Abstract
Initially, SBA-Propyl-Isatin-Malononitrile (SBA-Pr-IS-MN) was prepared by the reaction of SBA-Pr-Cl with IS-MN, which was synthesized by the reaction of isatin and malononitrile. This organic and inorganic hybrid material can be functionalized by the sequential reaction with dimedone to obtain the target product SBA-Pr-Is-MN-DM, which was investigated as a chemosensor by fluorescence spectroscopy to detect trace amount of Ag+ among several other cations in aqueous media.
Similar content being viewed by others
Introduction
SBA-15 is a mesoporous structure comprising pores and was modified via the functionalization process1,2,3,4. It has been deployed extensively in catalysis, for adsorption and separation, drug delivery, and chemical sensing because of its large surface area5,6 and being the first option to be employed as the sensor scaffold7. Structures with fluorophores with adequate binding sites, and mechanisms for connecting the two sites are termed chemosensors8,9.
Since Ag+ is a possible contaminant in drinking H2O10, it needs to be detected as Ag+ions in assorted samples. Some of the available methods such as atomic absorption spectroscopy11,12, and inductively coupled plasma-mass spectrometry13,14, are rather expensive and time-consuming. Consequently, chemosensors offer an ideal option endowed with excellent selectivity, and ease of instrument usage15.
In the past few years, the surface modification of the SBA-15 has been accomplished with a variety of organic compounds. Due to the high surface area and because of the presence of hydroxide groups on the surface, the SBA-15 has a superior ability to interact with organic compounds to generate organic-mineral hybrids. By fastening such organic compounds on the surface of SBA-15, the capability of these compounds is tremendously enhanced to react with different ions thus producing entities suitable as chemical sensors. As an example, such surface modification compound SBA-Pr-Is-TAP (SBA-propyl-isatin) was synthesized for deployment as a chemical sensor to detect Fe3+ and Cr2O72−ions16. In another example, the SBA-Pr-Is-MN was synthesized in 2021 and also served as a detector of Ag+ions. The surface of the SBA-Pr-Cl was reacted with isatin to give SBA-Pr-Is followed by the sequential reaction with malononitrile to generate SBA-Pr-Is-MN17.
Herein, the importance and capability of SBA-15 in connecting to organic compounds and the convenient assembly of organo-mineral hybrids that bind and identify metal ions has been explored in continuation of this ongoing research18,19,20,21,22,23. The synthesis of new compounds tethered to SBA-15 is presented including the identification, and application of the ensued product SBA-Pr-Is-MN-DM, which was investigated as a chemosensor.
Experimental section
Synthesis of SBA-Pr-Cl
It was synthesized according to a previously described report17.
Synthesis of malononitrile-isatin
The reaction mixture of malononitrile and isatin (1:1) was refluxed for 10 min in the presence of a suitable amount of SBA-Pr-SO3H catalyst in H2O. The resulting solid was dissolved in EtOH and filtered to separate the catalyst from this mixture to obtain the red crystals of isatin-malononitrile24. The SO₃H groups act as acid catalysts, facilitating key steps in the reaction pathway by providing protonation sites, which enhanced the synthesis efficiency of IsMN.
Synthesis of SBA-Pr-Is-MN
SBA-Pr-Cl (1 g) was stirred and dispersed in an anhydrous toluene solvent. Then, the ensuing product Is-MN (5 mmol, 0.9 g) and triethylamine (10 mmol, 1.39 mL) were added to the reaction mixture and refluxed for 48 h. The resulting mixture was dried and then treated with EtOH in a Soxhlet apparatus for 6 h and dried to obtain a brown solid SBA-Pr-Is-MN17.
Synthesis of SBA-Pr-Is-MN-DM
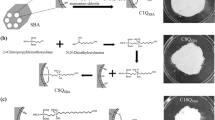
SBA-Pr-Is-MN (1 g) was mixed and dispersed in anhydrous toluene. Then, dimedone (1.5 mmol, 0.25 g) and Et3N were added to this mixture and refluxed for 72 h. The resulting mixture was filtered and then extracted with EtOH and dried to gain SBA-Pr-Is-MN-DM, which was characterized by FT-IR, TGA, XRD, and Fe-SEM.
