Abstract
Recently, colistin and carbapenem-resistant hypervirulent Klebsiella pneumoniae (CCR-hvKP) has been observed sporadically. The aim of this study was to report a nosocomial outbreak due to CCR-hvKP, so as to control the transmission of CCR-hvKP and prevent future outbreaks. The clinical characteristics of five involved cases were analyzed and infection prevention and control measures were documented. Five CCR-hvKP isolates were discovered from the five involved cases. Molecular features of the isolates including sequence type, capsule locus, antimicrobial resistance genes, virulence factors and phylogenetic relationship were analyzed by whole-genome sequencing. Validation of the role of the deleterious amino acid mutations to colistin resistance was examined by complementation assays. PCR was performed to identify insertion sequences within the mgrB gene. Mouse intraperitoneal infection models were used to assess virulence phenotype. Five cases infected with CCR-hvKP were identified with a high attributable mortality rate of 60% in the patients. The five outbreak isolates belonged to the high-risk ST11-KL64 clone and were closely clustered. They were highly resistant to commonly used antibiotics and showed hypervirulent in vivo. WGS revealed multiple antimicrobial resistance genes such as blaKPC-2 and blaCTX-M-65 and important virulence factors. Concerning colistin resistance, amino acid mutations G53S in pmrA gene, and T157P, T246A and R256G in pmrB gene were indentified. Among them, the deleterious mutation T157P in pmrB gene was validated to be responsible for the resistance phenotype of isolate KP01, KP03 and KP05. In addition, disruption of mgrB gene by insertion sequences of ISKpn26 and IS903B was indentified in isolate KP02 and KP04, respectively. This is the first report of an outbreak caused by CCR-hvKP. The study highlights infection prevention and control measures are key to successfully fight against CCR-hvKP dissemination and nosocomial infections. Continuous surveillance should be performed to limit the spread of these isolates.
Similar content being viewed by others
Introduction
Carbapenem-resistant Klebsiella pneumoniae (CRKP) can cause life-threatening infections, such as pneumonia, bacteremia, urinary tract infections, intra-abdominal infections and wound infections1. CRKP infections have been associated with high mortality between 33% and 42% due to limited options of antimicrobial therapy2,3. Recently, CRKP has been observed evolving into carbapenem-resistant hypervirulent Klebsiella pneumoniae (CR-hvKP) and hypervirulent carbapenem-resistant Klebsiella pneumoniae (hv-CRKP) by combination of hypervirulence and extreme drug resistance4,5. CR-hvKP and hv-CRKP usually cause invasive infections and leads to devastating clinical outcomes, owing to its high transmissibility, robust antibacterial resistance and significant virulence4,6.
Treatment options for infections caused by CR-hvKP and hv-CRKP are becoming extremely limited. Currently, polymyxins, including polymyxin B and colistin, are considered as last-line antibiotics for treating severe infections caused by them. Polymyxins are a group of cationic antimicrobial peptides that exert antibacterial activity by interacting with the negatively charged lipid A moiety of lipopolysaccharide (LPS) in the outer membrane (OM) of Gram-negative bacteria7.
Unfortunately, the increasing use of polymyxins in clinical settings has resulted in the emergence of colistin and carbapenem-resistant hypervirulent Klebsiella pneumoniae (CCR-hvKP), which has been reported sporadically8,9. Acquired colistin resistance in Enterobacteriaceae is mainly developed in the presence of modified lipid A with positively charged molecules, 4-amino-4-deoxy-Larabinose (L-Ara4N) and/or phosphoethanolamine (pEtN), leading to inefficient attachment of polymyxins to lipid A10. Studies have shown that the modification of lipid A is mainly mediated by two mechanisms, namely, chromosome-mediated pathways regulated by mutations in the two-component systems (PmrA/B, PhoP/Q or CrrA/B) and the MgrB protein which is a negative feedback regulator of PhoP/Q, or the acquisition of plasmid-mediated colistin-resistant genes (mcr-1 to mcr-10) encoding pEtN transferases11,12,13. Interestingly, one latest study suggests CrrAB may partly contribute to the virulence in K. pneumoniae14. In addition, the overexpression of efflux pumps and their related transcriptional regulators has been proven to play an important role in colistin resistance in K. pneumoniae recently15,16. Pantel et al. demonstrates that KexD, a class of multidrug efflux RND transporter, can confer high-level colistin resistance under the induction of its neighbor gene crrB17. CCR-hvKP raises a big challenge to clinical treatment and usually causes even more severe consequences. Therefore, monitoring the emergence of CCR-hvKP should be strengthened.
In the present study, we reported an outbreak of CCR-hvKP in a large teaching hospital in China. The epidemiology of the cases was investigated and infection prevention and control measures were implemented to promptly control the outbreak. Furthermore, molecular features and clonal relatedness of the CCR-hvKP isolates were analyzed by whole genome sequencing (WGS). The role of deleterious amino acid mutations in conferring colistin resistance was investigated through complementation assays. PCR was performed to identify insertion sequence (IS) elements in mgrB gene. In addition, the in vivo virulence of the isolates was evaluated by mouse intraperitoneal infection models. To the best of our knowledge, this is the first description of a nosocomial outbreak caused by CCR-hvKP.
