Abstract
Currently A(H5Nx) avian influenza viruses are globally widespread and continue to evolve. Since their emergence in 2020 novel highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b reassortant viruses have become predominant in the world and caused multiple infections in mammals. It was shown that some of A(H5N1) viruses mostly isolated from mammals contain an E627K mutation in the PB2 protein which can lead to adaptation of influenza viruses to mammalian cells. In 2023 in Russia we have isolated two highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b viruses from birds one of which contained an E627K mutation in the PB2 protein. This virus had increased virulence in mice. Limited airborne transmission of the virus with the PB2-E627K mutation was observed between ferrets, in which infectious virus was detected in the nasal washings of the three of the twelve recipient ferrets, and clinical symptoms of the disease were observed in one case. Both viruses showed dominant binding to avian-type sialoside receptors, which was most likely the reason for the limited transmissibility. Thus, this study indicates a possible limited increase in the pandemic potential of A(H5N1) 2.3.4.4b viruses and highlights the importance of continuous avian influenza surveillance for pandemic preparedness and response.
Similar content being viewed by others
Introduction
Influenza viruses are a well-known and widely described group of viruses which can cause disease in humans and various animal species. Influenza A viruses are the most significant representatives of the Orthomyxoviridae family due to their global distribution and ability to infect a wide range of susceptible hosts1. Influenza virus outbreaks pose a serious threat to agriculture and public health. Among the wide range of variants of this infectious agent, only some subtypes of influenza A virus have demonstrated the ability to cross the species barrier and cause disease in humans. The evolution of influenza A virus has led to the emergence of new highly pathogenic variants for humans and animals, the circulation of which in susceptible host populations can cause disease, often with lethal outcomes. This is evidenced by epizootics among animals, which cause great economic damage, and sporadic cases of human disease caused by highly pathogenic influenza viruses of the H5 subtype2,3.
To date, the most common and widespread variants of animal influenza viruses are A(H5Nx) highly pathogenic influenza viruses of clade 2.3.4.4. In the last three years, most outbreaks among birds and mammals have been caused by the novel A(H5N1) viruses of clade 2.3.4.4b worldwide, including Russia4,5,6. The spread of the novel A(H5N1) influenza viruses, which were first identified in late 20207 , has resulted in thousands of outbreaks among wild birds and poultry. In addition, many fatal cases of clade 2.3.4.4b H5 infections have been registered among mammals, including various species of carnivores and marine mammals. The viruses isolated from mammals often had genetic markers of adaptation to replication in mammalian cells8,9,10. Markers of increased pathogenicity to mammals were found in polymerases and included amino acid substitutions E627K and D701N in PB2, which have been well studied9,11. It has been shown that the adaptation of the polymerase complex of avian influenza virus to function in mammalian cells is important for the replication of influenza viruses in mammals4. An additional factor in the adaptation of avian influenza viruses to mammals may be a change in the binding specificity from avian α2.3-receptors towards α2.6-receptors of the human type4. Currently circulating A(H5N1) 2.3.4.4b viruses mainly have avian receptors-binding specificity, demonstrate a limited ability in infecting humans and have not demonstrated ability to transmit from person to person. Several sporadic cases of human infection with the A(H5N1) 2.3.4.4b virus have been reported. Additionally, serological studies indicate a larger extent of the occurrence of the infection of people with these viruses12.
It was shown that only few amino acid changes may substantially increase virus adaptation to mammalian host. In particular it was shown that E627K in PB2 may contribute to increased transmissibility of influenza viruses among mammals11. Evaluation of A(H5N1) 2.3.4.4b virus transmissibility is critical for estimation of its pandemic potential4. Currently only limited data is available on transmissibility of the A(H5N1) 2.3.4.4b viruses. Direct contact transmission in a ferret model was demonstrated for one A(H5N1) 2.3.4.4b virus with PB2-E627K mutation in a study in Canada13. In addition, during the mink farm outbreak in Spain in 2022 there was an indication of a possible contact transmission of A(H5N1) 2.3.4.4b virus between the animals; the identified virus had T271A mutation in PB2, which may be associated with adaptation to mammals10. There was a confirmation of the contact transmission in a ferret model for a virus isolated from a mink during this outbreak. The follow up study showed aerosol transmission of the virus in a ferret model, which was a first demonstration of 2.3.4.4b H5 virus transmission among mammals14. Two studies from the USA demonstrated that A(H5N1) 2.3.4.4b viruses which did not have the PB2-E627K mutation, did not show direct contact transmission in a ferret model5,15. Airborne transmission in a ferret model has not been demonstrated for A(H5N1) 2.3.4.4b viruses without PB2-E627K mutation in a previous study in the USA15 , and neither was it demonstrated for a A(H5N2) virus in another study16. A Canadian study of airborne transmission of an A(H5N1) 2.3.4.4b virus bearing the PB2-E627K mutation revealed a transfer of a virus by PCR detection of viral genetic material in recipient ferrets, but no infectious virus was recovered and no disease symptoms were observed13.
The goal of this study was to investigate virological properties and transmissibility of an avian influenza virus A(H5N1) 2.3.4.4b with E627K mutation in the PB2 protein, isolated in Russia in 2023.
Our study demonstrated airborne transmission of a highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus with the PB2-E627K mutation between ferrets, in which infectious virus was detected in the nasal washings of three of the twelve recipient ferrets, and clinical symptoms of the infection were observed in one recipient ferret, which did not survive the infection. The seroconversion was not detected in the recipient ferrets that survived the infection, indicating the limited nature of the transmission.
Results
Registration of the A(H5N1) outbreak
In 2023, emus (Dromaius novaehollandiae) and poultry deaths were registered on the territory of a biosphere reserve in the south-west of the Russian Federation. Two isolates were obtained by culturing samples in embryonated chicken eggs (ECE): one from emu (A/emu/Russia/372-2/2023 (A/Emu372-E627K)) and one from chicken (A/chicken/Russia/540 − 10/2023 (A/Ch540)). Based on the results of real-time RT-PCR and genome sequencing, the isolates A/Emu372-E627K and A/Ch540 were identified as A(H5N1) subtype influenza viruses.
Genetic analysis of the isolated A(H5N1) strains
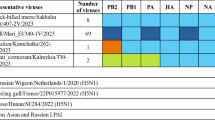
Phylogenetic analysis showed that A/Emu372-E627K and A/Ch540 viruses are closely related and belong to the phylogenetic clade 2.3.4.4b (Fig. S1, Table S1). Comparative genomic analysis of hemagglutinin (HA) segments of A/Emu372-E627K and A/Ch540 showed their close genetic similarity to A(H5Nx) 2.3.4.4b candidate vaccine viruses (CVVs) A/chicken/Ghana/AVL-763_21VIR7050-39/2021, A/Astrakhan/3212/2020 and A/American Wigeon/South Carolina/22-000345-001/2021 (Table 1). The HA protein of A/Ch540 differed from A/Emu372-E627K by one amino acid substitution R323K in polybasic cleavage site and was not reported as associated with changes of virus characteristics (Flusurver).
Analysis of the PB2 protein in A/Emu372-E627K showed the presence of E627K mutation associated with the virus adaptation to mammals and increased virulence in mammals17,18. Analysis of other genes did not reveal significant amino acid substitutions in A/Emu372-E627K compared to A/Ch540. Genetic analysis showed that all genome segments of A/Emu372-E627K and A/Ch540 viruses belong to the Eurasian lineage.
