Abstract
The lymphatic system is a crucial contributor to allograft rejection after corneal transplantation. However, no surgical procedures for the central pathway where conjunctival lymphatic vessels converge have been investigated. Therefore, we aimed to establish a murine model of lymphatic vessel ligation and evaluate its inhibitory effect on corneal allograft rejection. A tracer was used to visualise lymphatic vessels, and complications were evaluated. A surgical technique was developed to block the lymphatic vessels. Corneas from C57BL/6 mice were transplanted into BALB/c mice divided into two groups—one with and one without lymphatic vessel ligation, to evaluate their effects on allograft rejection. Graft opacity scores were evaluated for 8 weeks, and immunohistochemistry was used to quantify angiogenesis and lymphangiogenesis. 20% trypan blue used as a tracer showed clear inflow with no complications. The two sutures and cyanoacrylate glue combination demonstrated a blocking effect after 25 days and was thus used for lymphatic ligation. Three and nine out of fourteen eyes showed rejection at 8 weeks post-surgery in the lymphatic vessel ligation and control groups, respectively. Furthermore, neovascularisation and lymphangiogenesis significantly decreased in the lymphatic vessel ligation group. Overall, we present a novel therapeutic strategy for corneal transplantation.
Similar content being viewed by others
Introduction
Recently, corneal transplantation has progressed to selectively replacing diseased corneal layers1. However, penetrating keratoplasty (PKP) remains a prevalent form of corneal transplantation for various indications such as infection, regraft, and keratoconus2. Although the graft survival rate after 1 year in patients with PKP exceeds 90%, the rates differ with indications. For example, the survival rate at 10 years is also > 90% in patients with keratoconus; however, the survival rate of a failed previous graft is approximately 30%.3,4 Graft failure is often caused by immunologic graft rejection, and developing therapeutic strategies to improve graft survival is essential.
The lymphatic system is a key player in allograft rejection after corneal transplantation; however, excising cervical lymph nodes increases graft survival5. Lymphatic vessels play a role in graft rejection, and alloantigen and antigen-presenting cell migration to lymph nodes via lymphatic vessels may trigger graft rejection6. Consequently, inhibiting lymphatic vessel function could reduce the risk of graft rejection. Previous studies have regulated the lymphatic pathway, including inhibition of vascular endothelial growth factor (VEGF) expression7. However, no surgical procedures have been considered for the central pathway where conjunctival lymphatic vessels assemble. Therefore, in this study, we aimed to visualise the oculo-lymphatic pathway and demonstrate its blocking effect on allograft rejection after corneal transplantation using simple surgical techniques.
Results
Visualisation of lymphatic vessels and establishment of a lymphatic vessel ligation model
Although the Evans blue tracer was thoroughly rinsed, superficial punctate keratitis (SPK) was observed after administering a drop of the tracer. Therefore, the minimum concentration and method of tracer administration were evaluated. Table 1 shows the results of the confirmed influx into the superficial cervical and facial lymph nodes and complications at different concentrations.
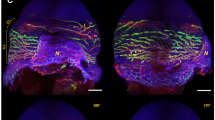
Figure 1 shows a schematic representation of the lymphatic vessel originating from the nasal aspect of the conjunctiva and culminating in cervical lymph nodes. The junction demarcated the peripheral and central pathways. The lymphatic vessels assembled from the limbus to the nasal side of the conjunctiva, and they were stained with trypan blue (Fig. 2A). Figure 2B shows the tracing lymphatic vessel with the tracer pooling into the cervical lymph nodes (Supplemental Figure S1 and Video S2). The results of the period during which lymphatic vessels were obstructed are shown in Table 2.
Illustration of lymphatic vessels. Lymphatic vessels from the conjunctival region assemble at the nictating membrane and flow from the nasal side of the conjunctiva into the superficial cervical and facial lymph nodes. The junction of lymphatic vessels divides into the peripheral and central pathways. LV: lymphatic vessel, Md: deep head of the masseter muscle Ms: superficial head of the masseter muscle, CLN: cervical lymph node.