Results
Outbreak description
Five different patients were involved in the outbreak event. They had overlapping hospital stay during the outbreak (Fig. 1). Patient 1, 2 and 3 shared the central intensive care unit (CICU) for 35 days. The firstly identified case (Patient 1) was a 58-year-old male who suffered from severe pneumonia. He was admitted to the CICU on 7th June 2021. A CCR-hvKP isolate (KP01) was identified from his sputum after a 23-day stay in CICU. The second case (Patient 2), a 28-year-old man who underwent cerebrovascular surgery in the Neurosurgery unit, was transferred to CICU on 22th June. After receiving invasive mechanical ventilation for 28 days, a CCR-hvKP isolate (KP02) was detected from his sputum on 20th July. The third case (Patient 3) was a 69-year-old female, who was transferred to CICU after intracranial surgery on 19th June. A CCR-hvKP (KP03) was isolated from her urine on 26th July after indwelling urinary catheters for 25 days. The fourth case (Patient 4) was a 22-year-old man, who had bone marrow transplantation in Hematology department on 7th July. He developed fever and productive cough 21 days after the transplantation, with a CCR-hvKP (KP04) detected from his sputum on 28th July. Patient 4 was consulted by the same infectious disease physician who consulted patient 2 in CICU earlier on 25th July. The fifth case (Patient 5) was a 45-year-old man, who received brain surgery in other hospital and was transferred to the neurosurgery intensive care unit (NICU) in our hospital on 28th July when Patient 3 was transferred to Neurosurgery unit from CICU. He had a CCR-hvKP (KP05) isolated from his sputum after receiving invasive mechanical ventilation for 13 days. Patient 3 and Patient 5 were managed by the same medical team. The epidemiology of the five cases infected with CCR-hvKP was shown in Fig. 1.
The epidemiology of the five cases infected by CCR-hvKP and infection prevention and control measures implemented to terminate the outbreak. Each horizontal line represents the timeline of one patient. The dates of admission, discharge, and strain isolation are marked above the corresponding icon. Clinical outcomes are noted on the right side of the discharge icon. The length of the antibiotic treatment icon represents the length of the antibiotic use. Abbreviations: CICU, central intensive care unit; NICU, neurosurgery intensive care unit; AK, amikacin; CAZ, ceftazidime; CTX, ceftriaxone; CZA, ceftazidime/avibactam; IPM, imipenem; LEV, levofloxacin; MEM, meropenem; PB, polymyxin B; SCF, cefoperazone/sulbactam; TGC, tigecycline; TZP, piperacillin/tazobactam.
The demographic and clinical data of the five cases were presented in Table S1. They had been administrated two or three types of antibiotics and underwent some invasive procedures, mainly including surgery and mechanical ventilation before CCR-hvKP isolates were detected from them. Four cases received polymyxin B via intravenous drip before having infections by CCR-hvKP. All the five cases had overlapping hospital stay. Their average length of hospital stay before the isolation of CCR-hvKP was 36.2 days, ranging from 16 to 75 days. Two of the five cases were discharged with improvement and three of them died of severe pulmonary infection.
Infection prevention and control measures
The infection prevention and control (IPC) measures as shown in Fig. 1 were promptly adopted to terminate the outbreak. Contact precautions were implemented after the five involved patients were identified to be infected with carbapenem-resistant Enterobacterales (CRE), including isolating them in a single room, placing CRE warning cards at the door of their rooms, informing CRE infected patients’ bed numbers via electronic display screen, dedicating nursing staff to care them during the same shift, wearing protective gowns and gloves when touching them and their surroundings, and establishing comprehensive protocols for their transport or transfer. Other measures were quickly implemented at the time of the outbreak was identified, including: (a) reinforcing hand hygiene and education of HCWs; preparing antiseptic hand sanitizer and disposable hand paper towel at every sink and wash-free hand sanitizer gel at patients’ bedsides, educating proper hand hygiene practices among them by posting posters and watching instructional videos on the World Health Organization (WHO) recommended seven-step hand washing technique; (b) enhancing environmental cleaning and disinfection during patients’ hospitalization and after discharge; increasing the frequency from once a day to twice a day, using a disinfectant with an effective chlorine concentration of up to 500 mg/L, and making a checklist to indicate the time and areas of cleaning and disinfection; (c) supervising the compliance of HCWs with contact precautions and hand hygiene by an infection control nurse; (d) strengthening antimicrobial stewardship programs by holding weekly meetings of clinicians, pharmacists and microbiologists. In addition, CRE screening surveillance was performed at the time of patients’ admission within 48 h and once a week during their hospitalization in high-risk units including CICU, NICU and Hematology department. Following the implementation of the infection prevention and control measures mentioned above in the three involved units, the outbreak was controlled successfully and surveillance was stopped at the end of November 2021.
Isolate identification, antimicrobial susceptibility and antimicrobial resistance genes
The five isolates were identified as K. pneumoniae by Matrix-Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF). They were also verified as K. pneumoniae by the average nucleotide identities (ANI) analysis with ANI value of 98.49-98.53% compared with reference type strain K. pneumoniae ATCC13883.
The antimicrobial susceptibility profiles of the five isolates were shown in Table 1. They had nearly identical resistant patterns. They were resistant to colistin with MIC of 16 mg/L or 32 mg/L. They also showed high resistance to piperacillin/tazobactam, cefepime, imipenem, ertapenem, amikacin, gentamicin, tobramycin, aztreonam, ciprofloxacin, levofloxacin and trimethoprim/sulfamethoxazole. Notably, they were all sensitive to ceftazidime/avibactam and four of them were sensitive to tigecycline, except for KP02, which showed intermediate to tigecycline (MIC = 4 mg/L).
The five outbreak isolates had Kleborate resistance score of 3. They carried an identical set of antimicrobial resistance genes which were consistent with their resistance patterns, including KPC-2, CTX-M-65 and TEM-1 (β-lactam resistance genes), qnrS1 (quinolone resistance gene), aadA3 and rmtB (aminoglycoside resistance gene), tet(A) (tetracycline resistance gene), sul2 (sulfonamide resistance gene) and dfrA14 (trimethoprim resistance gene). But no mobile colistin-resistance (mcr) genes were found in our study.
