Abstract
To enhance the adsorption efficiency of activated carbon for heavy metals, herein we synthesized a novel composite adsorbent by loading of nickel/aluminum layered double hydroxides (Ni/Al-LDH) onto a chemically modified Egyptian rice husk-derived activated carbon. The characterization techniques used for determining the chemical and surface structure of the prepared composite were including FTIR, XRD, SEM, and BET which confirmed the successful loading of LDH onto the prepared activated carbon surface. The modified activated carbon established significantly upgraded performance in eliminating lead ions from wastewater. Adsorption studies revealed that the process follows Freundlich isotherm and pseudo-second-order kinetics, indicating chemisorption as the rate-determining step. The maximum lead ions removal (using 50 ppm concentration solution) was 82% after 210 min at pH 7. The improved lead ions removal efficiency was attributed to the synergistic effect of the activated carbon’s surface chemistry and the LDH’s ion exchange properties. The presence of chelating groups like hydroxyl (–OH), amide (–CO–NH–), carboxylate (–COOH), and nitrogen-containing functional groups on the activated carbon surface, along with the hydroxide groups of the LDH, facilitates the complexation and adsorption of lead ions.
Similar content being viewed by others
Introduction
AC is produced from the conversion of high carbon content raw materials and is characterized by its high surface area, large types of pores, and amorphous in nature. Due to its remarkable mechanical and adsorptive properties, it is utilized in high mass in the industrial sector. A huge mass of AC is used per year as filler and adsorbents during the remediation of different types of pollutants from wastewater including gases, organic and metal ions threats1. As a result, the needs for renewable, sustainable, and cheap materials are required for the production of AC2,3. Agricultural wastes are the main resource for AC including wastes of different trees of palm4, almond, bamboo5, banana6, cassava7, coconut8, cotton9, grape10, oil palm11, olive12, walnut, rice husk, and peanut trees. Rice husk is the most agricultural waste produced around the world. Its chemical structure contains silicate in addition to the carbonaceous compounds. The presence of silicate can be diverse with the action of AC produced; hence removal of silicates is performed using alkaline treatment to increase the activity of the produced AC13,14,15.
Preparation of rice husk for production of AC involves crushing, washing, and drying of the husk. Then two types of processes are performed, physical and chemical activation16,17. Physical activation of activated carbon aims to increase its surface area and porosity, which increases its activity during its application processes. Physical activation proceeded via influence of heat at different temperature ranges, either at high temperatures (50 to 120 °C), or at elevated temperatures reach to carbonization (800 to 1000 °C) in absence of oxygen18. Steam, CO2, N2, or other gases may be used during the physical activation. The chemical activation of activated carbon utilizes saturated materials with specific chemical precursors including oxidant or dehydrate (ZnCl2, NaOH, KOH, K2CO3, H3PO4) using thermal treating at the range of 400 °C to 600 °C, to obtain activated carbon with proper porosity and functionality. The functionality with oxygen acquires the activated carbon its acidic character19. The functionalization occurred by treatment of activated carbon with sulfuric acid in high concentration. That increases the surface acidic sites and consequently the overall acidity of the activated carbon. Another form of activated carbon was the basic form or basic surface modification20,21. The basic nature of the activated carbon can be introduced by the introduction of functionalized nitrogen. The introduction of nitrogen groups can be performed using ammonia, nitric acid, or amines. Basic activated carbon was an important adsorbent for capturing acidic gaseous pollutants, metal ions and acidic dyes.
The harmfulness of Pb2+ ions reduces energy levels, disturbs the functionality of brain and various other organs of the human bodies such as lungs, liver, and kidneys22. In rivers, these ions cause distressing influence for ecological balance due to their neurotoxic effect on fish in the aquatic environment. In fish bodies, Pb2+ ions are interacted with blood to inhibit the biochemical reaction, which influence their communication with the surroundings23. In plant system, Pb2+ in soil decreases the production rate, and hinders the important processes of plant such as the darkening and deterioration of leaves, decrease of photosynthesis and water absorption by roots24. Due to their integral harmfulness and bio-magnification, metal ions and metallic compounds are recognized as fatal toxins contaminated with sources of water and have a great ability to accumulate in the food chain25. Metal ions, especially transition ions can harm the nervous system, and also harm the vital tissues leading to severe sicknesses26. Among the various toxic metal ions on the living organisms, fishes, birds, animals, and humans, lead is considered top of ions that had a direct impact on them. According to WHO, and EPA (the World Health Organization and US Environmental Protection Agency) recommendations and limitations, the allowed concentration of lead ions in drinking water (maximum limits) were 0.01 and 0.015 mg/l, respectively27.
The removal of metal ions from the aquatic system is achieved by using several techniques including: chemical precipitation, electrolytic recovery, ion exchange, chelation, membrane separation, and adsorption by adsorbents28,29. These technologies had advantageous and disadvantageous depending on the aim of the process, but adsorption process proclaimed as the most effective. Among the several advantageous of adsorption process of pollutants using the adsorbents, including: the high performance, low cost process, applicable at wide pH range of the medium, and easy to operate either in form of beads, columns, or after loading the adsorbent on a support to form sheets. Some disadvantageous can be appear at the surface, such as it is reversible and waste products produced after the process, in addition to the weak selectivity. To achieve the effective adsorbent that is active and rapid for pollution removal, the technology of adsorbent construction must be directed towards the field of nanotechnology to achieve an incredible applicability during the adsorption process.
Herein, we used a husk of rice as a starting for the preparation of AC in physical and chemical reactions. The silicate in the rice husk was alkaline treated to decrease its content in the produced AC. The prepared AC was chemically modified by loading nickel/aluminum-LDH on its structure to enhance its activity during the treatment of wastewater contaminated by Pb2+ ions. The chemical structures of the prepared AC and AC-Ni/Al-LDH will be characterized using different tools. Also, the adsorption and kinetic models were determined to describe the up-take process will be determined. The adsorption/interaction mechanism between Pb2+ ions and both AC and modified AC will be discussed.
Materials and methods
Preparation of AC from rice husk
A suitable amount of rice husk from El-Sharqia Governorate in Egypt was collected (1500 g) for assembling of AC. The collected amount was washed by flow of water several times to dirt, impurities, and dust removed, and open oven drying for 48 h at 313 °K30. Afterward, 500 g of clean and dried rice husk were crushed into fine irregular particles with an average particle size of 500 μm. The physically treated husk was chemically treated by soaking in 400 mL sodium hydroxide solution (2 M) (treated husk/NaOH = 1/3 weight ratio) for 6 h31. The chemically activated husk was washed by distilled water until the water effluent becomes at pH of 6.5–7 and dried for 12 h at 373 °K, followed by grinding into fine powder. To enhance the formation of the pores in the AC, the powder was placed in sunlight in a humid atmosphere before the pyrolysis step. The pyrolysis of the activated husk matrix was performed in a porcelain crucible in a muffle furnace at 1023 °K under a nitrogen atmosphere (nitrogen flow of 60 mL/min) for 100 min. The yielded AC was cooled to 298 °K, finely ground, soaked in 250 mL hydrochloric acid solution (1 M), and washed with bidistilled water to eliminate the residual impurities32. The produced AC was dried at 353 °K for 24 h for complete dryness (Fig. 1).