Images of lymphatic vessels stained with trypan blue in the right mouse eye. (A) The white triangle indicates peripheral conjunctiva. The white arrow shows the junction of the lymphatic vessels. (B) The blue triangle indicates lymphatic vessels flowing into the lymph nodes. The blue arrow indicates the cervical lymph node. (C) Images of the ligation technique. The lymphatic vessels were ligated at the junction in two places with 8 − 0 silk (red arrows). (D) Cyanoacrylate glue was used to fix the ligated area (blue circle).
The approaches of using two sutures with 8 − 0 silk (Fig. 2C) and cyanoacrylate glue (Fig. 2D) were selected because of their ability to block lymphatic vessels the longest.
Lymphatic were clearly observed extending from the nasal side of the conjunctiva to the cervical lymph nodes, as evidenced by trypan blue staining (Supplemental Figure S1 and Supplemental Video S2). Furthermore, these lymphatic vessels were anatomically independent of blood vessels, indicated by the independent staining patterns of LYVE-1 and CD-31, and were consistent with podoplanin staining (Supplemental Figure S3, S4). Co-staining with LYVE-1 and anti-podoplanin demonstrated that the trypan blue-stained tissue contained lymphatic vessels. Therefore, the positive staining trypan blue in the tissue indicates the presence of lymphatic vessels.
Clinical course of corneal allografts in a lymphatic vessel ligation model
Corneal transparency was maintained for 8 weeks in the control and lymphatic ligation groups without corneal transplantation. Corneal rejection was observed in 3 of 14 eyes in the ligation/Ag group, whereas 9 eyes exhibited rejection in the control group at 8 weeks post-surgery. The ligation/Ag group had significantly better graft survival than the control group at 8 weeks (log-rank test, p < 0.05). The ligation/Ag group showed > 50% survival at 8 weeks after surgery, and the median survival time (MST) was undefined. The MST in the control group was 3.0 weeks (Fig. 3).
Angiogenesis and lymphangiogenesis after corneal transplantation
Neovascularisation and lymphangiogenesis were significantly increased in the corneas with rejection (Figure A, B) compared with those in the corneas without rejection (Figure C, D). Figure 4E and F presents graphs quantifying neovascularisation and lymphangiogenesis and comparing the control (n = 13), ligation (n = 14), and ligation/Ag (n = 14) groups. Quantitative assessment could not be conducted in one eye in the control group because of suboptimal tissue conditions. The control group had a higher rejection rate than the ligation/Ag group and demonstrated a significant increase in neovascularisation and lymphangiogenesis (Steel–Dwass test, p = 0.008 and p = 0.004, respectively).
Neovascularisation and lymphangiogenesis images (A–D). (A) CD31 (blood vessels) and (B) LYVE-1 (lymphatic vessels) staining of the cornea exhibiting rejection in the control group. (C) CD31 and (D) LYVE-1 staining of the cornea without rejection in the ligation/Ag group. (E) The graph shows a comparison of the percent area of CD31 staining among the groups. (F) The graph represents a comparison of the percent area of LYVE-1 staining among the groups. * p < 0.05, **p < 0.01, *** p < 0.0001.
Discussion
In this study, we successfully visualised the oculo-lymphatic pathway—which contributes to allograft rejection—after corneal transplantation in mice. The results showed that 20% trypan blue is useful for detecting lymphatic vessels directly. Furthermore, blocking the oculo-lymphatic route using a simple surgical technique reduced the rate of allograft rejection after keratoplasty. Thus, we elucidated the anatomical structure of lymphatic flow in ophthalmology, developed a surgical technique to block a specific lymphatic vessel, and applied the technique to regulate allograft rejection after keratoplasty.
The dense lymphatic network around the eye is connected to the cervical lymph nodes, and thus is consistent with the present study results8. Evans blue is used to determine the localisation of lymphatic flow;9 however, in this study, Evans blue exerted superficial keratitis adverse effects in the mouse cornea. Therefore, other types of stains were considered for staining lymphatic vessels, and 20% trypan blue helped to successfully detect lymphatic vessels with equivalent visualisation.