Molecular mechanism of colistin resistance
We further screened for chromosomal mutations in colistin-resistant related genes (mainly, pmrA, pmrB, phoP, phoQ, crrA, crrB and mgrB). With reference to colistin-susceptible K. pneumoniae MGH78578 genome which contains these wild-type genes, one missense mutation (G53S) in pmrA gene and three missense mutations (T157P, T246A and R256G) in pmrB gene were identified in this study (Table 2). These mutations except for T246A in pmrB were predicted as deleterious by PROVEAN analysis (Table S2). The relationship between these deleterious mutations and colistin resistance was confirmed by complementation assays. As shown in Table 3, the mutation T157P in pmrB gene increased the colistin MIC by 16-fold compared with wild-type K. pneumoniae MGH78578 (colistin MIC = 1 mg/L). However, the mutations G53S in pmrA and R256G in pmrB did not alter the colistin MICs in MGH78578.
Regarding the mgrB gene, the PCR amplification products showed that all the five isolates were mgrB-positive and two isolates (KP02 and KP04) had an IS within mgrB. As shown in Fig. 2, an ISKpn26 of 1196 bp was inserted between nucleotides 74 and 75 within the mgrB gene in KP02. An IS903B of 1057 bp was located between 120 and 121 nucleotides of the mgrB sequence in KP04. The ISKpn26 and IS903B belonged to the IS5 family. The direct repeat (DR) sequences of ISKpn26 and IS903B were TTAA and TTTATTAAT, respectively.
Schematic representation of the mgrB gene alterations mediated by insertion sequences (ISs). (A) Intact wild-type mgrB immediate environment of K. pneumoniae MGH78578; (B) The mgrB gene disrupted by ISKpn26 at nucleotide + 74 in KP02; (C) The mgrB gene inactivated by IS903B at nucleotide position + 120 in KP04. The direct repeats (DRs) highlighted with underlines. The triangles flanking ISs represented left and right inverted repeats (IRLs and IRRs).
Virulence analysis
The five outbreak isolates had the same virulence score of 4 predicted by Kleborate. In terms of virulence factors, the five outbreak isolates carried a substantial amount of virulence factors, including type 1 fimbriae (fimA-I, fimK) and type 3 fimbriae adhesion genes (mrkABCDFHIJ), yersiniabactin (ybtAEPQSTUX, fyuA and irp1/2), aerobactin (iucABCD, iutA), salmochelin (iroE, iroN) and enterobactin (entABCDEFS, fepABCDG and fes).
The mouse intraperitoneal infection model was constructed to evaluate the in vivo virulence of the five isolates in this study. During the seven-day observation period after intraperitoneal injection of 107 CFU bacteria, mice infected with the hypervirulent control K. pneumoniae NTUH-K2044 all died within 2 days, whereas only one mouse died on the third day after injection with the low-virulence control strain K. pneumoniae ATCC700603. The survival rate of the mice injected with KP02, KP04 and KP05 was 20% (1/5), and survival rate of those injected with KP01 and KP03 was 40% (2/5). The five isolates in this study showed high virulence that was not significantly different from that of K. pneumoniae NTUH-K2044 (P>0.05, by log-rank test). No mice in the PBS group died during the 7 days (Fig. 3).
Survival curves of mice intraperitoneally infected with 1 × 107 CFU of the tested isolates. Hypervirulent K. pneumoniae NTHU-K2044, classic K. pneumoniae ATCC700603 and PBS were used as controls. There was no significant survival difference between mice infected with NTHU-K2044 and those infected with the five isolates in this study, while there was significant difference in survival rate of the mice infected with ATCC700603 and those infected with the five isolates in this study. P values from the log-rank (Mantel-Cox) test were indicated as follows: *, P value < 0.05; ns (no significant), P value > 0.05.
MLST, serotypes and clonal relatedness analysis
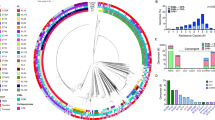
The genomic features of the five CCR-hvKP isolates were presented in Table S3. All the five isolates belonged to ST11 clone, capsular KL64 and O1/O2v1 antigen serotypes. The evolutionary relationships among the five ST11 CCR-hvKP isolates in our study, the typical hypervirulent K. pneumoniae (hvKP) strain SGH10 and ST11 CCR-hvKP isolates previously reported in different areas were further investigated. We conducted a search on PubMed and Web of Science databases using keywords such as “Klebsiella pneumoniae”, “colistin”, “carbapenem” and “hypervirulent”(until April 2024). There were only 12 available genomes of ST11 CCR-hvKP were downloaded from the NCBI GenBank database for clonal relatedness analysis together with genomes in this study and the genome of strain SGH10 (Fig. 4). The five isolates in this study were closely clustered together and did not belong to the same cluster with any isolate reported in other areas of China. The pairwise single nucleotide polymorphism (SNP) distance analysis on the core genome of our five isolates showed that they were genetically highly similar, only differed from each other by 1–11 SNPs (Table S4). As presented in Table S4, the SNP differences ranged from 38 to 113 between our five isolates and other ST11 CCR-hvKP.