Preparation of AC-Ni/Al-LDH
The loading of nickel/aluminum-LDH on the prepared AC was conducted via the co-precipitation technique (Fig. 2). In typical procedures33, 5 g of the prepared AC was scattered in 250 mL of bidistilled water and stirred at 800 rpm for 30 min. Aluminum nitrate nonahydrate (Al(NO3)3 9H2O, 0.03 mol, 11.25 g) was dissolved in 100 mL of bidistilled water, and another 100 mL solution of 0.015 mol nickel nitrate hexahydrate (Ni(NO3)2.6H2O) and 0.015 mol of magnesium nitrate hexahydrate (Mg(NO3)2 6H2O) in bidistilled water was prepared34. The two solutions were added to the AC suspension under strong stirring for 30 min at 303 °K. The pH of the medium was then adjusted at 9 by dropping 75 mL of an alkaline solution (1 M) consisting of sodium hydroxide and sodium carbonate for 1 h. The reaction medium was allowed to forceful mixing for 24 h at 313 °K, decanted to eliminate the clear layer, and then centrifuged to remove the aqueous medium. The product was washed away using deionized water several times until a pH of 6–7, and vacuum dry for 18 h at 343 °K to achieve the product of AC-Ni/Al-LDH.
Adsorption experiments
The screening of up-take process of lead ions (Pb2+) by AC, and AC-Ni/Al-LDH composites was studied by scattering a fixed weight of the two composites separately in 0.1 L of Pb2+ solution at a fixed conc. (in ppm( using a 500 mL reactor followed by stirring at 200 rpm at 298 oK and finally filtering21. The influence of the process duration (t, min( was determined by running the experiments for different times of 30–240 min using a 100 ppm solution of Pb2+ ions and 0.25 g of the two adsorbents AC, and AC-Ni/Al-LDH. The influence of Pb2+ ions conc. on the up-take process was recognized by using 0.25 g of AC, and AC-Ni/Al-LDH and 50–250 ppm of Pb2+ ions. The influence of adsorbents amounts on the process was determined by using 100 mL solution of Pb2+ ions (100 ppm) and different amounts of the adsorbents (0.1–0.4 g). The effect of pH on the adsorption process was determined at different values of 2 to 10, while, the zeta potentials of the two adsorbents were determined using potentiodynamic measurements. The adsorption process was performed at three different temperatures 298 °K, 308 °K, and 318 °K. The leftovers of Pb2+ ions in the filtered solutions were estimated using a flame atomic absorption spectrometer (PinAAcle-500, Perkin Elmer, USA).
Recycling and reusability of adsorbent
The reusability test allows knowing the ability of using the adsorbent several times with acceptable removal efficiency. Herein, accurate weight of the prepared AC-Ni/Al-LDH composite (0.3 g/L) was mixed with 50 ppm of Pb2+ solution under continuous stirring of 150 rpm at 25 °C for 120 min. Then the solution was filtered, and the concentration of the lead ions in the eluent was determined to calculate the adsorption efficiency. The used adsorbent was collected and treated by a solution of nitric acid (0.5 M) followed by washing with sodium hydroxide solution (0.5 M) for desorption of Pb2+ ions and regenerating the adsorbent35. These procedures were repeated for five cycles after compensating the loss in the adsorbent weight to investigate the reusability of the prepared adsorbent.
Results and discussion
Characterization
FTIR spectroscopic analysis
FTIR spectroscopic analysis were performed for the prepared compounds using Fourier transform infrared spectroscopy (Nicolet IS-10, USA) in the wavenumber range of 400–4000 cm− 1. The prepared compounds were mixed by proper amounts of completely dried KBr salt and pressed under piston into disks. Then, the samples were placed in a FTIR-holder in the path of the IR radiation source to achieve the resultant record of each compound.
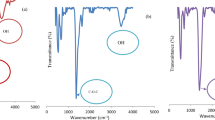
AC: Various FTIR absorption bands were assigned (Fig. 3A): 3415 cm− 1, 2928 cm− 1, 2850 cm− 1, 2362 cm− 1, 1740 cm− 1, 1621–1520 cm− 1, and 1120 cm− 136. The absorption band situated at 3415 cm− 1 is credited for –OH stretching, and also for the adsorbed water molecules. Bands of 2928 cm− 1 and 2850 cm− 1 designated the attendance of aliphatic –CH stretching37,38. The band at 2362 cm− 1 is pointed for the C ≡ C alkyne stretching39. The band at 1740 cm− 1 signifies the carbonyl/carboxyl groups, while the 1621 –1520 cm− 1 band evidenced the aromatic C = C ring40. The C–O stretching vibration of alcohols, phenols, ether, and esters was proved by the 1120 cm− 1 band41. Also, the bands at 1092 cm− 1 and 1195 cm− 1 represented the stretching vibration of Si-O groups in the SiO4 structure.
AC-Ni/Al-LDH: Fig. 3B represents the infrared spectra of the AC-Ni/Al-LDH composite. The bands located at 3390–3600 cm− 1 and 1650 cm− 1 are for stretch and bending vibrations of the –OH group. The band at 1092 cm− 1 and its shoulder around 1195 cm− 1 represented the asymmetrically stretching of Si–O groups in the SiO4 structure. The sharp band at 1391 cm− 1 is accredited to the asymmetric stretch of Si–O–Si groups. The band represented the symmetric Si–O stretching proved by the weak band at 797 cm− 1, while 475 cm− 1 designates the bend vibration of SiO4 groups42. The carboxyl group and C = C bond in aromatic rings were confirmed by 1621 cm− 1, and 1552 cm− 143,44.
XRD diffraction analysis
XRD measurements were performed on Panalytical Alpha-1 XRD instrument using copper radiation tube and nickel filter, while the relative intensity was recorded at 2θ of 4–80° at 40 kV and 40 mA.
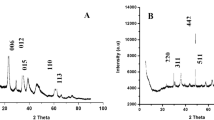
AC: The AC structure was studied using XRD diffraction, Fig. 4. The broad diffraction peaks located at 2θ of 15° to 30° are attributed to the amorphous structures of AC45. While the broad peaks in the range of 2θ = 40° to 50° were assigned to the graphite structures 34. The board peak at 22.3° indicated the organic components in the prepared AC46. The diffractogram of the prepared AC showed its amorphous nature.
AC-Ni/Al-LDH: The diffractogram showed three characteristic patterns at 10° (003), 21° (006), and 35° (009) represented the layered form of the Ni-Al LDH, Fig. 4. The AC-Ni/Al-LDH composite showed the diffraction peaks at 10°, 21°, 28°, 35°, and 62° corresponding to (003), (002), (006), (009), and (110) plans, respectively. That indicates the prepared composite has a layer structure, in addition to the carbon profile from the AC platform47. The diffractogram in Fig. 2 collected the characteristics of the AC and the Ni/Al-LDH composite, which is in agreement with the reported data48.
SEM study
SEM pictures of the prepared compounds were performed using Zeiss EVO LS15 SEM instrument with variable pressure secondary electron detector (VPSE G3).
