Abstract
The interfacial microstructure and corrosion behavior of oxide dispersion strengthening (ODS) FeCrAl alloy with 20 wt% Cr (e.g. 20Cr ODS FeCrAl alloy) exposed to static oxygen-saturated lead-bismuth eutectic (LBE) at 450 °C for 20 h, 40 h, 60 h, 80 h and 100 h as exposure time respectively have been investigated. The results show that multilayer corrosion films of 20Cr ODS FeCrAl alloy can exist at the corrosion interface. Due to formation of protective scales, the primary corrosion mechanism of 20Cr ODS FeCrAl alloy in LBE is oxidation rather than dissolution and penetration of liquid LBE. Specially, the spinel-shaped nano-sized dense external layer without any microcracks is obviously observed. Moreover, the dense and compact scale with micron-meters, i.e. Al-rich Fe-Cr spinel, generates at interface as the middle layer to effectively defend inward diffusion of oxygen and penetration of LBE. Meanwhile, a reinforced and continuous internal layer mixed with Cr2O3 scale and the ferrite inner oxidation zone (IOZ) existing in the form of the interlocking interface between the layers and the substrate can further promote the adhesion between corrosion layers and the substrate of 20Cr ODS FeCrAl.
Similar content being viewed by others
Introduction
As the main candidate material for coolant in LBE-cooled fast reactors and accelerator-driven system (ADS), lead-bismuth eutectic (LBE, Pb44.5Bi55.5, wt%) as one of the heavy liquid metal (HLM) coolants has superior neutronic, thermo-hydraulic, thermo-physical properties and minimal chemical reactivity1,2,3,4,5,6,7,8,9,10, which makes it greatly potential and commercialized applications in lead-cooled fast reactor (LFR).
However, the serious corrosion of structural materials exposed to liquid LBE has become the most significant factor to limit the widespread applications of LBE in the nuclear industry. The corrosion mechanisms of structural materials in LBE comprise liquid metal corrosion (LMC) and liquid metal embrittlement (LME). Materials exposed to liquid LBE are susceptible to severe degradation, including oxidation, dissolution, and flow erosion, which are influenced by numerous factors such as dissolved oxygen concentration, the thermodynamic and hydraulic states of liquid LBE, material characteristics and the interaction between materials and LBE1,3,6,7,8,11,12,13,14,15,16.
In comparison to other potential structural materials, such as refractory metals, MAX phase, SiC fiber-reinforced SiC matrix composites and so on, the commercial steels can offer lots of distinct advantages, including affordable fabrication costs, widespread availability of steels with controllable processing techniques, and satisfactory mechanical properties. Despite the neutron economy drawback inherent in all steels due to higher neutron absorption cross-section of iron, these disadvantages may be mitigated by the abundance of nuclear fuels and modifications to enhance irradiation-resistant properties3,6,7,8,10. Approximately, three classes of steels have been recognized as candidates for structural materials in LBE-cooled fast reactors: austenitic stainless steels (AuSSs), ferrite/martensite (F/M) steels and oxide dispersion strengthening (ODS) steels or alloys3,8,10,12,14,15,16,17,18.
AuSSs have demonstrated mature fabrication technology, satisfactory processibility, and favorable corrosion resistance3,10. Nevertheless, the limitations of their face-center cubic (FCC) crystal structure and high nickel content result in inadequate irradiation-resistant properties and neutron economy for fuel cladding materials in LBE-cooled fast reactors10,12,14,19. F/M steels, with body-centered cubic (BCC) crystal structure, exhibit good thermal physics properties, excellent irradiation swelling resistant performances and mechanical properties up to elevated temperatures. However, the instability of carbides in F/M steels at higher temperatures necessitates limiting operating temperature of liquid LBE to prevent thermal softening and other problems3,10,18,20,21,22,23. ODS steels, which introduce fine oxide particles (e.g. Y2O3) as strengthening phases, display enhanced high-temperature mechanical properties and irradiation-resistant properties due to the thermal stability of dispersed oxide particles which can pin dislocations and grain boundaries of alloys and act as the irradiation damage defect traps24,25,26,27. Additionally, proper addition of chromium and aluminum can promote the formation of dense and protective oxides (i.e. Cr2O3, Al2O3) on the alloy surface, thereby enhancing corrosion resistance to LBE due to the so-called third-element effect6,28,29,30,31.
Recently, some researchers have evaluated the corrosion resistances of some ODS FeCrAl alloys in liquid LBE32,33,34,35,36,37,38,39,40,41. Hosemann et al.32 conducted corrosion tests on five types of commercial ODS steels exposed to flowing LBE with the velocity of 2 m⋅s−1 at temperatures of 535 °C and 600 °C under an oxygen content of approximately 10−6 wt%. They found that the PM2000 ODS FeCrAl alloy with lots of high aluminum content exhibits the best corrosion resistance to LBE. Corrosion tests on ODS steels with varying Cr and Al contents simultaneously exposed to low-oxygen content and static LBE at 550 °C and 600 °C for thousands of hours have confirmed the essential additions by combining Cr with Al in proper ratios for their improved performances, rather than sole Cr or Al addition to prevent LMC33,34,35,36,37. Kim et al.34 carried out the static LBE corrosion test at 500 °C and 550 °C for 500 h, which suggests that ODS FeCrAl alloys exhibit better corrosion resistance in LBE than that of traditional FeCrAl alloys. Baker et al.38 employed the U-bend specimens and slow strain rate testing exposed to LBE, which proves that MA956 ODS FeCrAl alloy is not sensitive to LME due to the formation of continuous protective oxide scales (Al2O3 and Cr2O3) as blunting roles of LBE penetration. Consequently, the dense oxide films have been formed on the corrosion interfaces between LBE and the substrate of the ODS FeCrAl alloys in the temperature range of 500 °C to 650 °C over an extended duration, and the optimal Cr and Al ratios for corrosion resistance in LBE have been firmly identified by series of tests for ODS FeCrAl alloys.
In addition, the conventional ODS steels are prepared by mechanical alloying (MA) methods, which exhibits high number density and nano-sized oxide particles formed via non-equilibrium precipitation. Nevertheless, some significant factors may limit the widespread applications of ODS steels prepared by MA in some aspects owing to the expensive costs, complex fabrication process, limitations of sintering mold dimensions, potential in-service safety shortcomings, and so on42,43,44. Considering poor wettability between liquid steels and Y2O3, some researchers investigated the preparation of ODS steels via melting methods to improve the interface properties43,44. Meanwhile, other fabrications such as in-situ internal oxidation methods45,46, rapid solidification methods47,48 and alloying methods44, have been developed to reduce the shortcomings resulting from agglomeration and coarsening of oxide particles. Even so, the aforementioned ODS alloys still exhibited better high-temperature mechanical performances than that of the given oxide particles-free steels.