Phylogenetic tree (maximum likehood) of the five ST11 CCR-hvKP (in red), the typical hypervirulent strain SGH10 (CP025080) (in purple) and the 12 publicly available ST11 CCR-hvKP (in black) from different areas of China, using ST11 CRKP HS11286 (CP003200) (in blue) as the reference strain. The GenBank accession numbers of the 12 publicly available ST11 CCR-hvKP include A30 (DAFWKG010000000), TVGHCRE225 (CP023722), KP2509 (CP065949), KP15 (JAGQFM010000000), K28074 (NZ_CP104551), C2582 (CP079208), CRKP-Pus1 (JACYON000000000), FRPDR (CP063759), A225 (DAFWKA010000000), A221 (DAFWKL000000000), CT77 (CP080303) and 21,091,025 (CP095264). *The true branch length is 2.73. BALF, bronchoalveolar lavage fluid.
Discussion
In this study, we reported an outbreak caused by CCR-hvKP with a high attributable mortality rate, which to our knowledge is the first report of an outbreak caused by CCR-hvKP. The outbreak was controlled successfully by a bundle of the infection prevention and control measures. Five CCR-hvKP isolates from five severe patients were detected during a short period. The five isolates showed nearly identical resistant patterns and high virulence phenotype and were closely clustered.
It has been observed in several studies that the use of polymyxins is related to the development of colistin resistance, but the results are controversial18,19. In our study, four of the five cases involved in this outbreak received polymyxin B therapy before colistin resistant CRKP were obtained from them during hospitalization. All the five cases suffered from multiple comorbidities hospitalized in high-risk hospital units where infections often occur, thereby necessitating long-term antibiotic therapy. Colistin resistant CRKP has been reported to even cause fatal outbreaks in global clinical settings recently, which poses a great threat to clinical treatment and infection control20. Colistin resistance in CRKP is associated with high mortality rates between 51 and 66% in previous studies21,22. As shown in our study, three of the five patients (60%) died of severe pulmonary infection caused by CCR-hvKP. Given the very limited therapeutic options, careful resistance surveillance and antimicrobial stewardship are of great importance in an effort to prevent or reduce the dissemination of such isolates in high-risk hospital units.
In line with previous literature from other geographical areas23,24, chromosomal mutations associated with colistin resistance were found in all the five isolates in our study, but no plasmid-encoded mcr genes were detected. In K. pneumoniae, resistance to colistin is mainly through chemical modification of the lipid A portion of lipopolysaccharide (LPS), which is mediated by various mutations in pmrA, pmrB, phoP, phoQ, crrA, crrB and mgrB genes25. Interestingly, different colistin resistance mechanisms were observed in the five isolates which were closely clustered. The T157P substitution in pmrB was identified in KP01, KP03 and KP05, which was confirmed to increase colistin MIC of wild-type K. pneumoniae MGH78578 by 16-fold in our study and has been previously described to contribute to colistin resistance26. T157P lies in the core of the functional ___domain and has been found to disrupt the α-helical secondary structure of the mutated PmrB protein, resulting in the continuous activation of PmrA26,27. The amino acid substitution G53S (deleterious) in pmrA gene was only observed in isolate KP01. The T246A (neutral) and R256G (deleterious) substitutions in pmrB gene were found in all the five isolates. Liu et al. has also reported the PmrB mutation R256G in all the ST11 K. pneumoniae isolates with colistin resistance, along with the mutation T246A in approximately 85% of the isolates28. Though mutations G53S in pmrA and R256G in pmrB were predicted as deleterious by PROVEAN, they have been reported to be detected in colistin-resistant as well as susceptible isolates29. They did not increase colistin MICs of wild-type K. pneumoniae MGH78578 in our study, indicating that they alone may not be enough to elevate the MIC values for colistin and additional secondary factors are necessary alongside them, thus leading to colistin resistance.
The role of mgrB inactivation in colistin resistance has been well established in clinical CRKP24,30. The mgrB mutations were often mediated by various types of insertion sequences (ISs). Among the five isolates in our study, ISKpn26 and IS903B were detected in isolate KP02 and KP04, respectively. ISKpn26 and IS903B are members of the IS5 family, which is one of the major IS families responsible for inactivating mgrB24,30. Notably, the IS elements ISKpn26 and IS903B are most frequently detected in clinical samples from China31.
WGS has become a widely-used tool to provide a better understanding of molecular features including acquired antimicrobial resistance genes, virulence factors and clonal relatedness of pathogens. In our study, WGS revealed that the five CCR-hvKP isolates belonged to ST11-KL64 and harbored multiple resistance genes including blaKPC-2 and blaCTX-M-65. ST11-KL64 has become the dominant clone of KPC-2-carrying CR-hvKP, which may cause fatal infections and high mortality32. We also found the presence of virulence factors such as type 1 and 3 fimbriae, yersiniabactin, aerobactin, salmochelin and enterobactin in the five CCR-hvKP by WGS. They have been proven to be closely related to respiratory and urine tract infections with unfavorable outcomes33,34. In our study, four of the five involved cases had severe pneumonia and another one had urine tract infection, whose infection sites were consistent with the pathogenic characteristics of virulence patterns in the outbreak isolates. Additionally, the accurate clonal relatedness of the outbreak isolates was available by WGS-SNP analysis in our study. This highlights the prominent usage of WGS in outbreak investigations for better infection control management.
In our study, the outbreak caused by CCR-hvKP was identified by integrating case epidemiological data and genomic evolution information. The involved five cases had extended and overlapping inpatient stays. Among them, three cases (Patient 1–3) were hospitalized in CICU. They were managed by the same health care workers (HCWs), and there was potential for cross-infections. The putative transmission routes of Patient 4 in Hematology department and Patient 5 in NICU were investigated. Patient 4 was consulted by the same infectious disease physician who consulted Patient 2 in CICU earlier at the same day. In addition, the isolate KP04 from Patient 4 showed fewer SNP differences to KP02 from Patient 2 than other isolates, suggesting that KP04 showed a closer phylogenetic relationship to KP02. Therefore, it was possible that Patient 4 acquired the isolate from Patient 2. NICU and Neurosurgery unit in our hospital shared the same group of HCWs. Patient 5 was admitted to NICU and managed by the HCWs who also took responsibility for Patient 3 transferred to Neurosurgery unit from CICU. Furthermore, Patient 5’s isolate (KP05) showed fewer SNP differences to KP03 from Patient 3 than other three isolates. So we speculated that the isolate was probably transmitted to Patient 5 from Patient 3.