Figure 5A represents the SEM pictures of the activated carbon and AC-Ni/Al-LDH composites. It is clear that the activated carbon has different pore structures, which was confirmed by BET measurements. The elemental composition of the activated carbon was determined using EDX spectroscopy, and the following composition was pointed: C: 89.8%, O: 8.4%, Si: 1.6%, and Al: 0.2%. Modification of the activated carbon (Fig. 5B) showed decrease in the pore shapes (as represented in BET measurements) and the deposited LDH was also observed. That was confirmed by the EDX spectroscopy for the modified form as follows: C: 80.6%, O: 16.1%, Mg: 1.3%, Ni: 0.9%, Al: 0.99%, and Si: 0.2%. The decrease in C% and the increase in O%, in addition to the presence of Mg, Ni, and Al in the elemental composition represented the successful loading of Ni/Al-LDH on the activated carbon.
BET-surface area measurements
N2/adsorption-desorption isotherms were obtained using a Quantachrome-Nova-3200 instrument (USA) to study the surface area, pore volume and pore diameter of the prepared compounds. The degasification of the samples was performed for 12 h at 100 °C under vacuum (5–10 mmHg).
The N2-adsorption/desorption profiles of pure AC and AC loaded by Ni/Al-LDH composites are presented in Fig. 6. The BET profile isotherms corresponded to AC and the AC-Ni/Al-LDH composites were comprised IV type rendering to classification of IUPAC49,50, and accompanied by hysteresis loop of class H351. IV hysteresis is allied for the relatively large pore size composites and a high surface heterogeneity extent. The distinct heterogeneity of the composite surfaces is related to the diverse types of adsorption sites allocated on these surfaces, which consequently had different adsorption tendencies towards the dispersed materials in the medium. The extracted values of surface area and pore volume for both composites from the N2-adsorption/desorption profile are listed in Table 1. Different pore dimeter values were appeared in the pore diameter profile impeded in the BET figure indicating the presence of the three expected types of pores, with the majority of the micropores with pore diameters of 10.6, and 8.4 in case of AC and AC-Ni/Al-LDH composites, respectively52. The data in Table 1 indicated that the decrease in the pore volume and the surface area are ascribed to the arrangement of the formed Ni/Al-LDH on the AC framework, which leads to blockage of the AC pore volumes.
Figure 7 represents the effect of AC and AC-Ni/Al-LDH amounts variation from 0.1 to 0.4 g on the efficiency of Pb2+ ions removal process. It is clear that the small weight used of the two adsorbents (composites) exhibitd the lowest removal efficiency due to the limited adsorption sites prsented in the adsorbents used. The gradual increase in the adsorbents weight from 0.1 g to 0.25 g has a gradual upsurging influnce on the removal process53. That was ascribed to the gradual increase in the number of available active sites which capable to anchor Pb2+ ions from the medium. Further increase in the adsorbents amounts from 0.3 g to 04 g has no significant effect on the removal efficiency due to the comparatively higher number of the available aactive sites in the system whih interact by the constant amount of Pb2+ ions in the medium. The profile erpresented the optimum amount of the two adsorbents during the rmoval process of Pb2+ ions of 100 ppm at 0.25 g/100 mL.
The chemical structure of the activated carbon comprises arrangement of high abundance of carbon atoms and several surface functional groups such as hydroxyl, carbonyl, amino, and carboxylate groups. Similarly, AC-Ni/Al-LDH composite comprises a high concentration of hydroxyl groups. Consequently, the pH of the medium has a great influence on the surface charges of AC, and AC-Ni/Al-LDH composites. Figure 8 represents the zeta potential vs. pH of AC and AC-Ni/Al-LDH composites. It is clear that the two composites have positive zeta potentials values during the acidic range (pH 2–6). While, during the alkaline medium (pH > 7–11) the composites showed negative zeta potentials. The profile represented the pH value corresponding to the zero of point charges (pHpzc), which were pointed at 6.2 and 6.8 for AC and AC-Ni/Al-LDH composites, respectively. pHpzc points represented the pH of the medium at which the two composites comprised neutral surface.
The influence of pH of the medium on the adsorption efficiency of Pb2+ using AC and AC-Ni/Al-LDH composites was investigated during the pH range of 2–12 and profiled in Fig. 9. It is apparent from Fig. 7 that the acidic medium decreases the adsorption efficiencies of the adsorbents, while these efficiencies are gradually increased by moving towards the neutral medium. At pHpzc point, the adsorption efficiencies of the two composites are reached the maximum values at 71% and 82% for AC and AC-Ni/Al-LDH composites, respectively. Increasing the pH higher than pHpzc decreases the adsorption efficiencies considerably. At lower pH than pHpzc (acidic medium), the surface of the composites are acquiring positive charges due to the cation exchange and the protonation of the surface functional groups (adsorption active sites). That resulted in a repulsion interaction between the positively charged Pb2+ ions and the protonated adsorption sites which decreases the adsorption efficiency considerably54. At higher pH than pHpzc, anion exchange occurred and the counter ions (NO3−) of the composite surface are replaced by the anions from the alkaline medium. Furthermore, Pb2+ ions are oxidized in the alkaline medium and precipitated as PbO precipitate, which failed the adsorption efficiency as represented from Fig. 9.
Adsorption isotherm studies
This work presented the Pb2+ remediation from wastewater using AC-Ni/Al-LDH.
Figure 10A recognizes the Pb2+ ions conc. influence on the remediating efficiency of AC-Ni/Al-LDH composite. The initial increase in conc. of Pb2+ ions reduces the obtained remediation efficiency, which was attributed to the fullness of the adsorbent’s sites of adsorption by up-surging Pb2+ ions conc55. The obtained conc. vs. efficiency data were proceeded for defining the isotherm of Pb2+ ions remediation on AC-Ni/Al-LDH composite to afford the remediation statistics and mechanism, and the distribution of Pb2+ on the adsorbent surface. Several adsorption isotherms had been deduced during Pb2+ up-take on AC-Ni/Al-LDH composite such as Lm, and Fm isotherms56.
Lm isotherm supposed the uniformity of adsorptive centers and neglecting the repulsive interaction that occurred amongst the Pb2+ ions at surface saturation and the presented ionic species. This can be practical only when dealing with diluted solutions57. The lined form of Lm58 was expressed as follows:
\(\frac{{{C_e}}}{{{q_e}}}=\frac{1}{{{q_{\hbox{max} }}{k_l}}}+\frac{{{C_e}}}{{{q_{\hbox{max} }}}}\)
Ce: equilibrium conc. (ppm), qe: the adsorbed ion (mg/g), qmax is qe for a complete monolayer (mg/g), KL: constant related to the affinity of binding sites and is a measure of the adsorption energy (L/mg), respectively.
Lm was applied to elucidate the adsorption equilibrium of Pb2+ ions on AC and AC-Ni/Al-LDH in the medium. Relating the experimental data and the linear equation of Lm (Fig. 10B) for AC and AC-Ni/Al-LDH gave relationship profiles with R2 coefficients of 0.8839, and 0.9794, respectively (Table 2), which discloses the unfitting of the Lm model in defining the experimental data of Pb2+ ions up-take using the two adsorbents (as R2 value is far of unity). The deviation from Lm suggests an inhomogeneity and irregular activities of the effective adsorptive sites of the AC and AC-Ni/Al-LDH59.
Fitting the measured values of the up-take process was performed following Fm according to the following equation:
\(\ln {q_e}=\frac{1}{n}\ln {C_e}+\ln {k_f}\)
KF and n: constants related to adsorption capacity and intensity, respectively.