In this study, we also investigated the effects of blocking the oculo-lymphatic pathway on allograft rejection after corneal transplantation using a simple surgical technique. First, the central lymphatic duct was ligated using 10 − 0 nylon or 8 − 0 silk. The suturing technique alone was ineffective for more than 7 days. However, two sutures of the duct using 8 − 0 silk combined with cauterisation and cyanoacrylate glue use had a blocking effect for 14 and 25 days, respectively. Rejection is more pronounced in the early phase, approximately 2 weeks after surgery; therefore, 25 days of lymphatic occlusion can prevent rejection10,11. To the best of our knowledge, this is the first study to develop a surgical procedure that consistently blocks a specific lymphatic system.
Oculo-lymphatic vessels exist in the peripheral area of the conjunctiva12,13. Lymphatic vessels are essential for maintaining organ function in humans and mice by draining liquids. The importance of lymphatic vessels has been investigated in medical fields such as oncology, immunology, and pathology14,15. In ophthalmology, several studies have focused on the relationship between lymphatic vessels and inflammation during corneal transplantation, and inhibiting the lymphatic pathway and blocking lymph nodes can increase transplant survival7,16. Theoretically, three factors are responsible for the oculo-lymphatic pathway after corneal transplantation in mice. The first factor is the cervical lymph nodes, which our group first introduced5. However, surgically removing lymph nodes has not been recommended for clinical use owing to its invasive nature. The second factor is blocking of peripheral lymphangiogenesis. Corneal transparency is maintained by anti-angiogenic factors such as soluble VEGF receptor 1 (R1) or soluble VEGF-R217. VEGF plays a role in producing pathological blood vessels via VEGF-R1 and VEGF-R2. In contrast, VEGF contributes to producing lymphatic vessels via VEGF-R2 and VEGF-R318,19,20. VEGF is responsible for physiological or pathological angiogenesis, and previous studies have attempted to regulate the peripheral route by inhibiting VEGF expression. A pharmaceutical blocking approach using VEGF TrapR1R2 was developed,7 followed by related approaches to regulate VEGF, which is known as a VEGF trap20,21,22. The third factor is blocking the central pathway of the oculo-lymphatic route, that connects the peripheral area of the cornea or conjunctiva to the draining lymph node. Although the critical role of draining lymph nodes has been demonstrated by evaluating the effect of surgical lymphadenectomy in the immunology of corneal transplantation, no study has focused on blocking the central pathway of the oculo-lymphatic route. Therefore, in this study, we developed an approach for corneal transplantation for the first time.
In this study, the blocking approach using a ligation technique combined with cyanoacrylate glue resulted in reduced allograft rejection compared to control transplantation in normal-risk settings. Although further studies are required to evaluate immune responses in low- and high-risk settings in mice, the consistent blocking effect observed in the early phase after transplantation may have contributed to the significant improvement in allograft rejection in the sutured group compared to the control group. These results are in accordance with the findings of a previous study, which demonstrated that the pharmaceutical blocking approaches in corneal transplantation minimises allosensitisation23.
In conclusion, this study demonstrated that the surgical approach using suturing had a consistent effect and significantly improved the rejection rate after experimental corneal transplantation. Therefore, blocking the central pathway of oculo-lymphatic flow may be a novel strategy for regulating allosensitisation.
Methods
Mice and anaesthesia
Animal experimental protocols were approved by the Animal Care Committee of Nihon University (approval nos. P22-MED-028-1 and AP22-MED-062-3). All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research as well as in accordance with the ARRIVE guidelines. Male BALB/c mice (8–12 weeks old; Clea, Tokyo, Japan) were used to establish the lymphatic vessel ligation model. For corneal transplantation, BALB/c male mice (H-2, I-Ad, 8–12 weeks old; Clea) were used as recipients and C57BL/6 male mice (H-2, I-Ab, 8–12 weeks old; Clea) were used as donors. The mice were anesthetised using a mixture of medetomidine (1 mg/mL; ZENOAQ, Fukushima, Japan), midazolam (5 mg/mL; Sandoz, Tokyo, Japan), and butorphanol tartrate (5 mg/mL; Meiji, Tokyo, Japan). The mice were euthanised through carbon dioxide-induced hypoxia, followed by cervical dislocation. A total of 96 mice were randomly assigned to 24 cages (n = 4 mice per cage). There were no exclusions in our study.