Infection prevention and control measures played a significant role in preventing the spread of CCR-hvKP and terminating this outbreak rapidly. They included a variety of actions, such as reinforcing the importance of hand hygiene and rectal CRE screening. Those were the most common infection control measures described in outbreak reports of CRE infections35. As the nosocomial spread and cross-infections of K. pneumoniae were often caused by HCWs with contaminated hands36, strict implementation of hand hygiene was strongly recommended in successful management of multidrug resistant K. pneumoniae outbreaks37. Meanwhile, active surveillance of CRE rectal colonization was performed for all patients upon admission and once a week during their hospitalization in high-risk units to identify carriers for isolation to prevent CRE transmission. In our previous study, 18.4% CRE rectal carriers developed subsequent infections in hematological malignancy patients38. The importance of identification of CRE carriers as soon as possible has also been emphasized for infection control purposes by several studies39,40. In a word, a series of infection prevention and control measures should be combined to cut down the infection transmission and they all contributed to control this outbreak successfully. Therefore, continuous implementation of effective infection prevention and control measures was critical to prevent such outbreaks.
Our study has some limitations. All isolates were obtained at the physician’s discretion, so it was difficult to determine the precise time when the cases were infected by CCR-hvKP. It is imperative to strengthen surveillance and monitoring in order to rapidly identify multi-drug resistant isolates. Another is that it was a retrospective study so it did not provide timely assistance to control CCR-hvKP dissemination. If possible, genomic surveillance should be taken out to early identify the spread of high-risk pathogens and prevent future outbreaks.
Conclusions
In conclusion, we report an outbreak caused by ST11-KL64 CCR-hvKP for the first time. The successful containment of this outbreak was achieved by a bundle of the infection prevention and control measures. Given CCR-hvKP poses a major threat to public healthcare, it is urgent to detect colistin resistance and study the epidemiological characteristics in high-risk isolates to guide the rational use of this last-line class of antibiotics. Furthermore, this study highlights the importance of infection prevention and control measures to limit the transmission of CCR-hvKP and prevent future outbreaks.
Materials and methods
Study setting
This retrospective study was carried out in Xiangya Hospital of Central South University, a large tertiary healthcare and teaching hospital located in Changsha, China.
An outbreak caused by colistin resistant CR-hvKP occurred on 29th June 2021 and continued until 13th August 2021. Five cases were involved in this outbreak. Three of the five cases came from the CICU, one from Hematology department and another one from NICU. Four cases had severe pneumonia and another one had urine tract infection. The demographic and clinical details of each case were obtained from the clinical medical record data system. This study obtained ethical approval from our hospital in 2023.
Isolate identification and antimicrobial susceptibility testing
The strains were identified by MALDI-TOF mass spectrometry based on the MALDI Biotyper database version 2.3 (Bruker, Germany). The precise identification of subspecies was performed by comparative genomics analysis based on the genome average nucleotide identities (ANI) using BLASTn (ANIb) in JSpeciesWS (jspecies.ribohost.com/jspeciesws). Klebsiella pneumoniae ATCC13883 was used as reference type strain and an ANI threshold of ≥ 96% was adopted.
In vitro susceptibility of the isolates to eleven antimicrobial agents (piperacillin/tazobactam, cefepime, imipenem, ertapenem, amikacin, gentamicin, tobramycin, aztreonam, ciprofloxacin, levofloxacin and trimethoprim/sulfamethoxazole) was evaluated using VITEK 2 Compact System (BioMérieux, France). The minimum inhibitory concentrations (MICs) of colistin, tigecycline and ceftazidime/avibactam were performed by the broth microdilution method. The susceptibility to the above antimicrobials except tigecycline was determined using Clinical Laboratory Standards Institute (CLSI) breakpoints41. For tigecycline, the breakpoints recommended by the US Food and Drug Administration were used42.
Whole-genome sequencing (WGS) and molecular features analysis
Genomic DNA of the five CCR-hvKP was extracted using TIANamp Bacteria DNA Kit (TIANGEN BIOTECH, Beijing, China) and sequenced on the DNBSEQ-T7 Platform (Bioyi Biotechnology Co., Ltd., Wuhan, China). 150 bp paired-end reads were generated and raw reads were processed with fastp v0.21.0 (https://github.com/OpenGene/fastp) to remove adapter and low-quality sequences. Then, contigs were de-novo assembled using unicycler v0.4.8 (https://github.com/rrwick/Unicycler).
WGS data were used for molecular feature analysis. Multi-locus sequence typing (MLST) sequence type (ST), K and O antigen serotypes were identified by the Kleborate v0.3.0 (https://github.com/katholt/Kleborate). Antimicrobial resistance genes were determined by Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca/analyze). The virulence factors were predicted by virulence factors database (VFDB, http://www.mgc.ac.cn/cgi-bin/VFs/v5/main.cgi). The resistance and virulence scores were predicted by Kleborate v0.3.0.