Analysis of receptor binding specificity markers in HA protein revealed the presence of a QRG amino acid sequence at positions 222–224 (H5 numbering), which is associated with virus HA affinity to avian α-2.3-linked receptors19,20. At the same time, there were amino acids markers 123P, 133A, 156A which were previously reported as associated with the increased binding to mammalian α-2.6-linked receptors21,22,23,24.
Virological properties
Virulence and pathogenicity
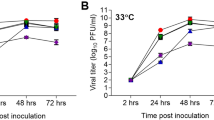
Cultivated on 10-day-old ECE, the viruses A/Emu372-E627K and A/Ch540 showed a high degree of virulence for chicken embryos, leading to their death within 48 h after infection. The infectious titers of the viruses in the allantoic fluid two days post inoculation were 9.7 and 10.3 lg EID50/mL, respectively (Table 2). These values were comparable to the infectious titers of a representative group of A(H5Nx) viruses previously isolated in Russia.
The studied viruses showed different degree of virulence in laboratory Balb/c mice upon intranasal infection. The A/Emu372-E627K had significantly lower 50% infective (ID50) and 50% lethal (LD50) doses than A/Ch540 strain, which was isolated during the same outbreak but did not contain the E627K mutation in the PB2 protein (Table 2, Fig.S2). The ID50 and LD50 of A/Emu372-E627K were also lower than values determined for several A(H5Nx) viruses which were collected in Russia in previous years and analyzed in our laboratory (Table 2).
The studied virus showed a high degree of virulence in ferrets. For intranasal infection, the 50% infective (ID50) and 50% lethal (LD50) doses for A/Emu372-E627K virus isolate were the same and equal to 0.14 ± 0.9 lg EID50 (Fig. S3).
Susceptibility to neuraminidase inhibitors
Study of drug susceptibility using a fluorescent neuraminidase inhibition assay showed that the tested strains were normally inhibited by zanamivir and oseltamivir. The half-maximum inhibitory concentrations (IC50) of zanamivir/oseltamivir determined for A/Emu372-E627K and A/Ch540 viruses were 0.33/1.81 and 0.32/2.1 nM respectively, which were comparable with typical values of A(H5N1) viruses from the 2.3.4.4b clade (Table 2).
Antigenic properties
Antigenic properties of the isolated viruses were investigated by hemagglutination inhibition assay (HIA) using reference antigens (including A(H5Nx) CVVs and influenza A(H5N1) viruses previously isolated in Russia) and the corresponding immune ferret blood antisera (Table 3). The results showed that A/Emu372-E627K and A/Ch540 strains have a high degree of antigenic similarity with the 2.3.4.4b A(H5N1) influenza viruses which circulated in Russia in 2022. At the same time, the analyzed strains were poorly recognized by sera raised against candidate vaccine strains of the clade 2.3.4.4 recommended by the WHO in previous years.
Receptor specificity
Receptor specificity of the viruses was evaluated by biolayer interferometry using influenza virus receptor analogs 3’-Sialyl-N-acetyllactosamine (α2.3-SA) and 6’-Sialyl-N-acetyllactosamine (α2.6-SA). The calculated equilibrium dissociation constants showed that A/Emu372-E627K and A/Ch540 viruses preferentially bound the avian-type α2.3-SA receptor analogs, while a binding to the human-type α2.6-SA receptor analogs was not detected (Table 4).
Transmissibility of A/Emu372-E627K strain
The airborne transmissibility of the A/Emu372-E627K was assessed in an experiment schematically shown in the Fig. 1.
Airborne transmission experiment for ferrets infected with the clade 2.3.4.4b influenza A(H5N1) virus A/Emu372-E627K. At 0 dpi, donor ferrets (n = 3 per dose) were inoculated with 4.7, 3.7, 2.7 and 1.7 lg EID50 of A(H5N1) virus. On the second dpi, groups of naïve ferrets were placed in separate cages of the TIEGEL ELC 04–60 dynamic aerobiology chamber (with directed airflow from donor to recipient ferrets) which were positioned at a distance from the cages of groups of donor ferrets to prevent direct contact. Joint exposure of the animals lasted 5 h daily for 5 days, starting on the second and ending on the sixth dpi. Clinical course of infection was monitored, and nasal wash samples were taken at indicated time points from both inoculated and contact ferrets. Blood was taken from all survived contact ferrets to check for seroconversion on 22 and 30 dpc.
Donor intranasally inoculated ferrets
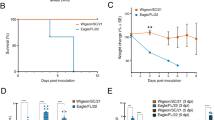
Results showed that all ferrets which were intranasally inoculated with various infectious doses of the A/Emu372-E627K (1.7–4.7 lg EID50) developed clinical signs of infection. In particular, ferrets inoculated with 1.7 and 2.7 lg EID50 showed a loss of appetite and reduced activity level (mean relative inactivity indexes (MRII) for these test groups were 2.6 and 3.3 respectively). Ferrets inoculated with 3.7 and 4.7 lg EID50 demonstrated loss of appetite, reduced activity level (MRII 3.6 and 3.8 respectively), wheezing, sneezing and nosebleeds (Table S2). All inoculated ferrets across all 4 experimental groups demonstrated increase in body temperature by 1.9–4.7 °C (a mean value 3 °C) during an acute phase of infection and 3–15% loss in body weight (a mean value 8%) prior to a death. Neurological symptoms (lethargy, paresis, paralysis) were not detected in inoculated ferrets. All inoculated ferrets died on days 4–7 post infection (dpi) (Fig. 2A). It was not possible to take blood samples from inoculated ferrets for seroconversion assessment. High loads of virus were detected in nasal washes of all inoculated ferrets during the whole observation period which was confirmed by RT-PCR (Table S3) and focus forming units (FFU) assay (Fig. 3A, C, E and G). Mean infectious virus titers in nasal washes collected from ferrets inoculated with 4.7, 3.7, 2.7 and 1.7 lg EID50 were 6.8, 7.3, 6.9 and 5.6 lg FFU/mL respectively.
Shedding of the virus A/Emu372-E627K by inoculated and airborne contact ferrets. A, С, E, G. Infectious viral titers in nasal washes of donor ferrets (n = 3 per dose) infected with 4.7, 3.7, 2.7 and 1.7 lg EID50 respectively. B, D, F, H. Infectious viral titers in nasal washes of recipient ferrets (n = 3 per dose) which were in an airborne contact with donor ferrets inoculated with 4.7, 3.7, 2.7 and 1.7 lg EID50 respectively. Columns represent individual animal’s titer (mean virus titer ± SD). Dashed lines indicate the lower limit of virus titer detection (1 lg FFU/mL). The asterisk sign indicates the washes from which the viral isolate was isolated in ECE.