Fm assumes the up-take of ions arises at heterogeneous surfaces, and this model is appropriate for diverse types of adsorbents and at a wide range of ions conc. The adsorption experimental data were corroborated using the Fm equation as represented in Fig. 10C, the Fm parameters itemized (Table 2). Principally, the correlation coefficient (R2) deduced was around the unity (R2 = 0.9999) for AC and AC-Ni/Al-LDH, which specifies the model validity concerning Fm. Consequently, the up-take mechanism of Pb2+ ions on both AC and AC-Ni/Al-LDH can be described as it occurs on their dissimilar adsorptive sites, while the adsorption strength is mainly dependent on their effective charges16,60. The inhomogeneity of the two adsorbents was originated from the amorphous structure of the AC crystal structure, which reflected also on the modified form61. Two important factors were delivered from the obtained parameters of Fm: n and kf. n value (0.98) indicates the strong attachment strength of Pb2+ ions on the AC-Ni/Al-LDH surface62, while kf (the capacity of adsorption of AC-Ni/Al-LDH at the equilibrium conc.) was 48.4 ppm, Table 2. Hence, the data extracted from the Fm revealed that the strength of Pb2+ ions adsorption takes place on AC-Ni/Al-LDH active sites based on their strength and activities62.
Tm isotherm model is described by the following equation:
\({q_e}=BLnA+BLn{C_e}\)
B = (RT/b), R: gas constant (8.314 J mol− 1 K− 1), T: temperature (K), Ce: equilibrium concentration (mg L− 1), and qe: adsorbed Pb2+ per unit adsorbent (mg g− 1) at equilibrium.
Tm isotherm is represented in Fig. 10D. The low R2 value of the obtained experimental results applied for AC and AC-Ni/Al-LDH composite (R2: 0.9257, 0.9627) revealed that the model is not suitable for describing the adsorption process. The values of the Tm constants were determined from the slope and intercept of the obtained equations of state, Table 2. In order to compare the studied adsorption isotherms of AC and AC-Ni/Al-LDH composite based on their R2 values, the adsorption process obeys Freundlich adsorption isotherm by having R2 values of 0.9882, and 0.9999, respectively.
Kinetics of the adsorption process
Figure 11A profiled the Pb2+ adsorption efficiency onto AC-Ni/Al-LDH composite in a time function. It was observed that the efficiency of up-take was regularly augmented upon up-surging the up-take process duration. The kinetics and rate-determining stages of Pb2+ ions up-take onto AC-Ni/Al-LDH were determined based on the data in Fig. 11A by realizing different kinetic models including PFO and PSO kinetics. It was reported that the up-take process comprises several stages including diffusion control, chemical reactions, and particle diffusion stages63,64. Elovich, PFO and PSO kinetic models are profiled according to the following equations (qt: Pb2+ ions conc. onto AC-Ni/Al-LDH (mg/g) at time t; k1 (1/min), k2 (g/mg.min), α (µg g− 1 h− 1), β (µg g− 1): constants of PFO, PSO, and Elovich models):
Elovich model was studied and applied for the experimental data. The represented profile of the model (Fig. 11B) showed the non-linear behavior with correlation coefficients of 0.9715 and 0.9948 for AC and AC-Ni/Al-LDH composite (Table 3). That considerably deviates from unity, indicating the unsuitability to describe the adsorption process by this model.
Figure 11C profiled the PFO model data of Pb2+ ions up-take on AC-Ni/Al-LDH composite. At 0–90 min time of the up-take process, the relation goes straight, but the line shape becomes unpredictable after 90 min. The model at the pre-stage of the process has R2 of 0.9998, while at the late stage of the process (at equilibrium) the correlation factor was decreased considerably to 0.1. That shows the validity of the PFO model at the first stage of the process, while the model drawback appears at the equilibrium of the process65.
For finding a proper kinetic model describing the up-take of Pb2+ ions on AC-Ni/Al-LDH composite, the measured data was utilized to fit the PSO model (Fig. 11D). The extracted variables from the PSO model were R2 parameter, equilibrium conc., and up-take process rate constant (R2, qe, and k2, Table 3. The applicability of the PSO model to fit the experimental data during the whole range of Pb2+ ions conc. and the process time was confirmed from almost unity of R2 value (R2 = 0.9999), and comparable qe to the experimental value. According to this model, the Pb2+ ions interact with the different adsorptive centers (functional groups) of the AC-Ni/Al-LDH composite66,67.
It is reported that the up-take process occurred through several steps including surface up-take, diffusion of particles into the pores of the adsorbents, and finally the precipitation and formation of multilayers in the core of the adsorbent pores. These processes were included in the interparticle diffusion model of adsorption. The interparticle diffusion stage has described the governing stage that determines the rate constant (kint, mg/g.min1/2) for the up-take process68, according to the following equation:
The qt vs. t1/2 plot was profiled (Fig. 11E) to calculate the interparticle diffusion rate (kint) and R2 parameter. Figure 11E points to three steps that occurred during the up-take process. Step I in Fig. 11E is linear, and represents the Pb2+ ions diffusion, while step II represents the equilibrium state between the adsorbed and the released Pb2+ ions and the formation of monolayer ions on the pore surfaces. Finally, step III showed the formation of multilayer precipitated Pb2+ ions in the pores of the adsorbent68. R2 values of the different stages were considered and listed in Table 3. The results recognize that the up-take process arose throughout multiple stages and the diffusion process of Pb2+ in the pores of AC-Ni/Al-LDH composite was the rate-determining stage69.
Temperature effect and thermodynamic parameters of the adsorption process
The influence of temperature on the adsorption of 100 ppm of Pb2+ ions on AC and AC-Ni/Al-LDH composites at pH of 7 for 120 min was profiled in Fig. 12 It is clear for both adsorbents that the adsorption efficiencies are increased by increasing the temperature from 25 °C to 45 °C. That can be attributed to the increase in the mobility of the free Pb2+ ions in the solution, and the high rate of diffusion of the positively charged ions towards the negatively charged centers allocated at the surfaces of the adsorbents70. The rate of diffusion of the positive ions towards the adsorption sites at 45 °C was slightly decreased than that occurred at 35 °C due to the decrease in the binding affinity of the Pb2+ ions on the adsorbents surfaces as the desorption process can be occurred due to the excessive increase in the temperature and the increase in the kinetic energies of the ions.
The influence of temperature on the adsorption process was used to evaluate the thermodynamic functions of the process. Figure 12 represents the endothermic nature of the adsorption process as the adsorption efficiencies are increased by increasing the temperature from 298 °K to 318 °K.
The Van’t Hoff plot of the adsorption process of Pb2+ ions on AC and AC-Ni/Al-LDH composites (Fig. 13) was used to determine the most important thermodynamic parameters including Gibbs free energy, enthalpy and entropy (ΔG, ΔH, ΔS) using Eqs. 1–371.
Kb, R: equilibrium constant and universal gas constant.
The thermodynamic parameters of the adsorption process of Pb2+ using AC and AC-Ni/Al-LDH composites were calculated according to Eqs. (1–3) using extracted Kb parameter from Fig. 13, and were listed in Table 4. The negative values of ΔG established the spontaneous progress of the adsorption process, which was increased by increasing the temperature from 298 °K to 318 °K. On the other hand, the endothermic nature of the adsorption process of Pb2+ on AC and AC-Ni/Al-LDH composites was confirmed by the positive enthalpy (ΔH) values at 47.157 kJ mol− 1 and 47.872 kJ mol− 1. The endothermic nature of the adsorption process comprises the gradual increase in the adsorption efficiency by the gradual rising in the temperature of the process72. Furthermore, the positive ΔS values of the adsorption process using AC and AC-Ni/Al-LDH composites (163.453 kJ mol− 1 K− 1, 169.796 kJ mol− 1 K− 1) is accompanied by a high degree of freedom for Pb2+ ions in the process medium and also at the interfaces of the adsorbents which facilitates the interaction between the ions and the adsorptive active sites.