However, the corrosion behavior and the underlying mechanism of ODS FeCrAl alloys in LBE has not been still clearly and well metallurgically revealed and clarified partly because of the complex compositions and the dependence of test conditions as well as difficult interfacial microstructural inspections. In present work, the corrosion tests on 20 wt.%Cr ODS FeCrAl alloy (e.g. 20Cr ODS FeCrAl alloy) fabricated by vacuum induction melting (VIM) method were performed in oxygen-saturated LBE at 450 °C. The corrosion interfacial morphologies and intrinsic microstructures have been systematically investigated by comprehensive characterizations to uncover the unique corrosion behaviors of 20Cr ODS FeCrAl alloy in LBE. Accordingly, the underlying corrosion mechanism of 20 wt.%Cr ODS FeCrAl alloy has been discussed and proposed based on the obtained results.
Results
Microstructure of as-annealed ODS FeCrAl alloys
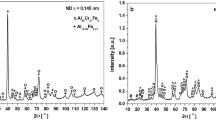
The X-ray diffraction (XRD) pattern and microstructures of the investigated ODS FeCrAl alloy are represented in Fig. 1. Prominent peaks corresponding to various crystal planes of ferrite (JCPDS 34–0396) are evidently observed in Fig. 1a, which indicates the microstructure of the alloy is single phase α-(Fe, Cr, Al) with body-centered cubic structure (i.e. BCC). The as-received microstructures and corresponding EDS mappings in Fig. 1b and f indicate the existence of many Y-Ti-O oxides precipitating with micron- and nano-sized particles formed by the reaction between Y2O3 and melting iron during VIM process (i.e. YTiO3, Y2TiO5, etc.)46,49,52, where some agglomerations of the Y-Ti-O particles (Fig. 1b and c specially selecting aggregated area with big particles in order to show the clear elemental distributions) can be found due to poor wettability of Y2O3 particles with liquid steels as well as difficult control for its dispersive distribution of nano-sized Y2O3 in melts43,44. Furthermore, the as-annealed TEM microstructures of the alloy are shown in Fig. 1g and l. The bright field (BF) images and selected area diffraction pattern (SADP) display high-density dislocations and some nano-sized and micron-sized particles distributed within the ferrite gains and at the grain boundaries (Fig. 1g and i), and both of the particles can pin the mobile dislocations. Besides, the SADP of oxide particle in Fig. 1j indicates the formation of nano-sized Yttrium Aluminium garnet (YAG) Y3Al5O12 oxide, which is also likely generated in the liquid steel via the reaction between Y2O3 and Al under low formation energy49,50,51. Furthermore, the coherent relationship of (060)YAG // (110)ferrite with a mismatch δ of approximate 1.5% can be further confirmed49, as represented in Fig. 1k and l of high-resolution transmission electron microscope (HRTEM) and Fourier fast transformation (FFT) images, which further indicates a novel pinning effect for the dislocations and grain boundaries via Orowan looping mechanism49,52,53.
XRD pattern and microstructures of as-annealed ODS FeCrAl alloy: (a) XRD pattern; (b) SEM morphology with low magnification; (c) SEM morphology with high magnification; (d-f) EDS mappings of Fig. 1c for Y, Ti, and O elements for Y-Ti-O particles, respectively; (g) TEM bright field (BF) morphology of oxide particles for YAG particle; (h) TEM BF morphology; (i) Selected area diffraction pattern (SADP) of α-(Fe, Cr, Al) matrix; (j) TEM BF image of YAG particle and its SADP; (k) High resolution (HR) image of phase boundary selected in Fig. 1j; (l) Fourier fast transformation (FFT) and its Inverse Fourier fast transformation (IFFT) image at phase interface.
Corrosion properties of the Alloy in LBE
Figure 2 shows the thickness loss in LBE and corrosion rate of the as-annealed ODS FeCrAl alloy exposed to oxygen saturated LBE at 450 °C as a function of exposure time. It is obvious that, with the increase of exposure time, the thickness loss of the alloy increases slightly and the corrosion rate decreases rapidly, which is likely to indicate the formation of dense, compact, and protective scales to defend severe dissolution corrosion of substrate.
Characterization of corrosion layers
The surface corrosion product morphologies and EDS mappings of the alloy on various stages are depicted in Fig. 3. As shown in Fig. 3a, the external corrosion products are observable only in regions where LBE is naturally spalling off the surface. The dense, extremely flat, and tens of nano-sized spinel-shaped scale can be clearly found beneath the coated LBE layer and particles on each stage (Fig. 3a and e), which exhibits no microcracks or any spallation. The natural surface scale features of the alloy suggest the adhesion and compactness of the obtained scales to demonstrate notable role in physically preventing the penetration and corrosion of LBE. The surface morphologies and grain sizes of the outmost scales have no distinct differences and changes during the 100 h (Fig. 3a and e). Clearly, the distribution of the average grain size for the external scale at 100 h is approximate 80 nm with a fairly uniform and compact surface, as shown in Fig. 3f. Moreover, Fig. 3g1-3g7 and Fig. 3h1-3h7 display the BSE morphologies and corresponding EDS mappings of samples exposed for 40 h and 60 h, respectively. Clearly, Pb and Bi primarily distribute in lighter areas of the image, which is identified as residual LBE (Fig. 3g1), and the uniform distributions of O, Fe, Cr, and Al elements in surface scales can be observed in thicker areas of Fig. 3g2-g7, which indicates the rapid generation of homogeneous oxide film during the tests before 20 h. Differently, the morphologies of surface scales formed in LBE after 60 h exhibit more flat and compact, and the elemental distributions of O, Fe, Cr, and Al are obviously observed (Fig. 3h1- 3h7), especially for Cr and Al elements, which infers that the spinel-shaped nano-sized dense Fe-Cr spinel can be generated. Therefore, more exposure time of alloy in LBE (e.g. 40 h and 60 h) can result in the dense and compact Fe-Cr spinel formed on the surfaces of the alloys according to elemental distribution in oxide scale areas (e.g. 60 h).