Results and discussion
Syntheses of SBA-Pr-Is-MN-DM
At first, the SBA-15 substrate surface was modified with (3-chloropropyl)triethoxysilane to produce SBA-Pr-Cl. Separately, the reaction between isatin and malononitrile led to the formation of Is-MN, which was treated with SBA-Pr-Cl to yield SBA-Pr-Is-MN. Finally, the SBA-Pr-Is-MN was treated with dimedone in the presence of Et3N to provide SBA-Pr-Is-MN-DM (Scheme 1). In this process, Et3N removed αH of ß-dicabonyl compound (Dimedone), which attacked SBA-Pr-Is-MN via the Micheal reaction, followed by cyclization to give SBA-Pr-Is-MN-DM.
Reaction mechanism
The synthesis mechanism of the SBA-Pr-Is-MN-DM compound is shown in Scheme 2. For the synthesis of this material, Et3N is first attacked by dimedone (a), and then compound (b) is produced by removing hydrogen. Then, by increasing compound (b) to SBA-Pr-Is-MN, product (d) is produced by cyclization followed by tautomerization to give the desired product SBA-Pr-Is-MN- DM.
Characterizations of SBA-Pr-Is-MN-DM
FT-IR spectra
The weak peaks appearing in the spectrum c in the regions (1470 cm−1) and (1710 cm−1) are associated with the C = C and C = O vibrations of isatin, respectively. In the spectrum c, the peaks appearing in the region (2192 cm−1) correspond to the stretching vibrations of the malononitrile C ≡ N group. The absorption band, which appears in the region (1627 and 1603 cm−1) is related to other carbonyl groups of dimedone compound, which can also be seen in the spectrum d. Additionally, the height of the peak representing the malononitrile group decreases in the region (2192 cm−1) thus affirming the presence of dimedone on the SBA-Pr-Is-MN, which was accomplished via multicomponent reactions on the surface of SBA-15. Therefore, the existence of these absorption bands is a confirmation of the placement of the desirable organic functional groups on the surface of SBA-15, the presence of C-O stretching band introduce the DM group at 1296 cm−1 (Fig. 1).
XRD Studies.
The XRD pattern of SBA-15 composition in the (100), (110), and (200) regions indicates regular mesoporous silica. The XRD spectrum of the final SBA-Pr-Is-MN-DM composition also had a similar pattern to that of the SBA-15, thus indicating that the new functional groups do not affect the entire structure of SBA-15 (Fig. 2).
FE-SEM image
SEM images of SBA-15 and SBA-Pr-Is-MN-DM show that after modifying the surface of SBA-15, the structure of the particles has not changed and there is no sign of disintegration (Fig. 3).
N2 adsorption-desorption study
The nitrogen adsorption-desorption diagram of SBA-15, SBA-Pr-Is-MN-DM is depicted in Fig. 4. Diagram a pertains to SBA-15, and the isothermal diagrams of type IV and H1 residual loop in diagram b are related to SBA-Pr-Is-MN-DM composition, wherein the structure of SBA-15 is preserved.
The physical characteristics of the two samples are presented in Table 1. According to this table, by functionalizing SBA-15, all three parameters of the specific surface area, the average diameter of the holes, and the total volume of porosity have decreased, which indicates that the inner surface of the mesoporous composition is covered with organic groups.
Thermogravimetric analysis (TGA)
Graphs for thermal gravimetric analysis of SBA-Pr-Is-MN and SBA-Pr-Is-MN-DM samples are shown in Fig. 5. The weight loss of the samples up to 150 °C is related to the water physically absorbed on the surface. Since the organic species are destroyed in the range of 200–700 °C, the percentage of weight loss for two combinations of SBA-Pr-Is-MN and SBA-Pr-Is-MN-DM is 20% and 27%, respectively. The amount of organic compounds present on the surface was calculated to be 0.718 mmol.g−1.
Energy dispersive X-ray analysis (EDX)
The main elements in the composition of SBA-Pr-Is-MN-DM include silicon, carbon, oxygen, and nitrogen, and the results of EDS analysis confirm their presence. Based on this, the weight% of elements C, Si, O, and N is 38.6%, 31.8%, 28.3%, and 1.3%, respectively, which is a confirmation of the successful surface modification of this composition (Fig. 6).
Fluorescence studies
To investigate the fluorescence response of the synthesized compound SBA-Pr-Is-MN-DM, 0.02 g of this substance was dispersed in 100 mL of H2O and its performance was evaluated in the presence of various cations of mercury, zinc, nickel, lead, manganese, and sodium, chromium, magnesium, cadmium, copper, calcium, aluminum, iron (II), iron (III), potassium, cobalt, and silver. 2.5 mL of the sample along with 200 µL of cations were poured into the corresponding cell and the fluorescence emission of each was analyzed separately. The results obtained as depicted in Fig. 7 show that the intensity of fluorescence emission after adding Ag+ ion to the SBA-Pr-Is-MN-DM compound increases strongly, while it does not show a significant change in the presence of other cations.