Airborne contact ferrets
The study of airborne contact ferrets showed that 10 out of 12 contact animals (ferrets 3R–12R) had at least one RT-PCR positive nasal washing specimen taken during the observation period (Table S3). The FFU-assay showed that infectious virus was detected in nasal washings of two out of the 12 recipient ferrets at titers 3.1 lg FFU/mL (ferret 3R) and 2.7 lg FFU/mL (ferret 6R) (Fig. 3B, D, F and H). Inoculation of ECE with the recipient ferrets’ nasal washes demonstrated the presence of infectious virus in samples taken from 2 out of 12 contact animals (ferrets 3R and 10R) (Fig. 3B and H). Egg isolates obtained from the 3R and 10R nasal washes had hemagglutination titers of 1:256 and 1:4096 HAU (hemagglutinating unit) respectively. Genome sequences were determined for these isolates.
Of the 12 tested contact ferrets, 11 ferrets survived and showed no clinical symptoms of disease (including ferrets 6R and 10R which showed transient presence of infectious virus in nasal washes) (Fig. 2B, Table S2). Survived contact ferrets demonstrated a small decrease in a body weight at day 1 post contact (dpc) (≤ 5%) followed by a slight upward trend of weight gain. Body temperature in these ferrets fluctuated on average by ± 1.2 °C from the initial (pre-exposure) body temperature. They remained normal level of physical activity (MRII 1.1–1.2). Blood sera taken from these ferrets on 22 and 30 dpc were seronegative against the A/Emu372-E627K strain based on the results of HIA and microneutralization assay (including ferrets 6R and 10R in which an infectious virus was detected in the upper respiratory tract).
Only one animal out of 12 recipient ferrets (ferret 3R) had clinical signs of disease. On 4 and 5 dpc, the 3R ferret developed loss of appetite and showed reduced activity level with inactivity score of 2 (the animal was alert but not playful)25. RII for this ferret was 1.8 (Table S2), which was lower than that of donor ferrets, but highest in the group of recipient ferrets. The lower RII may be due to the fact that the dose of virus infecting the ferret during the airborne contact was very low. This may have resulted in the somewhat lower mean effect of the virus on the ferret physical activity over the course of the disease compared to donor ferrets, which were infected with one time introduction of a higher dose of the virus. On 4 dpc body temperature has increased by 1.3 °C (t = 39.4 °C) followed by a 5.3 °C drop on 5 dpc (t = 33.9 °C). A body weight of the 3R ferret decreased by 4.3% from the initial mean value on 5 dpc. The 3R ferret died on 6 dpc (Fig. 2B), it was not possible to take its blood sample for seroconversion assessment.
Thus, combination of the FFU-assay and ECE inoculation methods allowed us to detect the infectious virus in the nasal washes of 3 recipient ferrets, of which one ferret became ill and died, and two ferrets had no symptoms of the disease, survived and did not seroconvert.
Genetic analysis of viruses isolated from the recipient ferrets
Egg viral isolates obtained from nasal washings taken from two recipient ferrets (3R, 10R) were taken for comparative genetic analysis with the initial viral stock of egg-propagated A/Emu372-E627K that was used for the infection of the donor ferrets.
In the sample from a deceased recipient ferret (3R), two substitutions were found in the HA protein: D155G substitution at a position that may play a role in receptor specificity26,27, , and N165S substitution which leads to a loss of the N-glycosylation site that may also affect receptor specificity27,28,29. No substitutions were detected in the remaining proteins.
In the sample from the second recipient ferret (10R), no substitutions were detected in the HA protein, but the N292S substitution was detected in the PB1 protein. In the other proteins no substitutions were found.
Single nucleotide polymorphisms (SNP) at amino acid positions 155 and 165 of the HA gene were not detected in the initial egg-propagated A/Emu372-E627K virus used to infect donor ferrets (SNPs with an occurrence frequency of more than 5% in a sample were considered). Protein PB1 292N/S polymorphism (major variant 292N – 91%, minor variant 292S – less than 9%) was detected in the A/Emu372-E627K isolate that was used for donor ferret inoculation in the airborne transmission studies.
Discussion
Due to the recent spread of the A(H5N1) virus 2.3.4.4 in many countries of the world, including Russia, these viruses pose an increasing threat to human health4,5,6. Monitoring studies make it possible to timely identify genetic changes in influenza viruses that lead to adaptation to mammals4,9,13. In 2023, during the outbreak of A(H5N1) 2.3.4.4b in Russia, influenza virus A/Emu372-E627K was isolated from infected emu. Genetic analysis of A/Emu372-E627K showed that hemagglutinin of the virus was genetically similar to HA of CVVs A/Astrakhan/3212/2020, A/chicken/Ghana/AVL-763_21VIR7050-39/2021, A/American Wigeon/South Carolina/22-000345-001/2021.
The virus A/Emu372-E627K contained an E627K amino acid substitution in the PB2 protein, which is responsible for adaptation of the virus to mammals17,18. Our study in Balb/c mice demonstrated high virulence of A/Emu372-E627K virus. It was significantly higher than the virulence of genetically similar A/Ch540 virus, which did not have an E627K amino acid substitution in the PB2 protein. Previously reported A(H5Nx) viruses isolated in Russia in 2014-2020 did not contain the E627K mutation and were less virulent to mice compared to A/Emu372-E627K virus with the E627K mutation4,30,31. Comparatively high virulence to mice was also observed for clade 2.3.4.4b A(H5N1) A/Red Tailed Hawk/ON/FAV-0473-4/2022 virus (RT.Hawk/ON/22) which was isolated in Canada and had the E627K mutation13. In addition, the study from Canada suggested a contribution of other genetic factors to enhanced virulence13. In particular, the study revealed that virulence of A(H5N1) viruses bearing a PB2-E627K substitution can vary depending on their reassortment genotype. Higher virulence was observed for the A(H5N1) virus containing NP, PB1, PB2 from the North American lineage compared to viruses with the genes of Eurasian lineage. Analogous results were showed in a study of A(H5N1) clade 2.3.4.4b viruses isolated in the USA, which did not contain the E627K mutation. It showed that the presence of NP and polymerase genes from the North American lineage resulted in higher virulence compared to viruses with all internal genes belonging to the Eurasian lineage5. It was especially noted that increased virulence was associated with severe neurological involvement. The lack of observed neurological symptoms in ferrets infected with Eurasian lineage A/Emu372-E627K, and results from the above-mentioned study from the USA may suggest a possibility of association of virus genotype with neurological involvement, which needs to be further investigated.
In addition to increased pathogenicity in mice, it was shown that RT.Hawk/ON/22 virus with PB2-E627K reproduced well in human nasal mucosal epithelial cell culture13. These properties of the virus can be considered as risk factors for spread among mammals.
Previously it was shown that PB2-E627K may increase transmissibility of avian influenza virus between mammals11. This amino acid substitution is more frequently detected in avian influenza viruses isolated from mammalian cases (including human cases) compared to avian cases9,30. The E627K amino acid substitution in the PB2 protein has been detected in clade 2.3.4.4b viruses isolated from mammals, in particular in A(H5N1) viruses isolated from wild carnivores (foxes, otter, polecat) in the Netherlands in 2021–20229 , in viruses isolated from seals in the USA8 and in A(H5N8) viruses collected from seals in Germany in 202131. The mutation E627K adapts the operation of PB2 to the mammalian organism9. The amino acid substitution has been shown to give the virus an advantage in replicating at the comparatively lower temperature of the upper respiratory tract of mammals compared to higher body temperatures in birds17. These properties probably may contribute to enhanced transmissibility of the viruses among mammals. In support of this, animal studies reported that the presence of E627K was found to increase transmission among guinea pigs11.