Figure 4 shows the interfacial morphologies and EDS mappings of the alloy exposed to liquid LBE. Clearly, three distinct layers including a thin external layer (~ 100 nm), a thick middle layer (~ 1 μm), and a thin internal layer (~ 100 nm) are observed at the corrosion interface corroded for 20 h (Fig. 4a and b). A detailed morphology of three different layers is typically exhibited by local naturally misaligned fractures of the layers in Fig. 4b, and there are a spinel-shaped external layer, a particularly dense middle layer, and a fine and compact internal layer which may be oxidized partially in the layers. In Fig. 4c, the interlocking interfaces are prominently observed in high magnified morphology, where the internal layer can enhance the adhesion of interfaces between substrate and layers significantly due to the interfacial bulging oxidation particles possibly. That means the interlocking interfaces can lead to the great improvement of protective capability of the dense layers and corrosion resistance of the alloy to LBE. Furthermore, the EDS mappings of these layers are represented in Fig. 4d1-4d6, where the segregation of oxygen indicates the generation of protective oxide film at corrosion interfaces, especially for the segregation of O, Cr and Al elements in the scales (Fig. 4d2-4d6). And the EDS analysis results (atomic percent) show the segregation of Cr in middle oxide layer (Point 1 in Fig. 4d1: 60.66% O, 10.25% Fe, 17.28% Cr, 5.17% Al, 6.64% Pb) and partial oxidation and segregation of Cr in sub-interface of substrate (Point 2 in Fig. 4d1: 18.16% O, 48.89% Fe, 23.13% Cr, 7.76% Al, 2.06% Pb). These results reveal the protective roles of middle oxide layer and preferred oxidation of Cr at corrosion interfaces due to high activity of Cr and low formation energy of Cr-rich oxides.
As depicted in Fig. 4e and f, the similar interfacial microstructures with thicker layers (40 h and 80 h, especially for 80 h) can be clearly shown, and the distributions of Fe, Cr and Al are found in oxide layers in Fig. 4e4-4e6 and 4f4-4f6, respectively. According to Fig. 4f5, the Cr concentration of layers close to scales/substrate interface is clearly somewhat higher than the upper layer (e.g. for 40–100 h), and the corresponding O concentration at internal interface is also enriched and segregated, which may be obviously confirmed as the Cr-rich layer at internal interface. Moreover, all the EDS point spectra results (atomic percent) accurately indicate the existences of Al-rich Fe-Cr spinel in the oxide layers (Point 3 in Fig. 4e1: 40.94% O, 31.43% Fe, 15.96% Cr, 9.56% Al, 2.11% Pb; Point 5 in Fig. 4f1: 40.87% O, 30.50% Fe, 14.43% Cr, 11.95% Al, 2.25% Pb) and IOZ in sub-interface of the substrates (Point 4 in Fig. 4e1: 3.93% O, 62.88% Fe, 17.85% Cr, 14.52% Al, 0.82% Pb; Point 6 in Fig. 4f1: 2.02% O, 60.59% Fe, 19.24% Cr, 17.50% Al, 0.65% Pb).
Additionally, Fig. 4g1-4g6 also show the similar microstructures of corrosion interface corroded for 100 h. The EDS mappings display the internal interfacial segregation of O and existence of Fe, Cr and Al in oxide layers, and the EDS analysis results (atomic percent) also indicate the formation of Al-rich Fe-Cr spinel in the layer (Point 7 in Fig. 4g1: 43.52% O, 29.68% Fe, 18.74% Cr, 3.60% Al, 2.53% Pb, 1.93% Bi). Compared to the EDS results at 20 h, the sub-interface of substrate with much lower Pb and Bi contents at 100 h (Point 8 in Fig. 4g1: 1.02% O, 71.00% Fe, 21.02% Cr, 6.80% Al, 0.16% Bi) further reveals better protective role of oxide layer than the layer exposed to LBE for 20 h to completely prevent the penetration of LBE into substrate (Fig. 4g1-4g6).
To further accurately investigate the microstructure and components of multilayer corrosion films at corrosion interfaces, EPMA mappings and line analysis of the cross-sectional morphologies for the alloy exposed to LBE for 60 h are clearly illustrated in Fig. 5. The stable three-layer oxide films are clearly confirmed at interfaces to resist this harsh LBE corrosion. EPMA mappings depict that the elemental segregations of O, Cr and Al in the middle layer take place to form a protective film, which effectively defends further inward diffusion of oxygen. Obviously, a preferential oxidation of Cr and Al is apparent in multilayer films based on EPMA mappings (i.e. Figure 5e and f). The accurate results of EPMA highlighted in red circle area in Fig. 5a also exhibit that the element composition (atomic percent) of middle layer at corrosion interface is 19.68% Fe, 14.74% Cr, 8.44% Al, and 57.14% O, which suggests the existence of an Al-rich Fe-Cr spinel layer (Fig. 5b and f). Notable segregation of Cr observed in the internal layer remarkably reveals the preferential oxidation of Cr at internal interfaces owing to the high Cr content in substrate and lower oxygen concentration (Fig. 5e). Moreover, the EPMA line analysis through the black arrow of Fig. 5a also indicates that the Cr-rich layer with approximately 0.45 μm in thickness (i.e. some Cr2O3) exists at the scale/substrate internal interface, while some ferrite with Cr depletion or consuming zone as the internal oxidation zone (i.e. IOZ) coexists at sub-interface beneath the Cr-rich layer according to the Cr and O distributions at oxide layer/substrate (Fig. 5g of EPMA line analysis). Differently, the Al distribution in the middle layer just exhibits somewhat increase by EPMA line analysis, which may be attributed to the uneven distribution of Al in this layer as well as no flatness of the whole layers detected EPMA probe through this middle layer (Fig. 5g). Besides, there is no nano or micro-sized particles aforementioned to be obviously observed at interfaces and also distinctly affect the oxide scales, which may be attributed the small size and stable chemistry of these particles (i.e. YAG or Y-Ti-O precipitates) to generate their spallation or probable interfacial corrosion during oxidation corrosion process in liquid LBE34,50,51. Actually, these particles dispersed in ODS alloys mainly contribute to the high-temperature strength as a block for mobile dislocations and act as a sink of point defects induced by radiation damage24,25,26,27,49. Therefore, it means that Cr and Al play key roles in generating multilayer films and protecting the 20Cr ODS FeCrAl alloy from the oxidation corrosion of liquid LBE.