Lymphatic vessel visualisation and evaluation
Lymphatic vessels were traced using vital staining. The minimum concentration of vital stain that did not cause corneal disorders was determined by evaluating different concentrations of Evans blue (#05604061; Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) and trypan blue (#20421102; Fujifilm Wako Pure Chemical Corporation). The 12 eyes were randomly assigned to four groups (n = 3 per group): (1) 1% Evans blue, (2) 10% Evans blue, (3) 10% trypan blue, and (4) 20% trypan blue. Both vital stains were administered in a single 0.1 mL eyedrop. The vital stains were thoroughly rinsed with phosphate-buffered saline (PBS) after 15 min. After shaving and disinfecting the surgical site with a 7.5% povidone-iodine surgical scrub, an incision was made in the skin at the maxillary region to confirm that the lymphatic vessels ran from the limbal to the nasal side. A longitudinal incision was made from the cervical region to the sternum after verifying the continuity of lymphatic vessels from the limbus to the maxillary region. Superficial cervical and facial lymph nodes were identified using a microscope, as previously reported5. The lymphatic vessels that were traced with vital staining ran from the limbus to the superficial cervical and facial lymph nodes. The cornea was observed once a week for 9 weeks using a slit-lamp biomicroscope to examine complications by applying a vital stain.
Lymphatic vessel inhibition in a normal murine model
After administering a drop of vital stain, 7.5% povidone-iodine was applied to the surgical site. The maxillary region was then partially skinned to reveal lymphatic vessels. Twenty-one eyes were divided into seven groups (n = 3 per group) to inhibit lymphatic vessel formation on the nasal side of the conjunctiva. The groups were as follows: (1) one suture with 10 − 0 nylon (MANI Ophthalmic Suture, Mani, Tochigi, Japan), (2) two sutures with 10 − 0 nylon, (3) bipolar cauterisation only, (4) two sutures with 10 − 0 nylon and bipolar cauterisation, (5) two sutures with 8 − 0 silk (CROWNJUN, Kono Seisakusho, Chiba, Japan), (6) two sutures with 8 − 0 silk and bipolar cauterisation, and (7) two sutures with 8 − 0 silk and cyanoacrylate glue (AlonAlpha A; Sankyo, Tokyo, Japan). The 8 − 0 silk was used to close the maxillary region in all the treated eyes. The lymphatic vessels between the two ligated lymphatic vessels were cauterised. Additionally, a small amount of cyanoacrylate glue was added to the ligated area. The tracer was administered following the same procedure described above on days 3, 7, 14, 21, 25, and 28 post-treatment, and the cervical lymph nodes were examined. The endpoint was determined based on the inflow of tracer into the superficial cervical and facial lymph nodes.
Evaluation of corneal rejection in the lymphatic vessel ligation model
As staining limits simultaneous lymphatic ligation and corneal transplantation owing to poor visibility, corneal transplantation was performed 1 week after lymphatic ligation. The methods of using two sutures with 8 − 0 silk and cyanoacrylate glue were used for lymphatic ligation. Four experimental groups were created to evaluate the effect of lymphatic vessel ligation: (1) full-thickness allograft corneal transplantation (control group, n = 14), (2) lymphatic vessel ligation (ligation group, n = 14), (3) full-thickness allograft corneal transplantation after lymphatic vessel ligation (ligation/Ag group, n = 14), and (4) untreated group without lymphatic vessel ligation or corneal transplantation (untreated group, n = 10).