Mutations in genes (pmrA, pmrB, phoP, phoQ, crrA, crrB and mgrB) related to colistin resistance were analyzed. Single nucleotide polymorphisms (SNPs) were identified by mapping sequence reads against the reference genome K. pneumoniae MGH78578 (Accession no. NC 009648.1) with Snippy v3.0 (http:/github.com/tseemann/snippy). The PROVEAN (Protein Variation Effect Analyzer) tool v.1.1.5 was used to predict whether protein function is affected by amino acid substitutions resulting from missense mutations in the above genes43.
Complementation assay
The contribution of the deleterious amino acid mutations (G53S in pmrA gene, T157P and R256G in pmrB gene) to colistin resistance was validated through complementation assays. Tetracycline-resistant plasmid pRK415 was used as the vector44. The gene sequences containing the above amino acid mutation sites were amplified by PCR using primers listed in Table S5. The amplified fragments were cloned into the BamHI site of pRK415 using the In-Fusion® Snap Assembly Master Mix (TaKaRa Bio USA, Inc.). Then, the recombinant plasmids were separately transformed into the E. coli TOP10 cells for amplification and cells carrying the recombinant plasmids were selected by the addition of 10 µg/mL tetracycline in Luria-Bertani (LB) agar. The presence of the cloned genes harboring mutations was further verified by PCR and sequencing. The recombinant plasmids in E. coli TOP10 were extracted and introduced into E. coli β2155 via electroporation, and individual colonies were selected as the donor strains of conjugation experiments by the addition of 10 µg/mL tetracycline and 0.5mM diaminopimelic acid in LB agar. Eventually, the conjugation experiments were performed with colistin-susceptible K. pneumoniae MGH78578 as the recipient strain. The transconjugants were selected by overnight incubation at 37 °C on LB agar supplemented with 10 µg/mL tetracycline and further validated by PCR to aquire K. pneumoniae MGH78578 transformants harboring mutations. The control strain was K. pneumoniae MGH78578 with empty vector pRK415. K. pneumoniae MGH78578 and its transformants were tested for colistin MIC using the broth microdilution method.
Determination of mgrB genetic sequence environment
The mgrB sequence environment analysis was performed by polymerase chain reaction (PCR) as previously reported45. PCR products were then visualized through agarose gel electrophoresis and were analyzed by subsequent Sanger sequencing (Sangon Biotech, Shanghai, China). The IS elements in the sequencing data were predicted using the ISfinder database platform (https://www-is.biotoul.fr).
Phylogenetic analysis
The phylogenetic tree based on core-genome SNPs(cgSNPs)was constructed using the maximum-likelihood method of the FastTree software with K. pneumoniae ST11 reference genome (strain HS11286, No. CP003200) and visualized with Interactive Tree of Life (iTOL) web server. The pairwise SNP distance matrix between the isolates was calculated using the snp-dists pipeline v.0.8.2. (https://github.com/tseemann/snp-dists). The threshold of 21 SNPs was used to determine clonal relationship, as reported elsewhere46.
Virulence evaluation in mouse intraperitoneal infection models
The mouse experiments were approved by the Laboratory Animal Ethics Committee of Xiangya Hospital of Central South University (Ethics no. 202409156). Female BALB/c mice at age of 5 to 6 weeks were obtained from Hunan SLAC Jingda Laboratory Animal Co. Ltd. (Hunan, China) and housed for a week before infection. Hypervirulent K. pneumoniae NTUH-K2044, classic K. pneumoniae ATCC700603 and phosphate-buffered saline (PBS) were used as controls. The mouse intraperitoneal infection models were established in accordance with previously described methodologies with minor modification47. Briefly, five mice were utilized for each group. Each mouse was intraperitoneally injected with 100µL bacteria dilution of 108 CFU/mL. The mortality rates of mice were continuously observed and recorded for up to seven days after infection. All the surviving mice were euthanized seven days subsequent to the injection. The in vivo virulence of strains was assessed by the survival of the infected mice. Survival curves were generated by Kaplan-Meier analysis and statistical analysis was performed using the log rank (Mantel-Cox) test with Prism 9.
Data availability
Sequence data that support the findings of this study have been deposited at NCBI repository under BioProject ID PRJNA1043816 with samples accession numbers (SAMN38353753, SAMN38353860-38353863) which correspond to isolates named KP01, KP02-KP05. All data are available from the corresponding authors upon reasonable request.
References
Wang, M. et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): A prospective, multicentre, cohort study. Lancet Infect. Dis. 22, 401–412. https://doi.org/10.1016/s1473-3099(21)00399-6 (2022).
Agyeman, A. A., Bergen, P. J., Rao, G. G., Nation, R. L. & Landersdorfer, C. B. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int. J. Antimicrob. Agents. 55, 105833. https://doi.org/10.1016/j.ijantimicag.2019.10.014 (2020).
Xu, L., Sun, X. & Ma, X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 16, 18. https://doi.org/10.1186/s12941-017-0191-3 (2017).
Gu, D. et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: A molecular epidemiological study. Lancet Infect. Dis. 18, 37–46. https://doi.org/10.1016/s1473-3099(17)30489-9 (2018).
Chen, Y. et al. Acquisition of plasmid with Carbapenem-Resistance Gene Bla(KPC2) in hypervirulent Klebsiella pneumoniae, Singapore. Emerg. Infect. Dis. 26, 549–559. https://doi.org/10.3201/eid2603.191230 (2020).
Xie, M. et al. Clinical evolution of ST11 carbapenem resistant and hypervirulent Klebsiella pneumoniae. Commun. Biol. 4, 650. https://doi.org/10.1038/s42003-021-02148-4 (2021).
Sabnis, A. et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife. 10 https://doi.org/10.7554/eLife.65836 (2021).
Huang, Y. H. et al. Emergence of an XDR and carbapenemase-producing hypervirulent Klebsiella pneumoniae strain in Taiwan. J. Antimicrob. Chemother. 73, 2039–2046. https://doi.org/10.1093/jac/dky164 (2018).