In order to further confirm the oxides of the alloys in LBE, the specific chemical valence states of elements in external layer of the alloy exposed to LBE for 20 h, 40 h, and 100 h measured by X-ray photoelectron spectroscopy (XPS) surface spectra to consider its chemical composition are shown in Fig. 6 in details. Similarly, in the three samples, the detected O 1s spectra should be mainly contributed by Metal-O peak and C-O peak, as shown in Fig. 6a1, 6b1 and 6c1, and the Metal-O peak (at 530.4 eV) indicates the metallic oxides, and the C-O peak (at 532.4 eV) is contributed to trace foreign organics54. Prominently, in Fig. 6a2, 6b2 and 6c2, the absence of Al peak suggests that the preferential oxidation of Al element only exists in the protective middle layer. The valences of Cr verified in Fig. 6c4 are + 3 and + 6 values, whose binding energy are 576.9 eV at 2p 1/2 and 587.2 eV at 3p 3/2 for Cr3+, and 579 eV at 3p 1/2 and 588.9 eV at 3p 3/2 for Cr6+, respectively55, which infers that some Cr takes part in the oxidation to form scales as main Cr3+ state while others may dissolve into LBE to form other products such as PbCrO4 with Cr6+ state58,59,60. At the range of Fe 2p in Fig. 6c3, Fe-Cr spinel (i.e. Fe3+ at 711.4 eV and Fe2+ at 710.8 eV) is distinctly verified, which exists in the external layer56. In addition, the valence of Bi verified in Fig. 6c6 is + 3 value, whose binding energy are 160.1 eV at 4f 7/257 as probable Bi2O3 product. To further identify the source of Cr + 6 state (i.e. Cr6+ cation), the range of Pb 4f (4f 5/2 at 143.7 eV and 4f 7/2 at 138.8 eV) is also strongly detected and accurately indexed, which is further greatly proved the existence of PbCrO4 lead chromate ceramic membrane formed by the reaction among dissolved Cr, Pb and O in liquid LBE as the earliest stage of ODS FeCrAl substrate for dissolution (e.g. Cr elemental dissolution)58,59,60. This detected and existing PbCrO4 lead chromate ceramic membrane in XPS results may be resulted from the abundant and residual products formed in the coated LBE layer owing to Cr dissolution from substrate and its subsequent oxidation in oxygen-saturated LBE. Furthermore, considering the high reactivity and corrosion of Pb atoms in LBE and high oxygen concentration dissolved in LBE, the previous dissolved Cr element in LBE at earliest stage can easily react with O and Pb atoms to form PbCrO4 product (namely previous Cr6+ cation). Therefore, the results mentioned above reveal the stable existence of Al-free Fe-Cr spinel from 20 h to 100 h, which is agreement with previous examinations and results aforementioned.
XPS spectra obtained from surface oxides of 20wt.% Cr ODS FeCrAl alloy exposed to LBE (i.e. including surface coated LBE): (a1-a6) XPS for 20 h; (b1-b6) XPS for 40 h; (c1-c6) XPS for 100 h. (The original data are shown in light grey lines, the fitting results are displayed in blue lines or green lines, the Fitting superimposed data are shown in red dotted lines, and the background is shown in black lines).
To investigate the refiner interfacial microstructure of oxide layers, TEM examinations of the alloy exposed for 60 h employed by FIB technique are demonstrated in Fig. 7. As shown in Fig. 7a, three layers are clearly observed between the prepared carbon coating and substrate, and among them there are no any cracks or holes in the layers, which is well agreed with the results presented in aforementioned Figs. 4 and 5a. The SADP of the middle layer in red circle area of Fig. 7a indicates the existence of Al-rich Fe-Cr spinel in middle layer without any other oxide crystal structure. The columnar external layer also suggests a gradient concentration of ions during its growth, combined with the dense compact morphology of the middle layer, which may be attributed to the outward diffusion of metallic cations. Moreover, the crystal structures of external layer and internal layer are identified by IFFT images and FFTs: external layer for Fe-Cr spinel (cubic, Fd-3 m) in Fig. 7c (Region A) and internal layer for Cr2O3 (hexagonal, R-3c) in Fig. 7d (Region B), respectively, which further suggests Fe-Cr spinel is formed on outmost layer while Cr2O3 scale can be formed on innermost layer, as shown in Fig. 7d.
TEM images on the corrosion interfaces: (a) Typical BF image of multi layers including columnar-shaped external layer and the SADP of middle layer; (b) Typical BF image of multilayers on the corrosion interfaces; (c) FFT and its IFFT image of region A marked in Fig. 7b; (d) FFT and its IFFT image of region B marked in Fig. 7b.
Discussion
According to the above-mentioned results, the main corrosion mechanism of the 20wt.% Cr ODS FeCrAl alloy exposed to static oxygen-saturated LBE at 450 °C should be oxidation corrosion owing to the formation of protective oxide scales at the corrosion interfaces. Figure 8 depicts the corrosion mechanism of the alloy in oxygen-saturated LBE at 450 °C, which suggests a corrosion progress through four stages as follows accordingly.
Initially, the alloy surface undergoes preferential oxidation of Cr and Al occurring at the corrosion interface, as illustrated in Fig. 8a. The dissolved oxygen from LBE acting as the oxidizing agent firstly diffuses into the substrate, leading to the adsorption and ionization as oxygen ions to oxidize Cr, Al and Fe atoms into Cr (+ 3), Al (+ 3) and Fe into Fe (+ 2) or Fe (+ 3) at the surface respectively by some involved selective oxidation processes of Cr and Al in following reaction after some minor dissolution of the substrate in LBE (i.e. elemental dissolution firstly).
Subsequently, a nano-sized layer with approximate hundreds of nanometers in thickness can be formed rapidly, as previously observed (Fe, Cr, Al)3O4 film. This layer is regarded to contribute to the further corrosion resistance of alloy to LBE.
As shown in Fig. 8a, the nano-sized layer of Al-rich Fe-Cr spinel acts as a physical barrier layer, preventing the penetration of LBE and controlling the inward diffusion of oxygen through grain boundaries. This results in the persistent preferential oxidation of Cr and Al within the layer. Concurrently, Fe and Cr atoms are oxidized to a hypervalent state cations and then constantly diffuse outward through the Al-rich Fe-Cr spinel layer, which contributes to the formation of a columnar Fe-Cr spinel with a complex spinel structure, as indicated in Fig. 8b. And its reaction may be given as the following transformation.
In this stage, a large number of Fe and Cr cation outward diffusion and oxidation through the layer may in turn accelerate the further outward diffusion of Fe and Cr atoms from the substrate so as to become various metal cations with different valence state. Some metallic cations (e.g. Fe2+ and Cr3+) could diffuse through the middle layer via lattice vacancies or other grain boundary defects of oxides, which provides a continuous supplying source for the growth of the external layer and thus controlling oxidation process. This leads to the formation of a columnar shaped layer by the outward diffusion of metallic ions through the Al-rich Fe-Cr spinel layer. Alternatively, the controlled inward diffusion of oxygen with the smallest anion radius through the grain boundaries of the layers may limit the further formation of the Al-rich Fe-Cr spinel. At much lower level of oxygen, preferential oxidation of Cr may occur alone at middle layer/substrate interface subsequently under high Cr chemical potential, and the reaction process may be controlled as follows.