Recycling and reusability
Figure 14 represents the reusability of the activated carbon and the prepared AC-Ni/Al-LDH composite after the adsorption of Pb2+ ions from the medium during several cycles. It is clear that the activated carbon showed steeper decrease in the removal efficiency during the first three cycles starting from cycle 1 at 76% to cycle 3 at 75%, while further usage decreases the removal efficiency to 69% after the 5th cycle. On the other hand, AC-Ni/Al-LDH composite has removal efficiency at 94% after the first cycle, while the second and third cycles decreased the removal efficiencies to 93% and 91% respectively. After the fifth cycle, the removal efficiency was decreased to 90%. The reusability of the adsorbents showed acceptable efficiencies until the third cycle with successful regeneration. That can be suggested the promising applicability of the prepared absorbents for Pb2 + removal from the contaminated medium.
Mechanism of adsorption
The adsorption of Pb2+ metal ions onto the prepared activated carbon improved by AC-Ni/Al-LDH can be described based on the analytical results, adsorption isotherm, and kinetic model points of view73,74. Firstly, the analytical methods used to elucidate the mechanism of the adsorption were: FTIR, XRD, and SEM-EDX spectroscopy.
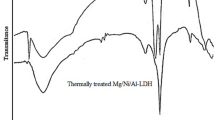
FTIR spectra of the AC-Ni/Al-LDH composite loaded by Pb2+ ions after adsorption of lead ions (Fig. 15) showed that the hydroxyl (–OH) band at 3446 cm− 1 was shifted to 3425 cm− 1 with a decrease in its intensity. This indicates the formation of interaction between the lead ions and the hydroxyl groups at the composite surface, i.e., complexation interaction. The decrease in the intensity of the hydroxyl groups in the profile can also be ascribed for the interaction between the lead ions and the hydroxyl groups. The decrease in the intensity of the hydroxyl groups can be referred to the chelation interaction occurred between the lead ions and the oxygen of the hydroxyl groups, and also for the interaction between the ions and these groups to form the lead hydroxide precipitates. The formed precipitates are physically adsorbed at the surface of the prepared composites and filling the composite pores75. The presented aromatic, and carbonyl moieties in the chemical structure of the activated carbon were showed a deep influence on the adsorption of lead ions onto the prepared composite. The peaks of the carbonyl C = O groups, and the aromatic groups (C = C) were appeared at 1621 cm− 1, and 1552 cm− 1 were shifted to 1616 cm− 1 and 1506 cm− 1 after adsorption of lead ions76. The shifting of the two bands can be ascribed for the interaction between the lead ions and the electron clouds of the benzene rings to form M-π interaction. While the lone pairs of oxygen at the carbonyl and hydroxyl groups form M-n interaction between the lone pair of electrons of oxygen and the metal ions (complexation interaction)77. The complexation mechanism is considered one of the effective mechanisms that occurred during the uptake of Pb2+ ions on the AC-Ni/Al-LDH composite due to the presence of active chelating groups such as hydroxyl (–OH), amide (–CO–NH–), carboxylate (–COOH), nitrogen groups (pyridine, amide, and di-amide derivatives, (Scheme 1) which can form stable Pb2+-complexes on the composite surface78,79,80. It is clear also that the intensities of the two peaks at 460 cm− 1 and 691 cm− 1 corresponded to Mg-O and Al-O was significantly decreased. That proves the ion exchange interaction between Pb2+ and Mg2+ of the LDH composite.
Several reports were reported that the main reason for the efficient metal ions adsorption with LDH was the formation of metal salts precipitates on the surface in forms of oxides, hydroxides, and carbonates81. XRD analysis of AC-Ni/Al-LDH composite after adsorption of lead ions (Fig. 16) was compared by its profile before adsorption and the results comprised the presence of four new characteristic patterns at 2θ = 21°, 26°, 36°, 42°, and 49° which were corresponding to the formed new phase of PbCO3 2H2O precipitate82. That provides an insight view about the precipitation mechanism occurred during the interaction between the lead ions and the prepared composite. Also, that evidenced the precipitation of the formed lead hydroxide precipitates in the pores of AC-Ni/Al-LDH composite. The precipitation of the formed lead hydroxide was further proved from the adsorption isotherm model describing the adsorption process. Precipitation is a remarkable process that occurs due to an interaction between Pb2+ ions and the hydroxyl groups on AC-Ni/Al-LDH composite surface, it also occurs continuously on the surface80. The agreement between the experimental adsorption data and the inter-particle diffusion kinetic model (precipitation mechanism) is describing the precipitation of the adsorbed species on the composite surface and subsequently the filling of the composite pores.
The XPS study was used to investigate the interaction between the lead ions and AC-Ni/Al-LDH composite. Figure 17 showed the full range survey of AC-Ni/Al-LDH composite before and after adsorption of lead ions from the medium. Figure 17 represents the binding energy of Pb2+ ions after adsorption onto the AC-Ni/Al-LDH composite, and revealed that the obtained binding energies of Pb2+ ions were 138.42 eV (Pb 4f7/2) and 143.4 eV (Pb 4f5/2). The binding energy values of Pb(NO3)2 were reported at 139.4 eV and 144.3 eV83. The obtained right shift was around 1.1 eV and suggests a strong interaction between Pb2+ ions and AC-Ni/Al-LDH composite. As shown in Fig. 17, the XPS of O 1s had a slightly binding energy shift by about 0.45 eV which represents the strong attractions between M(II)-O and Pb2+ ions. The binding energy value of Mg 2p before Pb2+ adsorption was 49.75 eV (Fig. 17), while it was shifted to 50.25 eV with a total positive shift by about 0.5 eV, which additionally proves the presence of Mg-O-Pb interaction at the surface of AC-Ni/Al-LDH composite. Inspecting the XPS full range survey of AC-Ni/Al-LDH composite before and after the adsorption of Pb2+ ions revealed that there is no change occurred in the binding energy of Ni 2p in the composite after the adsorption of Pb2+ ions suggesting that Ni ions had no benefits during the adsorption, precipitation, or complexation of Pb2+ ions. It was reported that the using of Ni ions in the composition of the LDH composite during the adsorption of metal ions enhances the electrochemical properties of the composite which improves its adsorption ability during the different adsorption mechanisms82,84.
SEM pictures of AC-Ni/Al-LDH composite after adsorption of lead ions represented in Fig. 18 showed the filling of the composite pore due to the precipitation of PbCO3.2H2O due to the interaction of Pb2+ ions by the hydroxyl groups on the surface of the adsorbent. The EDX analysis of the composite after the adsorption of Pb2+ ions showed the abundance of the elements as follows: C: 63.2%, O: 31.5%, Mg: 0.8%, Ni: 0.7%, Al: 0.2%, Pb: 3.6%. The elemental distribution recorded for the composite after adsorption of Pb2+ confirmed the successful adsorption of the Pb2+ ions with high adsorption capacity as represented in Fig. 8A.