Jin, X. et al. Resistance evolution of hypervirulent carbapenem-resistant Klebsiella pneumoniae ST11 during treatment with tigecycline and polymyxin. Emerg. Microbes Infect. 10, 1129–1136. https://doi.org/10.1080/22221751.2021.1937327 (2021).
Ahmed, E. S. Colistin and its role in the era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 9, 868–885. https://doi.org/10.1080/22221751.2020.1754133 (2020).
Macesic, N. et al. Emergence of polymyxin resistance in clinical Klebsiella pneumoniae through diverse genetic adaptations: A genomic, retrospective cohort study. Clin. Infect. Dis. 70, 2084–2091. https://doi.org/10.1093/cid/ciz623 (2020).
Ling, Z. et al. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J. Antimicrob. Chemother. 75, 3087–3095. https://doi.org/10.1093/jac/dkaa205 (2020).
Wang, C. et al. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 9, 508–516. https://doi.org/10.1080/22221751.2020.1732231 (2020).
Kim, S. J., Shin, J. H., Kim, H., Ko, K. S. & 14,, & Roles of crrAB two-component regulatory system in Klebsiella pneumoniae: Growth yield, survival in initial colistin treatment stage, and virulence. Int. J. Antimicrob. Agents. 63, 107011. https://doi.org/10.1016/j.ijantimicag.2023.107011 (2024).
Naha, S. et al. KPC-2-producing Klebsiella pneumoniae ST147 in a neonatal unit: clonal isolates with differences in colistin susceptibility attributed to AcrAB-TolC pump. Int. J. Antimicrob. Agents. 55, 105903. https://doi.org/10.1016/j.ijantimicag.2020.105903 (2020).
Li, Z. et al. Genetic diversity of polymyxin-resistance mechanisms in clinical isolates of Carbapenem-resistant Klebsiella pneumoniae: A multicenter study in China. Microbiol. Spectr. 11, e0523122. https://doi.org/10.1128/spectrum.05231-22 (2023).
Pantel, L. et al. Missense mutations in the CrrB protein mediate odilorhabdin derivative resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 65 https://doi.org/10.1128/aac.00139-21 (2023).
Drozdinsky, G., Ben-Zvi, H., Kushnir, S., Leibovici, L. & Yahav, D. Colistin exposure as a risk factor for infections caused by inherently colistin resistant Enterobacteriaceae-a case-control study. Clin. Microbiol. Infect. 24, 896–899. https://doi.org/10.1016/j.cmi.2017.11.022 (2018).
Huang, P. H. et al. Risk factors and mechanisms of in vivo emergence of colistin resistance in carbapenem-resistant Klebsiella pneumoniae. Int. J. Antimicrob. Agents. 57, 106342. https://doi.org/10.1016/j.ijantimicag.2021.106342 (2021).
Sharma, S., Banerjee, T., Kumar, A., Yadav, G. & Basu, S. Extensive outbreak of colistin resistant, carbapenemase (bla(OXA-48), bla(NDM)) producing Klebsiella pneumoniae in a large tertiary care hospital, India. Antimicrob. Resist. Infect. Control. 11, 1. https://doi.org/10.1186/s13756-021-01048-w (2022).
Giacobbe, D. R. et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin. Microbiol. Infect. 21, 1106e1101–1106e1108. https://doi.org/10.1016/j.cmi.2015.08.001 (2015).
Balkan, I. I. et al. Colistin resistance increases 28-day mortality in bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2161–2170. https://doi.org/10.1007/s10096-020-04124-y (2021).
Riquelme, M. P. et al. Chromosome-mediated colistin resistance in clinical isolates of Klebsiella pneumoniae and Escherichia coli: Mutation analysis in the light of genetic background. Infect. Drug Resist. 16, 6451–6462. https://doi.org/10.2147/idr.S427398 (2023).
Hamel, M. et al. Inactivation of mgrB gene regulator and resistance to colistin is becoming endemic in carbapenem-resistant Klebsiella pneumoniae in Greece: A nationwide study from 2014 to 2017. Int. J. Antimicrob. Agents. 55, 105930. https://doi.org/10.1016/j.ijantimicag.2020.105930 (2020).
Esposito, E. P. et al. Molecular epidemiology and virulence profiles of colistin-resistant Klebsiella pneumoniae blood isolates from the Hospital Agency Ospedale dei Colli, Naples, Italy. Front. Microbiol. 9, 1463. https://doi.org/10.3389/fmicb.2018.01463 (2018).
Jayol, A. et al. Resistance to colistin associated with a single amino acid change in protein PmrB among Klebsiella pneumoniae isolates of worldwide origin. Antimicrob. Agents Chemother. 58, 4762–4766. https://doi.org/10.1128/aac.00084-14 (2014).
Basu, S., Veeraraghavan, B. & Anbarasu, A. Impact of PmrB mutations on clinical Klebsiella pneumoniae with variable colistin-susceptibilities: Structural insights and potent therapeutic solutions. Chem. Biol. Drug Des. 103, e14381. https://doi.org/10.1111/cbdd.14381 (2024).
Liu, Y. et al. Molecular mechanisms of colistin resistance in Klebsiella pneumoniae in a Tertiary Care Teaching Hospital. Front. Cell. Infect. Microbiol. 11, 673503. https://doi.org/10.3389/fcimb.2021.673503 (2021).
Elias, R. et al. A phylogenomic approach for the analysis of colistin resistance-associated genes in Klebsiella pneumoniae, its mutational diversity and implications for phenotypic resistance. Int. J. Antimicrob. Agents. 59, 106581. https://doi.org/10.1016/j.ijantimicag.2022.106581 (2022).