Eventually, as schematically represented in Fig. 8b, c and a dense middle layer effectively defends the inward diffusion of oxygen, where residual oxygen may continuously oxidize metallic atoms at middle layer/substrate interface partially. Internal layer mixed with Cr2O3 scale and main Cr-depleted ferrite as inner oxidation zone (i.e. IOZ) can form with interlocking interface, which strongly promotes and consolidates the adhesion between oxide scales and the substrate.
Schematic corrosion mechanism of 20wt.% Cr ODS FeCrAl alloy exposed to LBE at 450 °C: (a) Initial stage showing adsorption of dissolved oxygen and the formation of Al-rich Fe-Cr spinel on surface in LBE, and also showing the formation of external FeCr2O4 after 20 h; (b) The second stage showing the growth of middle layer after 40 h; (c) Subsequent stage showing the formation of the internal mixed layer with Cr2O3 and ferrite as inner oxidation zone after 80 h; (d) Final stage showing the stable stage of multilayer oxide films after 100 h.
Therefore, Cr and Al outward diffusion and O-inward diffusion can dominate the first selective oxidation to form the middle layer of Al-rich Fe-Cr spinel as the first interface, i.e. Al-rich Fe-Cr spinel/substrate interface. Later, Cr and Fe outward diffusion continues to go on and simultaneous O-inward diffusion promotes the nano-sized columnar external Fe-Cr spinel, i.e. FeCr2O4 layer with very thin thickness to form on Al-depletion Fe-Cr spinel layer as the second interface (e.g. Al-rich Fe-Cr spinel/FeCr2O4 spinel interface) owing to lots of Al consumption of selective oxidation at first interface. Subsequently, Cr and Fe outward diffusion becomes difficult and inadequate owing the prolonged diffusion distance and the diffusion barrier layer of aforementioned layers. Thus, O-inward diffusion can strongly govern the formation of internal layer, partly because of Cr-rich at the internal Al-rich Fe-Cr spinel/substrate interface favourably and abundantly as well as the diffusion barrier layers to hinder Cr atom outward diffusion. Consequently, O-inward diffusion and internal interface reaction are slowly dominant for the internal layer mixed with Cr2O3 and IOZ as the third interface (i.e. substrate/ internal layer interface). Accordingly, the multilayer corrosion films of 20Cr ODS FeCrAl alloy can exist at the corrosion interfaces to effectively and greatly prevent the corrosion of liquid LBE.
The thermodynamic tendency of oxide formation is represented through the presented Ellingham diagram in Fig. 9. It illustrates the standard Gibbs free energy for the formation of oxides as a function of temperature at a given oxygen concentration in LBE describing in Refs57,58,61,62. It is surely confirmed that the formation of PbCrO4 in Pb-Cr-O system below 450 °C takes place and the thermal stability of PbCrO4 (around − 560 kJ∙mol−1) is fairly close to FeCr2O4 (around − 580 kJ∙mol−1) and FeAl2O4 (around − 590 kJ∙mol−1), which suggests the possible formation of PbCrO4 occurs under the dissolved Cr of the alloy with the liquid Pb and oxygen in liquid LBE45,46. Furthermore, the present examinations and observations also reveal that the Al2O3 and Cr2O3 scales with lower formation energies as indicated by the Ellingham diagram may initially generate at specific locations during the transient oxidation. Accordingly, the Al-rich Fe-Cr spinel controlled by preferential oxidation at low oxygen concentration can generate in present LBE conditions mentioned above.
Conclusions
The interfacial microstructures and corrosion behaviour of 20 wt% Cr ODS FeCrAl alloy exposed to static oxygen-saturated LBE at 450 °C for 20 h, 40 h, 60 h, 80 h and 100 h as exposure time respectively have been well investigated and the potential mechanism is discussed via scanning electron microscope (SEM) and transmission electron microscope (TEM). The main conclusions of present research are summarized as follows.
1) The 20 wt% Cr ODS FeCrAl alloy exhibits acceptable compatibility with static oxygen-saturated liquid LBE at 450 °C with the corrosion rate of 0.33 μm⋅h−1 after 100 h, which is greatly attributed to the beneficial multilayer films as the oxide layers at corrosion interfaces owing to oxidation corrosion of LBE.
2) The oxide layers at corrosion interfaces are composed of a compact spinel-shaped columnar external layer Fe-Cr spinel with grain size of approximate 80 nm and thickness of about 100 nm, a dense middle layer Al-rich Fe-Cr spinel with thickness of approximate 1 μm, and an internal layer (i.e. thickness of approximate 100 nm) mixed with Cr2O3 and ferrite inner oxidation zone (IOZ).
3) Preferential oxidation of Cr and Al is involved in internal interface at low oxygen level to form middle Al-rich Fe-Cr spinel layer, while a thin external Fe-Cr spinel can be generated by Fe and Cr outward diffusion in vacancies of Al-rich Fe-Cr spinel subsequently. And an internal layer mixed with Cr2O3 and IOZ can form by preferential oxidation of Cr partially at much lower oxygen content eventually.
4) The stable and compact three-layer oxides can resist the liquid LBE corrosion and effectively inhibit the penetration of LBE and oxygen inward diffusion. The corrosion mechanism of the alloy in the static oxygen-saturated LBE at 450 °C is oxidation-controlled corrosion, which is attributed to the emergence of protective multilayer scales.
Experimental and methods
Materials preparation
The 20 wt% Cr ODS FeCrAl alloys were melted by vacuum induction melting (i.e. VIM) in a water-cooled copper mold from a mixture of respective metals with purity exceeding 99.99%. After solidification, the as-cast alloy was rolled at 1150 °C into bars and then annealed at 980 °C for 2 h. The composition of ODS FeCrAl alloy is 20.00 wt.%Cr, 4.75 wt.%Cr, 0.40 wt.%Y2O3, 0.50 wt.%Ti, and 74.35 wt.%Fe. The corrosion medium was nuclear grade lead-bismuth eutectic (LBE, Pb44.5Bi55.5, wt%), in which total impurities are less than 10 ppm.