As seen from Fig. 9, with the increase of solution pH, the adsorption capacity increases gradually and reaches the equilibrium state at pH of 6–7. The adsorption efficiency of AC-Ni/Al-LDH composite was decreased considerably when the pH of the solution was about 2, due to the protonation of the surface hydroxyl groups. That resulted in repelling the positively charged Pb2+ ions and decrease of active adsorptive sites capable to coordinated with metal ions85. Additionally, the influence of pH on the metal removal process is related to the zero-charge point (pHpzc) of AC-Ni/Al-LDH composite86. As shown in Fig. 8, the pHpzc of AC-Ni/Al-LDH composite is 6.8. At pH values less than the corresponding pHpzc, the predominant surface charge of AC-Ni/Al-LDH composite is positive, which resulting in an electrostatic repulsion to the positively charged species in the medium87. While at higher pH value than the pHpzc, the predominant surface charge is negative which encourages the electrostatic attraction between lead ions and the adsorbent88.
In view of that, the conditions of the removal process play a vital role on electrostatic attraction mechanism of the adsorption process beside the other parallel mechanisms occurred during the adsorption mechanisms and represented in Scheme 2 including: metal-oxygen (n-electrons), complexation, ion exchange, and precipitation.
Cost estimation
The cost estimation is useful in deciding the feasibility of activated carbon production process. The factors affected the cost are: the raw material accessibility, conditions of treatment, and other requirements. Accordingly, considering the costs of the raw material (rice husk: free), and the activating chemicals such as sodium hydroxide and hydrochloric acid (3$), and electric power (0.9$), the cost production of 1 Kg of activated carbon can be calculated. The total cost to produce 1 kg of AC according to the laboratory scale was found 3.9 $.
The cost study of the prepared adsorbents during the remediation of Pb2 + metal ions from contaminated wastewater includes the adsorbents cost and their synthesis, handling cost, and the final output. The synthesis cost of AC-Ni/Al-LDH was 0.36 $/g. considering the concentration of Pb2+ metal ions in wastewater was 150 mg/L. The treatment cost of 1 L of wastewater is least than 4.5 cents per 1 L of wastewater.
Conclusion
Through the liquid-phase co-precipitation method, this study synthesized an effective composite material for removing Pb(II) using rice husk-derived activated carbon as the raw material. SEM-EDX, FTIR, and XRD results demonstrated that Ni/Al-LDH uniformly adhered to the surface of the activated carbon. The adsorption process was highly dependent on the pH solution, and pHpzc which determined the surface charge to AC, and AC-Ni/Al-LDH composite. The adsorption process of Pb(II) at the surface of both adsorbent AC and AC-Ni/Al-LDH composites took place by electrostatic attraction, metal-oxygen (n-electrons) interaction, complexation, ion exchange, and precipitation. The adsorption kinetics of Pb(II) by the AC-Ni/Al-LDH composite better fit the pseudo-second-order model. The intra-particle diffusion model for Pb(II) was divided into three stages. The Freundlich model fits the adsorption isotherm of Pb(II) at the surface of both adsorbent AC and AC-Ni/Al-LDH composites. The negative values of ΔG established the spontaneous progress of the adsorption process of Pb(II) at the surface of prepared composite, which was increased by increasing the temperature from 298 oK to 318 oK. Activated carbon prepared from agricultural waste can be used as an environmentally friendly material loaded with LDH for the remediation of heavy metal ion pollution as Pb(II).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
Abbreviations
- AC:
-
Activated carbon
- Fm :
-
Freundlich model
- Lm :
-
Langmuir model
- Tm :
-
Timken model
- LDH:
-
Layered double hydroxide
- PFO:
-
Pseudo first order
- PSO:
-
Pseudo second order
References
Liu, Q. et al. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 382, 121040 (2020).
Thithai, V., Gim, S. M., Mearaj, S. & Choi, J. W. Adsorption kinetics and capacity of activated carbon derived from non-woody waste biomass for water remediation. Int. J. Environ. Sci. Technol. https://doi.org/10.1007/s13762-024-05677-7 (2024).
Lu, Y. et al. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environ. Pollut. 239, 670–680 (2018).
Sun, K. et al. Microporous activated carbons from coconut shells produced by self-activation using the pyrolysis gases produced from them, that have an excellent electric double layer performance. New. Carbon Mater. 32, 451–459 (2017).
Hirunpraditkoon, S., Tunthong, N., Ruangchai, A. & Nuithitikul, K. Adsorption cpacities of activated carbons prepared from bamboo by KOH activation. World Acad. Sci. Eng. Technol. 78, 711–715 (2011).
Kabenge, I. et al. Characterization of banana peels wastes as potential slow pyrolysis feedstock. 11, 14–24 (2018).
Daud, Z. et al. Chemical composition and morphological of cocoa pod husks and cassava peels for pulp and paper production. 7, 406–411 (2013).
Rashidi, N. A., Yusup, S., Ahmad, M. M., Mohamed, N. M. & Hameed, B. H. Activated carbon from the renewable agricultural residues using single step physical activation: a preliminary analysis. APCBEE Procedia. 3, 84–92 (2012).
Abdolali, A. et al. Characterization of a multi-metal binding biosorbent: chemical modification and desorption studies. Bioresour Technol. 193, 477–487 (2015).
Pangavhane, D. R. & Tare, S. Grape stalk briquettes as an alternative feedstock of biomass gasifiers. 13, 11–20 (2012).
Uche Paul, O., Obanor, A., Aliu, S. & Ighodaro, O. Proximate and ultimate analysis of fuel pellets from oil palm residues. doi: (2019). https://doi.org/10.4314/njt.v36i3.44
Plaza, M. G., González, A. S., Pis, J. J., Rubiera, F. & Pevida, C. Production of microporous biochars by single-step oxidation: effect of activation conditions on CO2 capture. Appl. Energy. 114, 551–562 (2014).
Huang, Y. F., Chiueh, P. T., Kuan, W. H. & Lo, S. L. Microwave pyrolysis of lignocellulosic biomass: heating performance and reaction kinetics. Energy. 100, 137–144 (2016).
Gurten, I. I., Ozmak, M., Yagmur, E. & Aktas, Z. Preparation and characterisation of activated carbon from waste tea using K2CO3. Biomass Bioenerg. 37, 73–81 (2012).
Phiri, Z., Everson, R., Neomagus, H. & Wood, B. The effect of acid demineralising bituminous coals and de-ashing the respective chars on nitrogen functional forms. J. Anal. Appl. Pyrol. 125, (2017).
Sadeek, S. A., Mohammed, E. A., Shaban, M., Abou, M. T. H. & Negm, N. A. Synthesis, characterization and catalytic performances of activated carbon-doped transition metals during biofuel production from waste cooking oils. J. Mol. Liq. 306, 112749 (2020).
Mazlan, M. A. F. et al. Activated carbon from rubber wood sawdust by carbon dioxide activation. Procedia Eng. 148, 530–537 (2016).
Subramani, T. & Revathi, P. Production of activated carbon from agricultural raw waste. (2015).
Yahya, M. A., Al-Qodah, Z. & Ngah, C. W. Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew. Sustain. Energy Rev. 46, 218–235 (2015).