In this study, the airborne transmissibility of A/Emu372-E627K virus was investigated in a ferret model. Samples of nasal washings from the majority of recipient ferrets (10 of 12 from different groups) were positive in PCR test for the presence of influenza A virus RNA. Presence of infectious A(H5N1) virus was confirmed in 3 recipient ferrets, while symptoms of the disease were observed only in one ferret, which died from the infection. Seroconversion was not detected in the recipient ferret that died due to a short period of time between the beginning of the virus shedding and the fatal outcome. In the other 2 ferrets, a transient one-day virus shedding was detected, but no clinical signs of an illness and no seroconversion were registered. Similar cases were reported in other transmission studies32,33,34. There is a concern that in these cases an infectious virus could reach nasal epithelium of contact ferrets and even undergo a limited number of reproduction cycles, but was not able to induce a robust productive infection due to various factors such as insufficient airborne transmitted dose or effective innate immune response35. On the other hand, it is known that influenza infection in some cases does not result in detectable antibody response. This phenomenon could be associated with individual characteristics of an infected organism/species36 or with properties of a virus37.
Thus, this study is among the first, in which limited airborne transmissibility between mammals was shown for clade 2.3.4.4b A(H5N1) virus using a ferret model. For the first time limited airborne transmissibility for A(H5N1) 2.3.4.4b viruses was demonstrated in the ferret model using A/mink/Spain/3691-8_22VIR10586-10/2022 virus (A/mink (H5N1)) obtained by reverse genetics. A/mink(H5N1) virus, like A/Emu372-E627K, had a mammalian adaptation mutation in PB2 (T271A)14. The presence of adaptation mutations in PB2 may contribute to a limited increase in transmissibility of A(H5N1) clade 2.3.4.4b viruses. In addition, signs of possible airborne transmissibility in ferrets were demonstrated in a preliminary studies with RT.Hawk/ON/22 virus bearing a PB2-E267K mutation: the virus genetic material was detected in nasal washings from the recipient ferrets, but no infectious virus or disease symptoms were detected13. Further study of airborne transmissibility of influenza A(H5N1) clade 2.3.4.4b viruses is needed in order to evaluate the effect of the PB2-E627K and other mammal adaptive substitutions on transmissibility.
To our knowledge, airborne transmissibility has not yet been reported for the H5 viruses from the 2.3.4.4 clade which did not have mammalian adaptive mutations in PB2. In particular, the previously studied A(H5N2), A(H5N6) and A(H5N8) viruses which lacked such adaptive mutations did not transmit via airborne route16,24,38.
However, the studied clade 2.3.4.4b A(H5N1) viruses with adaptation mutations in PB2 had only limited transmissibility, which may be due to the absence of additional mutations, which are associated with effective airborne transmission, such as mutations that facilitate binding to α2.6-sialic receptors, as well as mutations that reduce the pH of membrane fusion39.
Analysis of hemagglutinin of A/Emu372-E627K virus showed the presence of genetic markers of preferential binding to avian-like α2.3-SA receptors (222–224 QRG) and also possible markers of increased binding with α2.6-SA receptors (123P, 133A, 156A)21,22,23,24. Nevertheless, the identified markers of increased binding with α2.6-SA receptors were initially described for H5 viruses from previously circulated clades. For the 2.3.4.4b clade, these amino acids became wild-type variants. Thus, phenotypic significance of these markers for 2.3.4.4b viruses needs further investigation. Evaluation of receptor binding properties using biolayer interferometry showed that A/Emu372-E627K binds to avian-like receptors with no detected affinity for the human-like receptors. Thus, our analysis suggests that A/Emu372-E627K retains avian properties.
The transmissibility properties of avian influenza viruses can change rapidly with the accumulation of additional mutations, especially when the viruses circulate in mammals39.
Our study showed that even during one transmission event the virus isolated from an airborne infected ferret acquired two amino acid substitutions in HA (D155G and N165S). In the other transmission event virus isolated from a contact ferret acquired a PB1-N292S substitution compared to the original virus used to infect donor ferrets. The effect of these mutations is not well understood. According to the analysis of the GISAID database, the HA-D155G mutation is extremely rare in avian influenza A(H5N1) clade 2.3.4.4b viruses. As described in the literature, the D155G substitution is located in the antigenic site40 and amino acid changes at this position can affect the receptor specificity of viruses26. The HA-N165S mutation in HA results in the loss of the N-glycosylation site, which may also affect receptor specificity27,28,29. A previous study of A/Hong Kong/486/97 (H5N1) virus showed that the N165S mutation can increase virulence in mice27. The virus isolated from another recipient ferret acquired the PB1-N292S mutation during reproduction in ferrets, which has not been described in the literature. According to the analysis of the GISAID database, this substitution in PB1 was present in a few cases of avian infection and one case of mammal infection (skunk, USA)41. Analysis of NGS sequencing data of the original virus used to infect donor ferrets showed the absence of single-nucleotide polymorphisms at amino acid positions 155 and 165 of the HA gene, whereas the polymorphism was present in the PB1 protein at position 292 N/S (292 N – 91%, 292 S < 9%). Thus, viral subpopulations carrying the PB1-N292S substitution could have been selected during virus reproduction in ferrets. These mutations identified in the recipient ferrets in this study may be associated with increased adaptability to mammals and should be investigated as possible markers of pathogenicity.
The study of avian influenza virus airborne transmissibility in ferrets is complicated by the influence of many experimental factors that must be considered when analyzing the data. Our experimental system is comparable to those used in previously published studies. Using the TIEGEL ELC 04–60 dynamic aerobiology chamber, we have previously demonstrated airborne transmissibility of seasonal influenza virus A(H1N1)pdm0916. The extremely low ID50value of A/Emu372-E627K (H5N1) in ferrets, lower than that for the pandemic virus A/California/07/200916 , and the high concentration of infectious virus in donor ferret’s swabs indicate that the infectivity of the virus is not a limiting factor.
Among the limiting factors of this study is the shortened exposure of the recipient ferrets to donor ferrets (5 h per day) due to technical limitations of the work with the aerobiology chamber and shortened time of exposure due to rapid death of the donor ferrets caused by the studied highly pathogenic virus. In our experiment, the length of co-exposure of donors and recipients lasted from 2 to 5 days due to early death of donor ferrets, which began being registered since the second dpc (which corresponds to the fourth dpi). In a similar experiment with A(H5N1) 2.3.4.4b virus a clear evidence of the infection were observed on the 9th day of continuous exposure, and several donor ferrets survived until the 14th day of the exposure14. In a previously conducted study of A(H5N2) 2.3.4.4b airborne transmissibility, donor ferrets did not die, and the exposure lasted for 14 days (5 h per day) in a similar conditions with the present study. However, for that studied virus, which did not have mammalian adaptive mutations in PB2, airborne transmissibility was not detected16.
Our study was conducted under temperature and humidity controlled conditions (21 ± 1 °C and 35 ± 5%), corresponding to standard living conditions. Previous studies in ferrets have shown that transmission occurred most frequently at 23 °C and 30% relative humidity42. Thus, the conditions in our experiment were optimal for transmissibility.