Corrosion procedures
Figure 10 represents the corrosion test in liquid LBE schematically. The corrosion samples were cut by using a wire electric discharge machine with the dimensions of 50 × 6 × 3 mm3 from the ODS FeCrAl bars. Before the corrosion tests, all corrosion samples were ground with silicon carbide sandpapers of from 400- to 2000-grit in sequence, and then manually polished using diamond pastes of 2.5 μm to achieve a mirror-like surface finish, and lastly rinsed with alcohol and acetone in an ultrasonic cleaner. The thickness of the samples was measured by using a micrometer for measurement of twelve times at multiple locations, and the average value was used later for the calculation of corrosion kinetics of the samples11,63. 0.5 kg LBE was melted in a pure alumina crucible heated by furnace. The temperature of liquid LBE was measured by a K-type thermocouple. As illustrated in Fig. 10a, approximately a 30 mm part of samples was exposed into the corrosion medium at 450 °C for 20 h, 40 h, 60 h, 80 h and 100 h. And the rest part of each sample was fixed by designed Mo clamp as a holder that was mounted on the top of crucibles so that samples could not float in the liquid LBE during the period of corrosion. Additionally, three specimens were tested simultaneously under the same conditions to ensure the experimental accuracy each time. The oxygen concentration in LBE was saturated because the liquid LBE was exposed to air directly. The oxygen content in LBE was 3.14 × 10−4 wt% calculated by Eq. (1)64.
Where C0 and T was the oxygen content (wt%) and temperature (K), respectively.
The tested samples were mounted in epoxy resin and polished to achieve a mirror-like surface finish. As depicted in Fig. 10b, the rest thickness of each tested sample after LBE corrosion was measured by optical microscope at 100 magnification times across the cross-section tens of times according to the method described in Refs65,66,67,68,69. Then, the thickness loss of the alloy corroded in LBE (i.e. the corrosion depth of the sample in LBE) and its corresponding corrosion rate of each tested sample was calculated by Eqs. (5) and (6).
Where Δd, R, a, b and t are the thickness loss (µm), corrosion rate (µm∙h-1), initial thickness (µm), residual thickness or uncorroded thickness (µm), and exposure time (h), respectively. The factor of 1/2 means the average corrosion rate of a dimension of each sample.
Characterization
The microstructure of as-annealing ODS FeCrAl alloy was carried out by using a JEM-2100 transmission electron microscopy (TEM) examining at 200 kV. X-ray diffraction (XRD) patterns of as-annealing ODS FeCrAl alloy was measured by X-ray diffractor with X’Pert software from 20° to 90° with the scanning speed of 0.1°/s and scanning step of 0.01° to confirm the crystal structure of the alloy with Cu-Kα radiation at 40 kV and 40 mA. The microanalysis of tested samples was conducted by Gemini 500 field scanning electron microscopy (FESEM) equipped with a secondary electron(SE), a back-scattered electron (BSE) detector, and an energy dispersed X-ray spectra (EDS) analyzer. The electron probe microscopic analyzer (EPMA) JXA-8230 was used to detect the elemental composition of thick middle layer qualitatively. An X-ray photoelectron spectroscopy (XPS) system equipped with XRC1000 X-ray gun and HSA3000 detector was applied to determine the corrosion products on the sample surfaces with monochromatic Al Kα radiation at 1486.8 eV. The calibration of binding energies was carried out by referring to the standard binding energy of the C 1 s peak (284.8 eV)54, and the fitting of peaks were conducted by OriginPro software. And the full width at half maximum (FWHM) of fitting results is given in Table 1.. In addition, a TEM sample contained scales and substrate of the tested sample was prepared by an ETHOS NX500 focus ions and electron beam system (FIB), and then a JEM-2100 TEM was utilized to elucidate the detailed microstructures of the corrosion interfaces.
Data availability
All data generated or analysed during this study are included in this published article. This data is available from this paper. If you need to have further information, then email to the corresponding author.
Change history
20 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-79831-z
References
Zhang, J. & Li, N. <ArticleTitle Language=“En”>Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 373, 351–377 (2008).
Sobolev, V. Thermophysical properties of lead and lead–bismuth eutectic. J. Nucl. Mater. 362, 235–247 (2007).
Gong, X., Short, M. P., Auger, T., Charalampopoulou, E. & Lambrinou, K. Environmental degradation of structural materials in liquid lead- and lead-bismuth eutectic-cooled reactors. Prog Mater. Sci. 126, 100920 (2022).
Gromov, B. F. et al. Use of lead-bismuth coolant in nuclear reactors and accelerator-driven systems. Nucl. Eng. Des. 173, 207–217 (1997).
Park, J. J., Butt, D. P. & Beard, C. A. Review of liquid metal corrosion issues for potential containment materials for liquid lead and lead–bismuth eutectic spallation targets as a neutron source. Nucl. Eng. Des. 196, 315–325 (2000).
Popovic, M. P. et al. A study of deformation and strain induced in bulk by the oxide layers formation on a Fe-Cr-Al alloy in high-temperature liquid Pb-Bi eutectic. Acta Mater. 151, 301–309 (2018).
Murty, K. L. & Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 383, 189–195 (2008).
Yun, D. et al. Current state and prospect on the development of advanced nuclear fuel system materials: A review. Mater. Rep. : Energy. 1, 100007 (2021).
Gorse-Pomonti, D. & Russier, V. Liquid metals for nuclear applications. J. Non-Cryst Solids. 353, 3600–3614 (2007).
Gong, X. et al. Opportunities for the LWR ATF materials development program to contribute to the LBE-cooled ADS materials qualification program. J. Nucl. Mater. 482, 218–228 (2016).
Cairang, W. et al. Oxidation mechanism of refractory Molybdenum exposed to oxygen-saturated lead-bismuth eutectic at 600°C. Corro Sci. 179, 1091320 (2021).
Schroer, C., Wedemeyer, O., Novotny, J., Skrypnik, A. & Konys, J. Selective leaching of nickel and chromium from Type 316 austenitic steel in oxygen-containing lead–bismuth eutectic (LBE). Corro Sci. 84, 113–124 (2014).
Ma, S. & Zhang, J. Interface morphologies and embrittlement behavior of α-Fe in liquid lead-bismuth eutectic at elevated temperature. Mater. Charact. 147, 43–49 (2019).
Hosemann, P., Frazer, D., Stergar, E. & Lambrinou, K. Twin boundary-accelerated ferritization of austenitic stainless steels in liquid lead–bismuth eutectic. Scr. Mater. 118, 37–40 (2016).