Seh-bardan, A. W. S. F. S. B. J. Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals. 967–976 (2014). https://doi.org/10.1007/s13762-013-0291-3
Zubair, M., Aziz, H., Ihsanullah, D., Azmier, M. & Al-Harthi, M. Biochar supported CuFe layered double hydroxide composite as a sustainable adsorbent for efficient removal of anionic azo dye from water. Environ. Technol. Innov. 23, 101614 (2021).
Farag, A. A. et al. Highly efficient elimination of pb(+ 2) and Al(+ 3) metal ions from wastewater using graphene oxide/3,5-diaminobenzoic acid composites: selective removal of pb(2+) from real industrial wastewater. ACS Omega 7, 38347–38360 (2022).
Singh Sankhla, M., Kumar, R. & Gulliya, S. New and advanced technologies in aquaculture to support environmentally sustainable development. in 249–263 (2020). https://doi.org/10.1007/978-981-15-2817-0_11
Singh, A. et al. Heavy metal contamination of water and their toxic effect on living organisms. in 1–19 (2022). https://doi.org/10.5772/intechopen.105075
Hafez, O., Mohamed, R., Abou kana, M., Mohamed, E. & Negm, N. Treatment of industrial wastewater containing copper and lead ions using new carboxymethyl chitosan-activated carbon derivatives. Egypt. J. Chem. (2021). https://doi.org/10.21608/ejchem.2021.82163.4050
Hoang, A. & Phạm, D. An investigation of remediation and recovery of oil spill and toxic heavy metal from maritime pollution by a new absorbent material. J. Mar. Eng. Technol. 20, 1–11 (2018).
Sayato, Y. WHO guidelines for drinking-water quality. Eisei Kagaku. 35, 307–312 (1989).
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metal toxicity and the environment. Exp. Suppl. 101, 133–164 (2012).
Mahmoud, D., Salleh, M. A. M. & Karim, W. A. W. A. Langmuir model application on solid-liquid adsorption using agricultural wastes: environmental application review. in (2012).
Riyanto, Astuti, R. & Mukti, B. Simple preparation of rice husk activated carbon (RHAC) and applications for laundry and methylene blue wastewater treatment. AIP Conference Proceedings vol. 1911 (2017).
Le Van, K., Luong, T., Ha, N. & Hoang, H. Activated carbon by KOH and NaOH activation: preparation and electrochemical performance in K2SO4 and Na2SO4 electrolytes. Russ J. Electrochem. 55, 900–907 (2019).
Zakaria, M. R., Ahmad Farid, M. A., Hassan, M., Ando, Y. & Ramli, I. Production of biochar and activated carbon from oil palm biomass: current status, prospects, and challenges. Ind. Crops Prod. 199, 116767 (2023).
Ahmad, N., Arsyad, S., Royani, F., Lesbani, A. & I. & Charcoal activated as template Mg/Al layered double hydroxide for selective adsorption of direct yellow on anionic dyes. Results Chem. 5, 100766 (2023).
Ravuru, S., Jana, A. & De, S. Synthesis of NiAl- layered double hydroxide with nitrate intercalation: application in cyanide removal from steel industry effluent. J. Hazard. Mater. 373, (2019).
Yu, J., Zhu, Z., Zhang, H., Qiu, Y. & Yin, D. Mg–Fe layered double hydroxide assembled on biochar derived from rice husk ash: facile synthesis and application in efficient removal of heavy metals. Environ. Sci. Pollut Res. 25, 1–12 (2018).
Xu, X., Cao, X. & Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92, 955–961 (2013).
Negm, N. A., Altalhi, A. A., Mohamed, S., Kana, N. E., Mohamed, E. A. & M. T. H. A. & Growth inhibition of sulfate-reducing bacteria during gas and oil production using novel Schiff base diquaternary biocides: synthesis, antimicrobial, and toxicological assessment. ACS Omega 7, 40098–40108 (2022).
Mohamed, E. A. et al. Two novel Schiff bases derived from 3-amino-1, 2, 4- triazole as corrosion inhibitors for carbon steel pipelines during acidizing treatment of oil wells : laboratory and theoretical studies. Energy Sources Part. Recover Util. Environ. Eff. 45, 3246–3265 (2023).
Negm, N. A., Farargy, E., Mohammad, A. F., Zaki, I. A., Khowdiary, M. M. & M. F. & Synthesis and inhibitory activity of Schiff base surfactants derived from tannic acid and their cobalt (II), manganese (II) and Iron (III) complexes against bacteria and fungi. J. Surfactants Deterg. 16, 767–777 (2013).
Altalhi, A., Hashem, H., Negm, N., Mohamed, E. & Azmy, E. Synthesis, characterization, computational study, and screening of novel 1-phenyl-4-(2-phenylacetyl)-thiosemicarbazide derivatives for their antioxidant and antimicrobial activities. J. Mol. Liq. 333, 115977 (2021).
Saka, C. & BET, TG–DTG, F. T. I. R. SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. J. Anal. Appl. Pyrol. 95, 21–24 (2012).
Xia, H., Liu, B., Li, Q., Huang, Z. & Cheung, A. S. C. High capacity Mn-Fe-Mo/FSM-16 sorbents in hot coal gas desulfurization and mechanism of elemental sulfur formation. Appl. Catal. B Environ. 200, 552–565 (2017).
Park, J. H. et al. Comparative sorption of pb and cd by biochars and its implication for metal immobilization in soils. Water Air Soil. Pollut 224, (2013).
Zhang, Z., Xu, M., Wang, H. & Li, Z. Enhancement of CO2 adsorption on high surface area activated carbon modified by N2, H2 and ammonia. Chem. Eng. J. 160, 571–577 (2010).
Zong, M. H., Duan, Z. Q., Smith, T. & Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green. Chem. 9, (2007).
Suganuma, S. et al. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 130, 12787–12793 (2008).
Wan, S., Wang, S. & Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 47, (2016).
Palapa, N. et al. CuAl LDH/Rice husk biochar composite for enhanced adsorptive removal of cationic dye from aqueous solution. Bull. Chem. React. Eng. Catal. 15, 525–537 (2020).
Amer, A. et al. Assessment of 3-amino-1H-1,2,4-triazole modified layered double hydroxide in effective remediation of heavy metal ions from aqueous environment. J. Mol. Liq. 341, 116935 (2021).
Altalhi, A. A., Morsy, S. M., Abou Kana, M. T. H., Negm, N. A. & Mohamed, E. A. Pyrolytic conversion of waste edible oil into biofuel using sulphonated modified alumina. Alexandria Eng. J. 61, 4847–4861 (2022).
Zuo, J., Wang, J. & Jiang, Y. Macro/meso failure behavior of surrounding rock in deep roadway and its control technology. Int. J. Coal Sci. Technol. 6, 301–319 (2019).
Sing, K. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 54, 2201–2218 (1982).
Mohrazi, A., Ghasemi-Fasaei, R., Mojiri, A. & Safarzadeh, S. Identification of influential parameters and conditions in heavy metals adsorption onto Cal-LDH-PC using optimization approaches of RSM and Taguchi. Sci. Rep. 14, 1–15 (2024).
Abdel-Hady, E. E. et al. Textural properties and adsorption behavior of Zn–Mg–Al layered double hydroxide upon crystal violet dye removal as a low cost, effective, and recyclable adsorbent. Sci. Rep. 13, 1–19 (2023).