Orthotopic corneal transplantation and defining graft rejection
Full-thickness corneal transplantation was performed as previously described5. Briefly, the recipient host bed was marked with a 1.5-mm trephine (Inami, Tokyo, Japan) and excised with a pair of micro-scissors (Vannas; Storz Instruments, San Dimas, CA, USA). The donor cornea was excised using a 2.0-mm trephine and transplanted into the host corneal bed with eight interrupted 11–0 needle sutures (Mani) in the right eye. After surgery, a gentamicin ointment was applied. The corneal sutures were removed 7 days after surgery. Eyes with postoperative cataracts, infections, or anterior chamber loss were excluded from the study. Corneal grafts were observed twice a week for 8 weeks using a slit-lamp biomicroscope. Graft opacity was scored on a standardised scale from zero to five, while neovascularisation was scored from zero to four24,25. Corneal rejection was defined as having consecutive opacity scores of 3 and a neovascularisation score of 2, according to previous studies24,25. Corneal angiogenesis and lymphangiogenesis were evaluated using whole-mount immunofluorescence staining 8 weeks after corneal transplantation.
Corneal flat mount immunostaining
The corneal flat mounts were excised after perfusion fixation and rinsed with PBS. The tissues were incubated with 20 mM ethylenediaminetetraacetic acid for 30 min at 37 °C to remove the corneal epithelium. The corneas were then rinsed thrice with PBS and lysed with 10% Triton X-100 (Sigma Chemical, St Louis, MO, USA) for 30 min at room temperature. The tissues were then blocked with 10% donkey serum albumin (#168665; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 30 min at room temperature. The tissues were incubated with purified rat anti-mouse CD31 (1:200; #557355; BD Biosciences Pharmingen, Franklin Lakes, NJ, USA), goat anti-mouse LYVE-1 (1:100; #AF2125; R&D Systems, Minneapolis, MN, USA), or rat anti-mouse podoplanin (1:50; ab318248; Abcam Cambridge, UK) overnight at 4 °C. The tissues were then rinsed with PBS and stained with Alexa Fluor 647-conjugated donkey anti-goat IgG (1:200; #A21447; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 488-conjugated donkey anti-rat IgG (1:200; #A21208; Thermo Fisher Scientific) secondary antibodies for 90 min at room temperature. Finally, corneal flat mounts were rinsed with PBS and covered with Vectashield mounting medium (Vector Laboratories, Newark, CA, USA). The immunofluorescence signals were evaluated using a fluorescence microscope (ZEISS Axio Imager Z2; Carl Zeiss Microscopy GmbH, Oberkochen, Germany). The area of the total cornea covered by blood or lymphatic vessels was analysed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
Kaplan–Meier analysis was used to construct survival curves, and the log-rank test was used to compare corneal graft survival. Immunostaining results for hemangiogenesis and lymphangiogenesis were compared between the groups using the Steel–Dwass test. Statistical significance was set at p < 0.05. All quantitative variables are expressed as mean ± standard deviation (SD). Statistical analyses were performed using the JMP Pro software v 15.0.0 (SAS Institute, Cary, NC, USA). Sample size was based on a previous study7.
Data availability
Data availability; The datasets used and/or analysed during the current study availablefrom the corresponding author on reasonable request.
References
Zhou, Y., Wang, T., Tuli, S. S., Steigleman, W. A. & Shah, A. A. Overview of corneal transplantation for the nonophthalmologist. Transpl. Direct. 9, e1434 (2023).
Pluzsik, M. T. et al. Changing trends in penetrating keratoplasty indications between 2011 and 2018 - histopathology of 2123 corneal buttons in a single Center in Germany. Curr. Eye Res. 45, 1199–1204 (2020).
Sangwan, V. S., Ramamurthy, B., Shah, U., Garg, P. & Sridhar, M. S. Rao, G. N. Outcome of corneal transplant rejection: A 10-year study. Clin. Exp. Ophthalmol. 33, 623–627 (2005).
Yamagami, S., Suzuki, S. & Tsuru, T. Risk factors for graft failure in penetrating keratoplasty. Acta Ophthalmol. Scand. 74, 584–588 (1996).
