261 (2020).
Montvilas, P., Solvig, J. & Johansen, T. E. Single-centre review of radiologically guided percutaneous nephrostomy using mixed technique: success and complication rates. Eur. J. Radiol.80 (2), 553–558 (2011).
Flukes, S. et al. Retrograde ureteric stent insertion in the management of infected obstructed kidneys. BJU Int.115 (Suppl 5), 31–34 (2015).
Wang, C. J., Hsu, C. S., Chen, H. W., Chang, C. H. & Tsai, P. C. Percutaneous nephrostomy versus ureteroscopic management of sepsis associated with ureteral stone impaction: a randomized controlled trial. Urolithiasis. 44 (5), 415–419 (2016).
Wu, K. et al. Management of staghorn stones by two stage antiureterolithotripsy through the mininephrostomy. J. Guangzhou Med. Coll.02, 13–16 (1993).
Helal, M., Black, T., Lockhart, J. & Figueroa, T. E. The Hickman peel-away sheath: alternative for pediatric percutaneous nephrolithotomy. J. Endourol. 11 (3), 171–172 (1997).
Gao, X. et al. Mini Percutaneous Nephrolithotomy Is a Noninferior Modality to Standard Percutaneous Nephrolithotomy for the Management of 20–40 mm Renal Calculi: A Multicenter Randomized Controlled Trial. Eur Urol.80, 114–21 (2021) (European urology, 80(6), e150).
Desai, M. R. et al. Single-step percutaneous nephrolithotomy (microperc): the initial clinical report. J. Urol.186 (1), 140–145 (2011).
Acknowledgements
This study was approved in education department of Ningbo No.2 Hospital, as a research project. The authors would like to thank Dr. Houmeng Yang and appreciate her support for the preparing of this manuscript.
Funding
This research is Supported by Zhejiang Rehabilitation Medical Association special Research, Project ID: ZKKY202406.
Author information
Authors and Affiliations
Contributions
Research conception and design: ZheBin Gao and HouMeng Yang. Data acquisition: Li Wang, Jiaren Pan, Xiao Shi. Statistical analysis: Huayang Zhang, Jing L, Li Wang, Jiaren Pan, Xiao Shi. Data analysis and interpretation: Huayang Zhang, Fei Zhang, Jing L, Li Wang. Drafting of the manuscript: Zhebin Gao, Huayang Zhang, Fei Zhang. Critical revision of the manuscript: Zhebin Gao, Huayang Zhang, Fei Zhang, Li Wang, Houmeng Yang. Approval of the final manuscript: all authors.
Corresponding authors
Ethics declarations
Ethics approval
This study solely used available summary data that was approved for human experimentation by Ningbo Second Hospital Medical Ethics Committee. Approved by Ningbo Second Hospital Medical Ethics Committee, informed consent is exempted. All methods were performed in accordance with the relevant guidelines and regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Z., Zhang, H., Zhang, F. et al. Micropercutaneous nephrostomy for intervention in acute upper urinary tract calculi obstruction with hydronephrosis and infection. Sci Rep 14, 25787 (2024). https://doi.org/10.1038/s41598-024-77078-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77078-2