Additionally, it is worth mentioning that our setup had a low-velocity air flow from donors to recipients, which may also have contributed to more favorable conditions for transmissibility.
Another factor that may affect airborne transmissibility is a possible sex bias in response of ferrets to virus infection. Only female ferrets were used in this study. Previously, airborne transmissibility analysis of an A(H7N9) virus demonstrated that results of experiment did not depend on the sex of ferrets43. However, in experiments studying the transmissibility of a mink A(H5N1) 2.3.4.4b virus, it was noted that aerosol transmissibility was observed only in males14. There is not enough statistical data for evaluation of the possible bias due to a small number of ferrets in the published experiments. Previous studies indicate that the course of influenza infection and immune response may differ between male and female ferrets44. Further studies are needed to elucidate possible sex disparities in airborne transmission among ferrets.
Airborne transmission of influenza viruses between mammals is considered as an important indicator of the pandemic potential. The WHO Tool for Influenza Pandemic Risk Assessment (TIPRA) uses 10 risk elements to assess the pandemic potential of influenza viruses, one of which is transmissibility along with other important characteristics of the virus such as pathogenicity and receptor specificity. It also takes into account population immunity and geographic distribution45. Limited airborne transmission identified for the two A(H5N1) 2.3.4.4b viruses indicates a possible increase in the pandemic potential of viruses of this clade, especially for viruses containing mammalian adaptation mutations in the PB2 protein24. At the same time, the lack of receptor binding of the virus to the human-type receptor, the absence of other mutations necessary for effective airborne transmissibility between mammals, limited recovery of live virus from recipient ferrets indicate a limited increase in pandemic potential of the virus and likely indicates insufficient conditions for efficient human-to-human transmission. Wide geographical circulation of A(H5N1) 2.3.4.4b viruses and ongoing evolution, particularly through frequently detected infections of mammalian species, may result in the accumulation of characterized or new mutations and reassortment, which can increase transmissibility among mammals.
This indicates the critical need for further monitoring and continuous evaluation of transmissibility and pandemic potential of highly pathogenic A(H5Nx) influenza viruses. These studies are necessary for initiating timely response measures to the possible emergence of a new pandemic virus, and for pre-pandemic preparedness activities.
Materials and methods
Influenza virus propagation and titration
The A(H5N1) influenza viruses were isolated from birds in 10-day-old embryonated chicken specific pathogen free (SPF) eggs (SPF-CE) at 37 °C for up to 48 h. Specimen collection and all the virus isolation procedures were carried out by the Federal State-Financed Institution “Federal Centre for Animal Health” (FGBI “ARRIAH”), Rosselkhoznadzor. Viral titers were determined by injecting 0.1 mL of 10-fold dilutions of virus into the allantoic cavities of 10-day-old SPF-CE and then calculating the 50% embryo infectious dose (EID50) by the method of Reed and Muench46. All experiments with the viruses were conducted in the Biosafety level 3 (BSL 3) containment laboratory.
Sequence analysis of influenza viruses
Sequencing was carried out at the Federal Budgetary Research Institute State Research Center of Virology and Biotechnology “Vector”, Rospotrebnadzor (SRC VB “Vector”). To determine the nucleotide sequences of the viral genes and genomes, viral RNA was isolated using the RIBO-sorb RNA/DNA Extraction Kit (InterLabService, Moscow, Russia) according to the manufacturer’s instructions. Reverse transcription was carried out with a mixture of primers (Uni12, Uni12.4, and Uni13)47 using the RT-M-MuLV-RH reagent kit (LLC BIOLABMIX, Russia). PCR amplification of cDNA was performed using the BioMaster LR HS-Taq PCR kit (2×) (LLC BIOLABMIKS, Russia) according to the manufacturer’s instructions. NGS sequencing was performed on an Illumina MiSeq using the MiSeq reagent kit v3 (Illumina, San Diego, CA, USA). The full-length genomes were assembled by the alignment of reads to known references with bwa-0.7.1548. The obtained nucleotide sequences were deposited in the Global Initiative on Sharing All Influenza Data (GISAID) database. Maximum likelihood phylogenetic analysis was performed in IQ-TREE v2.0.749 applying the best-fitted nucleotide substitution model selected by ModelFinder50 with 1,000 bootstrap replicates and visualized in FigTree v1.4.451. Reference strains HA sequences were used from GISAID (Table S1).
Hemagglutination inhibition assay
HIA was carried out at the SRC VB “Vector” as described previously52,53 using CVVs A/gyrfalcon/Washington/41088-6/2014 (H5N8) and A/chicken/Vietnam/NCVD-15A59/2015 (H5N6) which were kindly provided by Dr. R. Webby, St. Jude Children’s Research Hospital (Memphis, TN). Immune ferret antisera against CVVs and influenza A(H5Nx) viruses previously isolated in Russia (A/Astrakhan/3212/2020 (H5N8), A/dalmatian pelican/Astrakhan/213–2 V/2022 (H5N1) and A/chicken/Khabarovsk/24–1 V/2022 (H5N1)) were produced at the SRC VB “Vector”. Turkey red blood cells were used in the assay.
Receptor binding assay
Receptor binding assay was performed at the SRC VB “Vector”. A medium containing inactivated virus was centrifugated at low speed to remove large debris and was then filtered using a membrane filter (0.45 μm). Virus particles were next pelleted by ultracentrifugation and resuspended in phosphate-buffered saline (PBS). Size-exclusion chromatography was performed using Sepharose CL-4B resins (GE Healthcare). Finally, influenza A virions were concentrated using Amicon® Ultra-15 Centrifuge Filters Ultracell®100KDa (Merck Millipore). The concentrations of purified viruses were determined by HA assays. Concentrations determined as number of hemagglutination units per mL (HAU/mL) were converted to the nmol/L (nM) measurement units (taking into consideration that one HAU typically corresponds to 106viral particles, and 1 viral particle has approximately 1000 HA glycoprotein spikes; concentration measured in HAU/mL was converted to spikes/mL, and then converted to nmol/mL (by dividing by Avogadro constant) as previously described54.
The kinetics of the interaction of influenza virions with biotinylated trisaccharides 6’- Sialyl-N-acetyllactosamine receptor analog 0997-BP (Lectinity) and 3’-Sialyl-N-acetyllactosamine receptor analog was measured by biolayer interferometry using an Octet RED96e (ForteBio). The sugars were loaded onto streptavidin biosensors at a concentration of 0.5 µg/mL and then viruses at 10–100 nM were added. To inhibit neuraminidase, oseltamivir (20 nmol/L) was added to viruses. Obtained data were analyzed by Octet software.
Animal experiments
Ethics statement
All applicable international, national and institutional guidelines for the care and use of animals were followed. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). Animal experiments were carried out in accordance with Russian legislation and under bioethics protocol № 1 (28 June 2022) and № 9 (10 November 2023) issued by BioEthics Committee at FBRI SRC VB Vector Rospotrebnadzor. All experimental protocols for work with animals were approved by bioethics protocol № 1 (28 June 2022) and № 9 (10 November 2023) issued by BioEthics Committee at FBRI SRC VB Vector Rospotrebnadzor.