The aforementioned combination of CN and NH2 positions can trap the silver ions. The proposed mechanism of silver ion interaction with the SBA-Pr-Is-MN-DM compound is presented in Fig. 8.
The competitive test was performed to further evaluate the synthesized compound for the detection of silver ions. The fluorescence spectrum of the compound SBA-Pr-Is-MN-DM was recorded in the presence of a mixture of Ag+ ions and other cations. By examining the data in Fig. 9, it can be concluded that the intensity of silver ions has increased significantly in the presence of most cations. Only in the presence of mercury ions, this increase is insignificant and causes some interference.
Fluorescence emission spectrum of SBA-Pr-Is-MN-DM dissolved in distilled water (0.2 g L−1) in presence of the mixture of Fe3+ ions (200 µL × 10−2 M) and several tested cations containing Pb2+, Hg2+, Cr3+, Ni2+, Zn2+, Cu2+, Co2+, Cd2+, Mn2+, Ca2+, K+, Al3+, Mg2+, Na+, and Fe2+ ions (200 µL of Mn+ × 10−2 M).
In the next step, a titration test was performed for this compound, the obtained results are depicted in Fig. 10. This test shows the effect of silver ion concentration on the emission intensity of the compound SBA-Pr-Is-MN-DM, which appears to increase with the increase of Ag+ concentration.
linear relationship between Ag+ concentration and emission intensity of the SBA-Pr-Is-MN-DM with a regression coefficient of 0.9863, with detection limit 2.219 × 10−7 M was illustrated at Fig. 11. This detection limit indicates that the synthesized sensor is capable of detecting silver ions even in low concentrations.
Comparison study
The sensing properties of SBA-Pr-Is-MN-DM was compared SBA-Pr-IS-MN as shown in Table 2. It was proved that the SBA-Pr-Is-MN-DM catalyst has high catalytic activity in this reaction.
Conclusion
The surface modification of the SBA-15 compound was performed via a multicomponent reaction, which led to the production of the final product, SBA-Pr-Is-MN-DM. In this study, multi-component reaction was performed on the surface of SBA-15. The identification of intermediates and this organic-mineral hybrid was performed, and all these analyses indicated the successful surface modification of the SBA-15. Finally, the fluorescence response of this compound was investigated in the presence of different ions, and a different and significant fluorescence response was observed exclusively in the presence of silver ions.
Data availability
Data will be made available on request to corresponding Author (GMZ).
References
Margolese, D., Melero, J., Christiansen, S., Chmelka, B. & Stucky, G. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 12, 2448–2459 (2000).
Colilla, M., Balas, F., Manzano, M. & Vallet-Regí, M. Novel method to enlarge the surface area of SBA-15. Chem. Mater. 19, 3099–3101 (2007).
ALOthman, Z. A. A review: fundamental aspects of silicate mesoporous materials. Materials 5, 2874–2902 (2012).
Puputti, J., Jin, H., Rosenholm, J., Jiang, H. & Lindén, M. The use of an impure inorganic precursor for the synthesis of highly siliceous mesoporous materials under acidic conditions. Micropor Mesopor Mat. 126, 272–275 (2009).
Zhao, D., Huo, Q., Feng, J., Chmelka, B. F. & Stucky, G. D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Amer Chem. Soc. 120, 6024–6036 (1998).
Walcarius, A., Etienne, M. & Lebeau, B. Rate of Access to the binding sites in organically modified silicates. Chem. Mater. 15, 2161–2173 (2003).
Park, S. S., Kong, J., Selvaraj, M. & Ha, C. S. Functionalized Mesoporous Silica for Highly Selective Sensing of Iron Ion in Water. J. Nanosci. Nanotechnol. 21, 4406–4411 (2021).
Czarnik, A. W. Chemical communication in water using fluorescent chemosensors. Acc. Chem. Res. 27, 302–308 (1994).
Fu, L., Mei, J., Zhang, J. T., Liu, Y. & Jiang, F. L. Selective and sensitive fluorescent turn-off chemosensors for Fe3+. Luminescence 28, 602–606 (2013).
Peng, J. Y., Botelho, M. & Matinlinna, J. Silver compounds used in dentistry for caries management: a review. J. Dent. 40, 531–541 (2012).