Schroer, C., Konys, J., Furukawa, T. & Aoto, K. Oxidation behaviour of P122 and a 9Cr–2 W ODS steel at 550°C in oxygen-containing flowing lead–bismuth eutectic. J. Nucl. Mater.Bold">398, 109–115 (2010).
Kondo, M. & Takahashi, M. Corrosion resistance of Si- and Al-rich steels in flowing lead–bismuth. J. Nucl. Mater. 356, 203–212 (2006).
Xiao, J. et al. A refined oxidation mechanism proposed for ferritic-martensitic steels exposed to oxygen-saturated liquid lead-bismuth eutectic at 400°C for 500 h. J. Nucl. Mater. 549, 152852 (2021).
Ye, Z. et al. Oxidation mechanism of T91 steel in liquid lead-bismuth eutectic: with consideration of internal oxidation. Sci. Rep. 6, 35268 (2016).
Gossé, S. Thermodynamic assessment of solubility and activity of iron, chromium, and nickel in lead bismuth eutectic. J. Nucl. Mater. 449, 122–131 (2014).
Stergar, E. et al. Influence of LBE long term exposure and simultaneous fast neutron irradiation on the mechanical properties of T91 and 316L. J. Nucl. Mater. 473, 28–34 (2016).
Gong, X., Marmy, P. & Yin, Y. The role of oxide films in preventing liquid metal embrittlement of T91 steel exposed to liquid lead-bismuth eutectic. J. Nucl. Mater. 509, 401–407 (2018).
Gong, X. et al. Liquid metal embrittlement of an Fe10Cr4Al ferritic alloy exposed to oxygen-depleted and -saturated lead-bismuth eutectic at 350°C. Corro Sci. 165, 108364 (2020).
Wang, H. et al. Liquid metal embrittlement of 12Cr ferritic/martensitic steel thin-walled tubes exposed to liquid lead-bismuth eutectic. Corro Sci. 195, 110024 (2022).
El-Genk, M. S. & Tournier, J. M. A review of refractory metal alloys and mechanically alloyed-oxide dispersion strengthened steels for space nuclear power systems. J. Nucl. Mater. 340, 93–112 (2005).
Shi, Z. & Han, F. The microstructure and mechanical properties of micro-scale Y2O3 strengthened 9Cr steel fabricated by vacuum casting, Mater. Des. –2015) 66, 304–308 (2015) (1980).
Yan, X. et al. Highly stable nanocrystalline oxide dispersion strengthened alloys with outstanding helium bubble suppression. J. Nucl. Mater. 557, 153283 (2021).
Ukai, S. & Fujiwara, M. Perspective of ODS alloys application in nuclear environments. J. Nucl. Mater. 307–311, 749–757 (2002).
Zhang, Z., Zhang, X., Sheng, L. & Teng, X. The Effect of the Third Element Cr on Oxidation Behavior of Fe-xCr-10Al(at. %), the Open Corro. J. 2, 37–44 (2009).
Wagner, C. Passivity and inhibition during the oxidation of metals at elevated temperatures. Corro Sci. 5, 751–764 (1965).
Stott, F. H., Wood, G. C. & Stringer, J. The influence of alloying elements on the development and maintenance of protective scales. Oxid. Met. 44, 113–145 (1995).
Gong, X. et al. A comparative study on liquid metal embrittlement susceptibility of three FeCrAl ferritic alloys in contact with liquid lead-bismuth eutectic at 350°C. Corro Sci. 183, 109346 (2021).
Hosemann, P., Thau, H. T., Johnson, A. L., Maloy, S. A. & Li, N. Corrosion of ODS steels in lead–bismuth eutectic. J. Nucl. Mater. 373, 246–253 (2008).
Takaya, S. et al. Corrosion behavior of Al-alloying high Cr-ODS steels in lead–bismuth eutectic. J. Nucl. Mater. 386–388, 507–510 (2009).
Lim, J., Nam, H. O., Hwang, I. S. & Kim, J. H. A study of early corrosion behaviors of FeCrAl alloys in liquid lead–bismuth eutectic environments. J. Nucl. Mater. 407, 205–210 (2010).
Takaya, S. et al. Corrosion resistance of Al-alloying high Cr–ODS steels in stagnant lead–bismuth. J. Nucl. Mater. 398, 132–138 (2010).
Kimura, A. et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 417, 176–179 (2011).
Takaya, S. et al. Al-containing ODS steels with improved corrosion resistance to liquid lead–bismuth. J. Nucl. Mater. 428, 125–130 (2012).
Baker, B. W. & Brewer, L. N. Evaluation of liquid metal embrittlement susceptibility of oxide dispersion strengthened steel MA956. J. Nucl. Mater. 453, 239–246 (2014).
Gao, R. et al. Oxidation resistance in LBE and air and tensile properties of ODS ferritic steels containing Al/Zr elements. J. Nucl. Mater. 455, 407–411 (2014).
Lambrinou, K., Koch, V., Coen, G., Van den Bosch, J. & Schroer, C. Corrosion scales on various steels after exposure to liquid lead–bismuth eutectic. J. Nucl. Mater. 450, 244–255 (2014).
Hojna, A., Hadraba, H., Gabriele, F., Di & Husak, R. Behaviour of pre-stressed T91 and ODS steels exposed to liquid lead-bismuth eutectic. Corro Sci. 131, 264–277 (2018).
Serrano, M., Hernández-Mayoral, M. & García-Junceda, A. Microstructural anisotropy effect on the mechanical properties of a 14Cr ODS steel. J. Nucl. Mater. 428, 103–109 (2012).
Verhiest, K. et al. Experimental study on the contact angle formation of solidified iron–chromium droplets onto yttria ceramic substrates for the yttria/ferrous alloy system with variable chromium content. Ceram. Int. 40, 2187–2200 (2014).
Odette, G. R., Alinger, M. J. & Wirth, B. D. Recent developments in irradiation-resistant steels. Ann. Rev. Mater. Res. 38, 471–503 (2008).
Hong, Z., Zhang, X., Yan, Q. & Chen, Y. A new method for preparing 9Cr-ODS steel using elemental yttrium and Fe2O3 oxygen carrier. J. Alloy Compd. 770, 831–839 (2019).
Moghadasi, M. A., Nili-Ahmadabadi, M., Forghani, F. & Kim, H. S. Development of an oxide-dispersion-strengthened steel by introducing oxygen carrier compound into the melt aided by a general thermodynamic model. Sci. Rep. 6, 38621 (2016).
Mirzababaei, S., Ghayoor, M., Doyle, R. P. & Pasebani, S. In-situ manufacturing of ODS FeCrAlY alloy via laser powder bed fusion. Mater. Lett. 284, 129046 (2021).