Animals
Female ferrets aged 6–8 months and Balb/c mice weighing 16–18 g were obtained from the breeding facilities of laboratory animals of the SRC VB “Vector”. Animals were kept in individually ventilated cages on a standard diet. Serological testing was conducted to confirm the absence of virus-specific antibodies in animals before the start of the study and verify virus transmission from infected to healthy animals during the experiment. After experiments and during experiments upon necessity animals’ euthanasia was carried out using an automated compact CO2 system for humane output from the experiment of laboratory animals (Euthanizer, Russia). The concentration of carbon dioxide (30% at the 1st stage, 70% at the 2nd stage) and gas supply rate satisfy the requirements of the American Veterinary Medical Association (AVMA) 2020. All animal experiments were conducted under the Animal Biosafety level (ABSL 3) conditions.
Virulence
To determine the virulence of avian influenza viruses the female Balb/c mice were intranasally infected. Mice were divided into 12 groups of six animals each to determine the 50% infectious and lethal doses. Animals were lightly anesthetized with a combination of Zoletil 100 (Delpharm Tours, France) and Xyla (Interchemie, Estonia) before intranasal inoculation with 0.05 mL of six virus dilutions. Three days after inoculation six groups of mice were humanely euthanized and lung samples were taken and studied for the presence of influenza virus using 10-day-old chicken embryos as described above. For sample preparation 900 µl of DMEM culture medium with antibiotic and antimycotic were added to 100 µg of lung sample. The resulting lung samples were loaded into a TissueLyser LT homogenizer. Homogenization was performed for 5 min at 50 Hz. The resulting homogenates were centrifuged for 10 min at 10,000 g. The supernatant was collected in a clean tube to obtain 10% lung homogenate. The resulting lung homogenates were stored at -80 °C for further studies. Based on data on the presence of the virus in the lungs of the studied animals, a 50% infectious dose (ID50) was calculated. The remaining six groups were observed for 14 days for clinical signs and mortality to calculate the 50% lethal dose (LD50). ID50 and LD50were calculated by the method of Reed and Muench46.
To determine the virulence of avian influenza viruses the female ferrets were intranasally infected. Ferrets were divided into 7 groups of three animals each to determine the 50% infectious and lethal doses. Animals were lightly anesthetized with a combination of Zoletil 100 (Delpharm Tours, France) and Xyla (Interchemie, Estonia) before intranasal inoculation with 0.05 mL of seven virus dilutions. Nasal swab samples were collected under light anesthesia on days 2, 4, 6, and 8 after inoculation. Based on data on the presence of the virus in the swabs of the studied animals, a 50% infectious dose (ID50) was calculated. The animals were then observed for 14 days for clinical signs and mortality to calculate the 50% lethal dose (LD50). ID50 and LD50were calculated by the method of Reed and Muench46.
Transmissibility
To study transmissibility of influenza A viruses, an original ELC 04–60 aerobiology chamber (TIEGEL GmbH, Radeberg, Germany) was used as previously described16.
To assess the transmissibility of the A/Emu372-E627K (H5N1) virus, ferrets were divided into 8 groups: 4 groups of donor ferrets (3 ferrets per group) and 4 groups of recipient ferrets (3 ferrets per group). Groups of donor ferrets were intranasally infected with four different doses 104.7, 103.7, 102.7, 101.7 EID50 in a volume of 0.5 mL (0.25 mL in each nasal passage) under anesthesia. To assess airborne transmission of the virus, recipient ferrets (3 ferrets per group) were placed separate from the donor ferrets in the cage of aerobiology chamber TIEGEL ELC 04–60. The distance between cages was 10 cm, which excluded direct contact between infected animals and recipients. The airflow was directed from the infected donor ferrets towards the recipient ferrets and its velocity was 3 cm/sec. Joint exposure of the animals lasted 5 h daily for 5 days, starting on the second day and ending on the sixth day after the infection of donor ferrets.
Two days after infection, donor ferrets (3 heads) of each infecting dose were placed in one cage of one of the ELC 04–60 TIEGEL chambers16 , after which recipient ferrets (3 heads) were placed in another cage of the same chamber. The remaining groups of animals were placed similarly.
To assess the course of the infectious process, nasal washings were taken from donor ferrets from days 2 to 6 after infection. In all washings obtained on days 2–6 after infection, influenza virus RNA was determined by RT-PCR, as well as the presence of infectious virus by titration by FFU in MDCK cell culture.
To assess airborne transmission of the virus to recipient ferrets, nasopharyngeal washings were taken from recipient ferrets on days 2, 4, 6, 8 after the first joint exposure to donor ferrets. In the nasal washings of recipient ferrets obtained on all of the abovementioned days, the presence of the influenza virus RNA was determined by RT-PCR, and in addition, in samples positive for RT-PCR, the presence of the infectious virus was determined by titration methods in MDCK cell culture by counting FFU, as well as by inoculation of ECE.
To exclude unintentional contamination, nasal washings were taken from ferrets separately: donor ferrets – daily after airborne contact, recipient ferrets – daily before airborne contact.
Throughout the experiment, temperature and weight were measured daily for donor and recipient ferrets, and all ferrets in the study were assessed for symptoms and physical activity. Ferret activity level was assessed using a scoring system described by Reuman et al25. In short, a score was assigned from 0 to 3 based on activity ranging from alert and playful to neither alert nor playful. Relative inactivity index was calculated using an equation: Σ(day 1 to day X) [score + 1]n/Σ(day 1 to day X) n; X – total number of days of observation, n– total number of observations. Relative inactivity index characterizes a mean degree of influenza illness in a ferret over the whole experiment observation period, from 1 to 4, where 1 – normal physical activity and 4 – lack of physical activity even with stimulation55. At the same time, individual inactivity score characterizes level of activity on a particular day of the experiment.
Genomes of the viruses isolated from nasopharyngeal washings obtained from donor ferrets and recipient ferrets were sequenced using NGS.
Determination of viral load in biological samples in MDCK cells using FFU assay
MDCK cell monolayer was grown in 96-well plates. Tenfold dilutions of nasal washings from laboratory animals were prepared. Growth medium was removed from flat-bottom plate wells with cell monolayer, cells were washed twice with supportive growth medium (DMEM, 100 units/mL penicillin, 100 µg/mL streptomycin, 0.25 µg/mL amphotericin, 25 mM HEPES, 0.2% bovine serum albumin, 2 µg/mL TPCK-treated trypsin according to a protocol of “Manual for the laboratory diagnosis and virological surveillance of influenza” (WHO, 2011), Part 2. С53 ). Serial dilutions of test samples were added. For each sample, the starting material and its 10-fold serial dilutions (up to 10−4) were added in triplicate in a volume of 100 µl per well. Cells were incubated for 1 h at 37 °C and 5% CO2. The medium was then removed from wells, and cells were washed with supportive medium once. A total of 150 µL of supportive medium was added to all wells. The medium was removed after 18–20-h incubation at 37 °C and 5% CO2, and 200 µL of 80% cold acetone (− 20 °C) was added, followed by incubation for 20–30 min. Acetone was removed, and the wells were dried. Next, 50 µL of diluted 1:1000 mouse monoclonal antibodies to influenza virus nucleoprotein was added to each well. The plate was incubated at 37 °C for 1 h, wells were washed three times with PBS, and secondary rabbit anti-mouse IgG antibodies conjugated to Alexa Fluor 488 were added at a ratio of 1:1000. After 1 h incubation, wells were washed three times with PBS. Using an imaging system, stained foci of influenza A virus reproduction in a monolayer were counted, and the virus titer was calculated as the number of focus-forming units (FFU) per 1 mL of nasal washings (FFU/mL).