Chakrapani, G., Mahanta, P. L., Murty, D. S. R. & Gomathy, B. Preconcentration of traces of gold, silver and palladium on activated carbon and its determination in geological samples by flame AAS after wet ashing. Talanta 53, 1139–1147 (2001).
Dadfarnia, S., Haji Shabani, A. M. & Gohari, M. Trace enrichment and determination of silver by immobilized DDTC microcolumn and flow injection atomic absorption spectrometry. Talanta 64, 682–687 (2004).
Aragay, G., Pons, J. & Merkoçi, A. Recent trends in Macro-, Micro-, and Nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 111, 3433–3458 (2011).
Coskun, A. & Akkaya, E. U. Ion sensing coupled to Resonance Energy transfer: a highly selective and sensitive ratiometric fluorescent Chemosensor for Ag(I) by a Modular Approach. J. Am. Chem. Soc. 127, 10464–10465 (2005).
Iyoshi, S., Taki, M. & Yamamoto, Y. Rosamine-based fluorescent Chemosensor for selective detection of silver(I) in an aqueous solution. Inorg. Chem. 47, 3946–3948 (2008).
Mohammadi Ziarani, G., Akhgar, M., Mohajer, F., Badiei, A. & Luque, R. SBA-Pr-Is-TAP functionalized nanostructured silica as a highly selective fluorescent chemosensor for Fe3+ and Cr2O72– ions in aqueous media. Nanomaterials 11, 2533 (2021).
Mohammadi Ziarani, G., Akhgar, M., Mohajer, F. & Badiei, A. SBA-Pr-IS-MN synthesis and its application as ag + optical sensor in aqueous media. Res. Chem. Intermed. 47, 2845–2855 (2021).
Mohammadi Ziarani, G., Lashgari, N. & Badiei, A. Application of organoamine-functionalized mesoporous silica (SBA-Pr-NH2) as a nano base catalyst in organic reactions. Curr. Org. Chem. 21, 674–687 (2017).
Mohammadi Ziarani, G. et al. SBA-Pr-SO3H-catalyzed synthesis of bispyrazole compounds as anti-bacterial agents and inhibitors of phosphorylated RET tyrosine kinase. J. Iran. Chem. Soc. 16, 1401–1409 (2019).
Karimi, M., Badiei, A. & Mohammadi Ziarani, G. A single hybrid optical sensor based on nanoporous silica type SBA-15 for detection of pb2+ and I– in aqueous media. RSC Adv. 5, 46 (2015).
Moradi, R., Mohammadi Ziarani, G., Badiei, A. & Mohajer, F. Synthesis and characterization of mesoporous organosilica supported palladium (SBA-Pr-NCQ-Pd) as an efficient nanocatalyst in the mizoroki–heck coupling reaction. Appl. Organomet. Chem. 34, e5916 (2020).
Mohajer, F., Mohammadi Ziarani, G. & Badiei, A. Decorated palladium nanoparticles on mesoporous organosilicate as an efficient catalyst for Sonogashira coupling reaction. J. Iran. Chem. Soc. 18, 589–601 (2020).
Mohammadi Ziarani, G., Rohani, S., Ziarati, A. & Badiei, A. Applications of SBA-15 supported Pd metal catalysts as a nanoreactor in C-C coupling reactions. RSC. Adv. 8, 41048–41100 (2018).
Lashgari, N., Badiei, A. & Mohammadi Ziarani, G. A novel functionalized nanoporous SBA-15 as a selective fluorescent sensor for the detection of multianalytes (Fe3+ and Cr2O72−) in water. J. Phys. Chem. Solids, 103, 238–248 (2017).
Acknowledgements
The authors were grateful for the research council’s support of Alzahra University.
Funding
There is no fund to mention.
Author information
Authors and Affiliations
Contributions
Author contributions: Ghodsi Mohammadi Ziarani: Project Administration-Lead, Supervision-Lead, Editing-Lead.Fatemeh Mohajer: Writing original paper, Editing the original draft and Modifying data.Fatemeh Javadi: Doing experimental part and Data calculation. Alireza Badiei: Review & Editing, Software-Supporting. Rajender S. Varma: Editing & Review of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent of publication
All authors read and approved the final manuscript.
Ethical approval
It does not apply to this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohammadi Ziarani, G., Mohajer, F., Javadi, F. et al. SBA-Pr-IS-MN-DM: a new fluorescent chemosensor for trace detection of silver in aqueous solutions. Sci Rep 14, 29072 (2024). https://doi.org/10.1038/s41598-024-80406-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80406-1