Cannatelli, A. et al. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob. Agents Chemother. 58, 5696–5703. https://doi.org/10.1128/aac.03110-14 (2014).
Fordham, S. M. E., Mantzouratou, A. & Sheridan, E. Prevalence of insertion sequence elements in plasmids relating to mgrB gene disruption causing colistin resistance in Klebsiella pneumoniae. Microbiologyopen. 11, e1262. https://doi.org/10.1002/mbo3.1262 (2022).
Huang, N. et al. Hypervirulent carbapenem-resistant Klebsiella pneumoniae causing highly fatal meningitis in southeastern China. Front. Public. Health. 10, 991306. https://doi.org/10.3389/fpubh.2022.991306 (2022).
Bachman, M. A. et al. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect. Immun. 79, 3309–3316. https://doi.org/10.1128/iai.05114-11 (2011).
Stahlhut, S. G., Struve, C., Krogfelt, K. A., Reisner, A. & 34,, & Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol. Med. Microbiol. 65, 350–359. https://doi.org/10.1111/j.1574-695X.2012.00965.x (2012).
Magiorakos, A. P. et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control. 6, 113. https://doi.org/10.1186/s13756-017-0259-z (2017).
Dashti, A. A., Jadaon, M. M., Gomaa, H. H., Noronha, B. & Udo, E. E. Transmission of a Klebsiella pneumoniae clone harbouring genes for CTX-M-15-like and SHV-112 enzymes in a neonatal intensive care unit of a Kuwaiti hospital. J. Med. Microbiol. 59, 687–692. https://doi.org/10.1099/jmm.0.019208-0 (2010).
Tacconelli, E. et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 20 (Suppl 1), 1–55. https://doi.org/10.1111/1469-0691.12427 (2014).
Chen, X., Wen, X., Jiang, Z. & Yan, Q. Prevalence and factors associated with carbapenem-resistant Enterobacterales (CRE) infection among hematological malignancies patients with CRE intestinal colonization. Ann. Clin. Microbiol. Antimicrob. 22, 3. https://doi.org/10.1186/s12941-023-00554-6 (2023).
Kiddee, A. et al. Risk factors for gastrointestinal colonization and acquisition of carbapenem-resistant gram-negative bacteria among patients in intensive care units in Thailand. Antimicrob. Agents Chemother. 62 https://doi.org/10.1128/aac.00341-18 (2018).
Ambretti, S. et al. Screening for carriage of carbapenem-resistant Enterobacteriaceae in settings of high endemicity: a position paper from an Italian working group on CRE infections. Antimicrob. Resist. Infect. Control. 8, 136. https://doi.org/10.1186/s13756-019-0591-6 (2019).
CLSI. Performance standards for antimicrobial susceptibility testing, 31nd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute. (2021).
FDA. U. Tigecycline – Injection products. https://www.fda.gov/drugs/development-resources/tigecycline-injection-products (2013).
Choi, Y. & Chan, A. P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 31, 2745–2747. https://doi.org/10.1093/bioinformatics/btv195 (2015).
Keen, N. T., Tamaki, S., Kobayashi, D. & Trollinger, D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 70, 191–197. https://doi.org/10.1016/0378-1119(88)90117-5 (1988).
Olaitan, A. O. et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: An epidemiological and molecular study. Int. J. Antimicrob. Agents. 44, 500–507. https://doi.org/10.1016/j.ijantimicag.2014.07.020 (2014).
Hadjirin, N. F. et al. Dissemination of carbapenemase-producing enterobacterales in Ireland from 2012 to 2017: A retrospective genomic surveillance study. Microb. Genom. 9 https://doi.org/10.1099/mgen.0.000924 (2023).
Russo, T. A. et al. An Assessment of siderophore production, mucoviscosity, and mouse infection models for defining the virulence spectrum of hypervirulent Klebsiella pneumoniae. mSphere 6 (2021). https://doi.org/10.1128/mSphere.00045-21
Acknowledgements
The authors would like to thank the staff from CICU, Haematology department, NICU, Infection Control department and Pharmacy department for their contribution in the outbreak management. In addition, we would like to express our gratitude to Professor Zhou Tieli from the First Affiliated Hospital of Wenzhou Medical University for the kind provision of Klebsiella pneumoniae MGH78578.
Funding
This study was supported by Hunan Province Natural Sciences Foundation of China under Grant 2021JJ31116.
Author information
Authors and Affiliations
Contributions
WL, QY, and ZJ conceived and designed the project; ZJ, YL, and ZW participated in the sample collection and isolate identification; ZJ and LZ collected the clinical details of each case; ZJ and YL were responsible for antimicrobial susceptibility testing, complementation assay, PCR and virulence phenotype analysis; ZJ and ZW conducted bioinformatics analysis. ZJ wrote the manuscript. All authors have reviewed and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Xiangya Hospital, Central South University with the ethical code number 202303034. Written informed consent for publication of their details was obtained from the patients or their next of kin. This study was conducted in accordance with the Declaration of Helsinki. The mouse experiments were approved by the Laboratory Animal Ethics Committee of Xiangya Hospital of Central South University (Ethics no. 202409156). This study is reported in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Informed consent
Written informed consent for participation in this study and publication of their details was obtained from the patients or their next of kin.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jian, Z., Liu, Y., Wang, Z. et al. A nosocomial outbreak of colistin and carbapenem-resistant hypervirulent Klebsiella pneumoniae in a large teaching hospital. Sci Rep 14, 27744 (2024). https://doi.org/10.1038/s41598-024-79030-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79030-w