Park, D. J. et al. Fabrication and mechanical properties of an oxide-dispersion-strengthened FeCrAl alloy. Fusion Eng. Des.Bold">139, 81–85 (2019).
Wu, S. J., Li, J., Li, W. H. & Liu, S. Characterization of oxide dispersoids and mechanical properties of 14Cr-ODS FeCrAl alloys. J. Alloy Compd. 814, 152282 (2022).
Fabrichnaya, O., Seifert, H. J., Ludwig, T., Aldinger, F. & Navrotsky, A. The assessment of thermodynamic parameters in the Al2O3-Y2O3 system and phase relations in the Y-Al-O system. Schad J. Metall. 30, 175–183 (2001).
Ukai, S., Sakamoto, K., Ohtsuka, S., Yamashita, S. & Kimura, A. Alloy design and characterization of a recrystallized FeCrAl-ODS cladding for accident-tolerant BWR fuels: An overview of research activity in Japan. J. Nucl. Mater. 538, 154508 (2023).
Wu, S. J. et al. Preliminary study on the fabrication of 14Cr-ODS FeCrAl alloy by powder forging. J. Mater. Sci. Technol. 83, 49–57 (2021).
Zhang, L. et al. Cr-promoted formation of B2 + L21 composite nanoprecipitates and enhanced mechanical properties in ferritic alloy. Acta Mater. 243, 118506 (2023).
Shi, H. et al. Corrosion behavior of Al-containing MAX-phase coatings exposed to oxygen containing molten Pb at 600°C. Corro Sci. 201, 110275 (2022).
Desimoni, E., Malitesta, C., Zambonin, P. G. & Rivière, J. C. An x-ray photoelectron spectroscopic study of some chromium–oxygen systems. Surf. Interface Anal.Bold">13, 173–179 (1988).
Oku, M. & Hirokawa, K. X-ray photoelectron spectroscopy of Co3O4, Fe3O4, Mn3O4, and related compounds. J. Electron. Spectrosc. Relat. Phenom. 8, 475–481 (1976).
Dharmadhikari, V. S., Sainkar, S. R., Badrinarayan, S. & Goswami, A. Characterisation of thin films of bismuth oxide by X-ray photoelectron spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 25, 181–189 (1982).
Sahu, S. K., Ganesan, R. & Gnanasekaran, T. Standard molar Gibbs free energy of formation of Pb5CrO8(s), Pb2CrO5(s), and PbCrO4(s). J. Chem. Thermodyn. 42, 1–7 (2010).
Sahu, S. K., Ganesan, R. & Gnanasekaran, T. Studies on phase diagram of Pb–Cr–O system. J. Nucl. Mater. 376, 366–370 (2008).
Pederson, L. R. Two-dimensional chemical-state plot for lead using XPS. J. Electron. Spectrosc. Relat. Phenom. 28, 203–209 (1982).
Lim, J., Mariën, A., Rosseel, K., Aerts, A. & Bosch, J. Van den. Accuracy of potentiometric oxygen sensors with Bi/Bi2O3 reference electrode for use in liquid LBE. J. Nucl. Mater. 429, 270–275 (2012).
Schroer, C. & Konys, J. Physical Chemistry of Corrosion and Oxygen Control in Liquid Lead and Lead Bismuth Eutectic, 17–25Germany, (2007).
Schroer, C. & Konys, J. Quantification of the Long-Term Performance of Steels T91 and 316L in Oxygen-Containing Flowing Lead-Bismuth Eutectic at 550°C. J. Eng. Gas Turbines Power-Trans ASME. 132, 082901 (2010).
Li, N. Active control of oxygen in molten lead–bismuth eutectic. J. Nucl. Mater. 300, 73–81 (2001).
Wang, Y. et al. Interface characterization and erosion–corrosion behavior of directional Fe-3.5 wt.% B steel in flowing liquid zinc at various temperatures. Corro Sci. 104, 260–268 (2016).
Wang, W., Lin, J., Wang, Y. & Chen, G. The corrosion of Fe3Al alloy in liquid zinc. Corro Sci. 49, 1340–1349 (2007).
Wang, W., Lin, J., Wang, Y., Zhang, Y. & Chen, G. Isothermal corrosion of TiAl-Nb alloy in liquid zinc. Mater. Sci. Eng. A. 452, 194–201 (2007).
Wang, W., Lin, J., Wang, Y. & Chen, G. Isothermal corrosion Fe3Si alloy in liquid zinc. J. Univ. Sci. Technol. Beijing. 14, 52–55 (2007).
Liu, X. et al. Liquid metal corrosion of 316L, Fe3Al, and FeCrSi in molten Zn-Al baths. Metall. Mater. Trans. A. 36A, 2049–2058 (2005).
Acknowledgements
The authors appreciate the financial support for this work from the National Natural Science Foundation of China under (Grants No. 52071254 & 52271069), the Innovative Scientific Program of CNNC (J202107006-02) and the Technology Innovation Leading Program of Shaanxi (Program No. 2022GXLH-01-28 & 2024QCY-KXJ-001).
Author information
Authors and Affiliations
Contributions
Y. L and X.-T. W. conducted the LBE corrosion experiments. Y. L. and X.-T. W. performed the characterization of samples. P. L. and Y.-S. L. performed TEM results and analyzed the original data. S.-Q. M. and J.-D. X. proposed the original problem, wrote and supervised the investigations and analysis. L.-L. L. proposed the preparation method of alloy and also prepared TEM-foil. Y. L. wrote the original manuscript with assistances from all authors. All authors contributed to all the discussions in the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Acknowledgements section. “The authors appreciate the financial support for this work from the National Natural Science Foundation of China under (Grants No. 52071254 & 52271069), the Innovative Scientific Program of CNNC (J202107006-02) and the Technology Innovation Leading Program of Shaanxi (Program No. 2022GXLH-01-28 & 2021QCY-KXJ-001).” now reads: “The authors appreciate the financial support for this work from the National Natural Science Foundation of China under (Grants No. 52071254 & 52271069), the Innovative Scientific Program of CNNC (J202107006-02) and the Technology Innovation Leading Program of Shaanxi (Program No. 2022GXLH-01-28 & 2024QCY-KXJ-001).”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Y., Ma, S., Lv, P. et al. Interfacial microstructure and corrosion behavior of ODS FeCrAl alloy in oxygen-saturated lead-bismuth eutectic at 450 °C. Sci Rep 14, 26175 (2024). https://doi.org/10.1038/s41598-024-77786-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77786-9