Yamagami, S. & Dana, M. R. The critical role of draining lymph nodes in corneal alloimmunization and graft rejection. Invest. Ophthalmol. Vis. Sci. 42, 1293–1298 (2001).
Hou, Y., Bock, F., Hos, D. & Cursiefen, C. Lymphatic trafficking in the eye: Modulation of lymphatic trafficking to promote corneal transplant survival. Cells. 10, 1661 (2021).
Cursiefen, C. et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol. Vis. Sci. 5, 2666–2673 (2004).
Lohrberg, M. & Wilting, J. The lymphatic vascular system of the mouse head. Cell. Tissue Res. 366, 667–677 (2016).
Maloveska, M. et al. Dynamics of Evans blue clearance from cerebrospinal fluid into meningeal lymphatic vessels and deep cervical lymph nodes. Neurol. Res. 40, 372–380 (2018).
Plsková, J., Kuffová, L., Holán, V., Filipec, M. & Forrester, J. V. Evaluation of corneal graft rejection in a mouse model. Br. J. Ophthalmol. 86, 108–113 (2002).
Hegde, S. & Niederkorn, J. Y. The role of cytotoxic T lymphocytes in corneal allograft rejection. Invest. Ophthalmol. Vis. Sci. 41, 3341–3347 (2000).
Wu, Y. et al. Organogenesis and distribution of the ocular lymphatic vessels in the anterior eye. JCI Insight. 5, e135121 (2020).
Subileau, M. et al. Eye lymphatic defects induced by bone morphogenetic protein 9 deficiency have no functional consequences on intraocular pressure. Sci. Rep. 10, 16040 (2020).
Dieterich, L. C., Tacconi, C., Ducoli, L. & Detmar, M. Lymphatic vessels in cancer. Physiol. Rev. 102, 1837–1879 (2022).
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science. 369, eaax4063 (2020).
Cursiefen, C. et al. Lymphatic vessels in vascularized human corneas:Iimmunohistochemical investigation using LYVE-1 and podoplanin. Invest. Ophthalmol. Vis. Sci. 43, 2127–2135 (2002).
Ambati, B. K. et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 443, 993–997 (2006).
Cursiefen, C. et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050 (2004).
Amadio, M., Govoni, S. & Pascale, A. Targeting VEGF in eye neovascularization: What’s new? A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol. Res. 103, 253–269 (2016).
Albuquerque, R. J. C. et al. Alternatively spliced VEGF receptor-2 is an essential endogenous inhibitor of lymphatic vessels. Nat. Med. 15, 1023–1030 (2009).
Zhang, W., Schönberg, A., Bock, F., Cursiefen, C. & Posttransplant VEGFR1R2 trap eye drops inhibit corneal (lymph)angiogenesis and improve corneal allograft survival in eyes at high risk of rejection. Transl Vis. Sci. Technol. 11, 6 (2022).
Dohlman, T. H. et al. VEGF-trap Aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 99, 678–686 (2015).
Chen, L. et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat. Med. 10, 813–815 (2004).
Chen, G. L. et al. Evaluation of corneal graft survival in mice model. Int. J. Ophthalmol. 6, 578–583 (2013).
Ma, D. et al. Conditions affecting enhanced corneal allograft survival by oral immunization. Invest. Ophthalmol. Vis. Sci. 10, 1835–1846 (1998).
Acknowledgements
The study is reported in accordance with ARRIVE guidelines.
Funding
This research was supported by the Charitable Trust Fund for Ophthalmic Research in the Commemoration of Santen Pharmaceutical’s Founder and Japan Cornea Society.
SPK, superficial punctate keratitis.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript. A.I, and T.H. were writing, reviewing, and editing of the manuscript. T.S. was doing curation of the data and editing of the manuscript. K.Y. was reviewing and editing of the manuscript. S.Y. was responsible for supervision and validation. All authors critically checked the manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Igarashi, A., Hayashi, T., Shimizu, T. et al. Inhibiting corneal transplantation rejection via lymphatic vessel ligation in a novel murine model. Sci Rep 14, 25692 (2024). https://doi.org/10.1038/s41598-024-77160-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77160-9