Sun, S. & Wang, A. Adsorption properties of N-succinyl-chitosan and cross-linked N-succinyl-chitosan resin with pb(II) as template ions. Sep. Purif. Technol. 51, 409–415 (2006).
Ahmad, M., Manzoor, K., Venkatachalam, P. & Ikram, S. Kinetic and thermodynamic evaluation of adsorption of Cu(II) by thiosemicarbazide chitosan. Int. J. Biol. Macromol. 92, 910–919 (2016).
Ünlü, N. & Ersoz, M. Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J. Hazard. Mater. 136, 272–280 (2006).
Septhum, C., Rattanaphani, S., Bremner, J. B. & Rattanaphani, V. An adsorption study of Al(III) ions onto Chitosan. J. Hazard. Mater. 148, 185–191 (2007).
Varma, A. J., Deshpande, S. V. & Kennedy, J. F. Metal complexation by Chitosan and its derivatives: a review. Carbohydr. Polym. 55, 77–93 (2004).
Abdel Wahab, M. M. et al. Synergistic effects of graphene oxide grafted with barbituric acid nanocomposite for removal of heavy metals from aqueous solution. Nanotechnol. Environ. Eng. 8, 347–359 (2023).
Garg, R., Sabouni, R. & Ahmadipour, M. From waste to fuel: challenging aspects in sustainable biodiesel production from lignocellulosic biomass feedstocks and role of metal organic framework as innovative heterogeneous catalysts. Ind. Crops Prod. 206, 117554 (2023).
Coelho, T., Laus, R., Mangrich, A. & Fávere, V. Effect of heparin coating on epichlorohydrin cross-linked chitosan microspheres on the adsorption of copper (II) ions. React. Funct. Polym. 67, 468–475 (2007).
Li, Y. H. et al. Adsorption thermodynamic, kinetic and desorption studies of Pb2 + on carbon nanotubes. Water Res. 39, 605–609 (2005).
Mohamed, E. A. et al. Novel magnetic chitosan Schiff base impregnated with ZnO for removal of malachite green dye from aqueous environment. Int. J. Environ. Sci. Technol. https://doi.org/10.1007/s13762-024-06016-6 (2024).
Ho, Y. S. & McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum Moss peat. Water Res. 34, 735–742 (2000).
Hasan, M., Ahmad, A. L. & Hameed, B. H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 136, 164–172 (2008).
Ozcan, A., Öncü, E. & Özcan, A. Kinetics, isotherm and thermodynamic studies of adsorption of acid blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. Physicochem. Eng. Asp. 277, 90–97 (2006).
Febrianto, J. et al. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J. Hazard. Mater. 162, 616–645 (2009).
Abo El-Reesh, G. Y., Farghali, A. A., Taha, M. & Mahmoud, R. K. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for cr(VI) removal. Sci. Rep. 10, 1–20 (2020).
Yadav, B. S. & Dasgupta, S. Effect of time, pH, and temperature on kinetics for adsorption of methyl orange dye into the modified nitrate intercalated MgAl LDH adsorbent. Inorg. Chem. Commun. 137, 109203 (2022).
Ayawei, N., Ebelegi, A. & Donbebe, W. Modelling and interpretation of Adsorption isotherms. Hindawi J. Chem. 201, 11 (2017).
An, Y. et al. Multi-functionalized self-floating microspheres for dyes capture: amphoteric adsorption and rapid surface solid-liquid separation. J. Clean. Prod. 296, 126535 (2021).
Wang, B., Lan, J., Bo, C., Gong, B. & Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon: review. RSC Adv. 13, 4275–4302 (2023).
Raji, Z., Karim, A., Karam, A. & Khalloufi, S. Adsorption of heavy metals: mechanisms, kinetics, and applications of various adsorbents in wastewater remediation—a review. Waste 1, 775–805 (2023).
Yang, X. et al. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem. Eng. J. 366, 608–621 (2019).
Lai, K. C., Lee, L. Y., Hiew, B. Y. Z., Thangalazhy-Gopakumar, S. & Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 79, 174–199 (2019).
Wang, H. et al. Grafting of β-cyclodextrin to magnetic graphene oxide via ethylenediamine and application for cr(VI) removal. Carbohydr. Polym. 113, 166–173 (2014).
Li, H. et al. Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178, 466–478 (2017).
Supong, A. et al. Experimental and theoretical insight into the adsorption of phenol and 2,4-dinitrophenol onto Tithonia diversifolia activated carbon. Appl. Surf. Sci. 529, 147046 (2020).
Liang, X., Chi, J. & Yang, Z. The influence of the functional group on activated carbon for acetone adsorption property by molecular simulation study. Microporous Mesoporous Mater. 262, 77–88 (2018).
Pavlovic, I., Pérez, M., Barriga, C. & Ulibarri, M. A. Adsorption of Cu2+, Cd2 + and Pb2 + ions by layered double hydroxides intercalated with the chelating agents diethylenetriaminepentaacetate and meso-2,3-dimercaptosuccinate. Appl. Clay Sci. 43, 125–129 (2009).
Cheng, S., Zeng, X. & Liu, P. One-step synthesis of magnetic N-doped carbon nanotubes derived from waste plastics for effective cr(VI) removal. Arab. J. Chem. 17, 105956 (2024).
Qin, X. et al. Preparation of pyrolysis products by catalytic pyrolysis of poplar: application of biochar in antibiotic wastewater treatment. Chemosphere 338, 139519 (2023).
Li, S. S. et al. Competitive adsorption behavior toward metal ions on nano-Fe/Mg/Ni ternary layered double hydroxide proved by XPS: evidence of selective and sensitive detection of pb(II). J. Hazard. Mater. 338, 1–10 (2017).
Wang, Y. et al. EDTA functionalized Mg/Al Hydroxides modified biochar for pb(II) and cd(II) removal: adsorption performance and mechanism. Sep. Purif. Technol. 335, (2024).
Khalil, A. K. A. et al. Insights into the adsorption of lead ions by Mg-Al LDH doped activated carbon composites: implications for fixed bed column and batch applications. Chem. Eng. Sci. 281, 119192 (2023).
Mo, W. et al. Adsorption behavior of Mg–Al layered double hydroxide on Pb(II), zn(II), cd(II), and as (V) coexisting in aqueous solution. Mater. Today Sustain. 27, 100861 (2024).
Zhao, M. et al. Comparative study of layered double hydroxides intercalated by MoS42 – and Mo3S132 – on removal of Cu(II) and pb(II): adsorption mechanism and environmental stability. J. Water Process. Eng. 62, 105414 (2024).
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through TU-distinguished scientific publishing program and international appearance, Project number (TU-DSPP-2024-265). Also, the authors would like to express appreciation to Liwa College and its leaders for their support and sponsorship.
Author information
Authors and Affiliations
Contributions
N. A. Negm, and E. A. Mohamed: Performed the experimental part, participated in the discussion of the experimental data, and wrote the original draft of the manuscript.H. M. Ahmed: interpreted the results, and discussed the experimental data. A. A. Altalhi: Supervised all steps of the research, edited, and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Negm, N.A., Altalhi, A.A., Ahmed, H.M. et al. Synergistic effect of rice husk-derived activated carbon modified by Ni/Al-layered double hydroxides for lead removal from industrial wastewater. Sci Rep 14, 28411 (2024). https://doi.org/10.1038/s41598-024-77569-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77569-2