Determination of virus RNA load in biological samples by RT-PCR
Influenza virus RNA was detected in nasal washings and analytical filters by quantitative RT-PCR with real-time data acquisition. The RIBO-sorb kit (InterLabService Ltd., Moscow, Russia) was used for RNA isolation. Reverse transcription was carried out using the Reverta-L kit (InterLabService Ltd., Moscow, Russia) in an incubator. The resulting influenza A cDNA fragments were amplified using the AmpliSens Influenza virus A-FL (H5N1) kit (InterLabService Ltd., Moscow, Russia). Real-time PCR and data registration were conducted on a RotorGene 6000 real-time cycler.
Data availability
Virus genome sequences generated in the study are available in GISAID (https://gisaid.org/, ID numbers EPI_ISL_19305433 and EPI_ISL_19305434).
References
Krammer, F. et al. Influenza. Nat. Rev. Dis. Prim. 4, 3. https://doi.org/10.1038/s41572-018-0002-y (2018).
Short, K. R. et al. One health, multiple challenges: the inter-species transmission of influenza a virus. One Health. 1, 1–13. https://doi.org/10.1016/j.onehlt.2015.03.001 (2015).
Shi, J. et al. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 12, 2155072. https://doi.org/10.1080/22221751.2022.2155072 (2023).
Yamaji, R. et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev Med Virol. 30, e2099. https://doi.org/10.1002/rmv.2099 (2020).
Kandeil, A. et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat. Commun. 14, 3082. https://doi.org/10.1038/s41467-023-38415-7 (2023).
Marchenko, V. Y. et al. Review on the epizootiological situation on highly pathogenic avian influenza around the world and in Russia in 2022. Probl. Particularly Danger. Infections. 1, 48–55. https://doi.org/10.21055/0370-1069-2023-1-48-55 (2023). (In Russ.).
Cui, P. et al. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic analysis of the ones detected in China. Emerg. Microbes Infect. 1, 1693–1704. https://doi.org/10.1080/22221751.2022.2088407 (2022).
Puryear, W. et al. Highly pathogenic avian influenza A(H5N1) virus outbreak in New England Seals, United States. Emerg. Infect. Dis. 29, 786–791. https://doi.org/10.3201/eid2904.221538 (2023).
Vreman, S. et al. Zoonotic mutation of highly pathogenic avian influenza H5N1 virus identified in the brain of multiple wild carnivore species. Pathogens. 12, 168. https://doi.org/10.3390/pathogens12020168 (2023).
Agüero, M. et al. October. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill Bull Eur sur les Mal Transm = Eur Commun Dis Bull. 28, 2300001. https://doi.org/10.2807/1560-7917.es.2023.28.3.2300001 (2023).
Steel, J. et al. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 5, e1000252. https://doi.org/10.1371/journal.ppat.1000252 (2009).
Gomaa, M. et al. We are underestimating, again, the true burden of H5N1 in humans. BMJ Glob Heal. 8, e013146. https://doi.org/10.1136/bmjgh-2023-013146 (2023).
Kobasa, D. et al. Transmission of lethal H5N1 clade 2.3.4.4b avian influenza in ferrets. Res. Square. https://doi.org/10.21203/rs.3.rs-2842567/v1 (2023).
Restori, K. H. et al. Risk assessment of a highly pathogenic H5N1 influenza virus from mink. Nat. Commun. 15, 4112. https://doi.org/10.1038/s41467-024-48475-y (2024).
Pulit-Penaloza, J. A. et al. Pathogenesis and transmissibility of north American highly pathogenic avian influenza A(H5N1) virus in ferrets. Emerg. Infect. Dis. 28, 1913–1915. https://doi.org/10.3201/eid2809.220879 (2022).
Gudymo, A. et al. Quantitative assessment of airborne transmission of human and animal influenza viruses in the Ferret model. Atmos. (Basel). 14, 471. https://doi.org/10.3390/atmos14030471 (2023).
Hatta, M. et al. Molecular basis for high virulence of Hong Kong H5N1 influenza a viruses. Science. 293, 1840–1842. https://doi.org/10.1126/science.1062882 (2001).
Peng, X. et al. Amino acid substitutions HA A150V, PA A343T, and PB2 E627K increase the virulence of H5N6 influenza virus in mice. Front. Microbiol. 9, 453. https://doi.org/10.3389/fmicb.2018.00453 (2018).
Shi, Y., Wu, Y., Zhang, W., Qi, J. & Gao, G. F. Enabling the host jump: structural determinants of receptor-binding specificity in influenza A viruses. Nat. Rev. Microbiol. 12, 822–831. https://doi.org/10.1038/nrmicro3362 (2014).
Zhang, W. et al. An airborne transmissible avian influenza H5 hemagglutinin seen at the atomic level. Science. 340, 1463–1467. https://doi.org/10.1126/science.1236787 (2013).
Yang, Z-Y. et al. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science. 317, 825–828. https://doi.org/10.1126/science.1135165 (2007).
Gao, R. et al. T160A mutation-induced deglycosylation at site 158 in hemagglutinin is a critical determinant of the dual receptor binding properties of clade 2.3.4.4 H5NX subtype avian influenza viruses. Vet. Microbiol. 217, 158–166. https://doi.org/10.1016/j.vetmic.2018.03.018 (2018).
Cao, L. et al. [Epidemiological and genetic characteristics of H5 subtype avian influenza virus in Guangzhou, 2014–2019]. Zhonghua Liu Xing Bing Xue Za Zhi. 41, 1115–1120. https://doi.org/10.3760/cma.j.cn112338-20190730-00565 (2020).
Yamaji, R. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev Med Virol. 30, e2099. https://doi.org/10.1002/rmv.2099 (2020).
Reuman, P. D., Keely, S. & Schiff, G. M. Assessment of signs of influenza illness in the ferret model. J. Virol. Methods. 24, 27–34. https://doi.org/10.1016/0166-0934(89)90004-9 (1989).
Wang, W. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J. Virol. 84, 6570–6577. https://doi.org/10.1128/JVI.00221-10 (2010).
Matsuoka, Y. et al. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J. Virol. 83, 4704–4708. https://doi.org/10.1128/JVI.01987-08 (2009).
Mir-Shekari, S. Y., Ashford, D. A., Harvey, D. J., Dwek, R. A. & Schulze, I. T. The glycosylation of the influenza a virus hemagglutinin by mammalian cells. A site-specific study. J. Biol. Chem. 272, 4027–4036. https://doi.org/10.1074/jbc.272.7.4027 (1997).
Deom, C. M., Caton, A. J. & Schulze, I. T. Host cell-mediated selection of a mutant influenza a virus that has lost a complex oligosaccharide from the tip of the hemagglutinin. Proc. Natl. Acad. Sci. U S A. 83, 3771–3775. https://doi.org/10.1073/pnas.83.11.3771 (1986).
Subbarao, E. K., London, W. & Murphy, B. R. A single amino acid in the PB2 gene of influenza a virus is a determinant of host range. J. Virol. 67, 1761–1764. https://doi.org/10.1128/JVI.67.4.1761-1764.1993 (1993).
Postel, A. et al. Infections with highly pathogenic avian influenza a virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg. Microbes Infect. 11, 725–729. https://doi.org/10.1080/22221751.2022.2043726 (2022).
Belser, J. A. et al. Mammalian pathogenicity and transmissibility of low pathogenic avian influenza H7N1 and H7N3 viruses isolated from North America in 2018. Emerg. Microbes Infect. 9, 1037–1045. https://doi.org/10.1080/22221751.2020.1764396 (2020).
Belser, J. A., Sun, X., Pulit-Penaloza, J. A. & Maines, T. R. Fatal Infection in ferrets after ocular inoculation with highly pathogenic avian influenza A(H5N1) virus. Emerg. Infect. Dis. 30, 1484–1487. https://doi.org/10.3201/eid3007.240520 (2024).
Belser, J. A. et al. Genetically and antigenically divergent influenza A(H9N2) viruses exhibit Differential Replication and Transmission phenotypes in mammalian models. J. Virol. 94, e00451–e00420. https://doi.org/10.1128/JVI.00451-20 (2020).
Maines, T. R. et al. Local innate immune responses and influenza virus transmission and virulence in ferrets. J. Infect. Dis. 205, 474–485. https://doi.org/10.1093/infdis/jir768 (2012).
Wong, S. S. et al. Activated CD4 + T cells and CD14hiCD16 + monocytes correlate with antibody response following influenza virus infection in humans. Cell. Rep. Med. 2, 100237. https://doi.org/10.1016/j.xcrm.2021.100237 (2021).
Wong, S. S. et al. H5N1 influenza vaccine induces a less robust neutralizing antibody response than seasonal trivalent and H7N9 influenza vaccines. npj Vaccines. 2 https://doi.org/10.1038/s41541-017-0017-5 (2017).
Herfst, S. et al. A Dutch highly pathogenic H5N6 avian influenza virus showed remarkable tropism for extra-respiratory organs and caused severe disease but was not transmissible via air in the ferret model. mSphere. 8, e0020023. https://doi.org/10.1128/msphere.00200-23 (2023).
Richard, M., Fouchier, R. A. & Influenza A virus transmission via respiratory aerosols or droplets as it relates to pandemic potential. FEMS Microbiol. Rev. 40, 68–85. https://doi.org/10.1093/femsre/fuv039 (2016).
Siddique, N., Naeem, K., Abbas, M. A., Ahmed, Z. & Malik, S. A. Sequence and phylogenetic analysis of highly pathogenic avian influenza H5N1 viruses isolated during 2006–2008 outbreaks in Pakistan reveals genetic diversity. Virol. J. 9, 300. https://doi.org/10.1186/1743-422X-9-300 (2012).
Elsmo, E. J. et al. Pathology of natural infection with highly pathogenic avian influenza virus (H5N1) clade 2.3.4.4b in wild terrestrial mammals in the United States in 2022. Emerg. Infect. Dis. 29, 2451–2460. https://doi.org/10.3201/eid2912.230464 (2023).
Gustin, K. M. et al. Environmental conditions affect exhalation of H3N2 seasonal and variant influenza viruses and respiratory droplet transmission in ferrets. PLoS One. 10, e0125874. https://doi.org/10.1371/journal.pone.0125874 (2015).
Belser, J. A., Eckert, A. M., Tumpey, T. M. & Maines, T. R. Complexities in Ferret Influenza Virus Pathogenesis and Transmission models. Microbiol. Mol. Biol. Rev. 80, 733–744. https://doi.org/10.1128/MMBR.00022-16 (2016).
Wang, C. et al. Sex disparities in influenza: a multiscale network analysis. iScience. 25, 104192. https://doi.org/10.1016/j.isci.2022.104192 (2022).
Organization, WH. Tool for Influenza Pandemic Risk Assessment (TIPRA). (2020). https://www.who.int/publications/m/item/tool-for-influenza-pandemic-risk-assessment-(tipra)
Reed, L. J. M. & Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 27, 493–497. https://doi.org/10.1093/oxfordjournals.aje.a118408 (1938).
Kawaoka, Y. & Neumann, G. Influenza virus methods and protocols. New York: Humana Press; 2012. (Methods in molecular biology, 865). Available from: https://lib.ugent.be/catalog/ebk01:3390000000031639
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv. 16, 1303. https://doi.org/10.48550/arXiv.1303.3997 (2013).
Minh, B. Q. et al. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534. https://doi.org/10.1093/molbev/msaa015 (2020).
Kalyaanamoorthy, S. et al. Fast model selection for accurate phylogenetic estimates. Nat. Methods. 14, 587–589. https://doi.org/10.1038/nmeth.4285 (2017).
Rambaut, A. Figtree ver 1.4.4. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh (2018). http://tree.bio.ed.ac.uk/software/figtree
Spackman, E. & Killian, M. L. Avian influenza virus isolation, propagation, and Titration in Embryonated Chicken Eggs. Methods Mol. Biol. 2123, 149–164. https://doi.org/10.1007/978-1-0716-0346-8_12 (2020).
Manual for the laboratory diagnosis and virological surveillance of influenza» (WHO, 2011). https://www.who.int/publications/i/item/manual-for-the-laboratory-diagnosis-and-virological-surveillance-of-influenza
Fei, Y. et al. Characterization of receptor binding profiles of Influenza A viruses using an ellipsometry-based label-free glycan microarray assay platform. Biomolecules. 5, 1480–1498. https://doi.org/10.3390/biom5031480 (2015).
Zitzow, L. A. et al. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76, 4420–4429. https://doi.org/10.1128/JVI.76.9.4420-4429.2002 (2002).
Acknowledgements
This research was funded by the state assignment of FBRI SRC VB VECTOR, Rospotrebnadzor, Russia.
Funding
This research was funded by the state assignment of FBRI SRC VB VECTOR, Rospotrebnadzor, Russia.
Author information
Authors and Affiliations
Contributions
M.V.: Ideas; formulation of overarching research goals and aims, original draft preparation, supervision. P.A.: writing-reviewing and editing. K.N.: PCR analysis, N.G.S. experiments, bioinformatics analysis and data interpretation, writing-reviewing and editing. D.A., B.N., S.K.: PCR analysis, N.G.S. experiments, bioinformatics analysis and data interpretation. G.A.: animal experiments and data interpretation, original draft reviewing and editing. P.O.: animal experiments. S.S.: drug susceptibility analysis, antigenic properties investigating and data interpretation, writing-reviewing and editing. V.N., E.M.: influenza virus propagation and titration, data interpretation. O.G., K.M.: receptor binding analysis and data interpretation. M.A.: determination of viral load in biological samples in MDCK cells. Z.P., Z.N.: sample collection and preparation, PCR analysis. A.D., C.I.: original draft preparation, supervision, writing-reviewing and editing. R.A.: conceptualization the overall study and coordinated the investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marchenko, V.Y., Panova, A.S., Kolosova, N.P. et al. Characterization of H5N1 avian influenza virus isolated from bird in Russia with the E627K mutation in the PB2 protein. Sci Rep 14, 26490 (2024). https://doi.org/10.1038/s41598-024-78175-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78175-y