Abstract
Functional capacity is an important objective health metric, and relies on the maintenance of physiological homeostasis. Autonomic nervous system is known to coordinates the maintenance of multi-organ homeostasis. The objective of this study was to examine the association of autonomic nervous system function with functional capacity in adults aged 55 years and older. A cohort of 542 adults (mean age of 70.1 years) received repeated measurements of heart rate variability, an autonomic nervous system function marker, and chair rise time, a functional capacity measure. Linear mixed models analysis showed that 1 SD lower powers in low-frequency range at baseline was associated with a 0.11 (95% CI 0.01–0.21) s/year faster increase in chair rise time during the follow-up, whereas 1 SD increase in powers in high-frequency range and 1 SD decrease in the ratio of powers in low-frequency range to powers in high-frequency range during the follow-up were associated with a 0.22 (95% CI 0.06–0.39) s and 0.17 (95% CI 0.01–0.33) s increase in chair rise time. In conclusion, autonomic nervous system function and its changes were longitudinally associated with changes in functional capacity in older adults.
Similar content being viewed by others
Introduction
Functional capacity is an important objective health metric for older adults1,2. Its preservation is also of high propriety from older adults’ perspective3. Unfortunately, functional capacity becomes more susceptible to decline as an individual ages1,2. Evidence shows that a declined functional capacity in physical domains, by itself, is associated with a higher risk of multiple adverse health events and high care needs4,5,6,7. As the population of older adults grows, preserving functional capacity as an individual ages is becoming a major goal of care and represents an unmet healthcare need.
Despite this, detailed mechanisms contributing to the decline of functional capacity in late life remain incompletely understood. As an aggregate health measure that typically declines with age, an increasing amount of evidence suggests that the functional capacity relies on the maintenance of physiological homeostasis by multiple organ systems, including cardiovascular, metabolic, and immune systems, in the face of stress and its decline as an individual age may result from a failure of this hemostasis maintenance2,5,8,9,10,11,12,13,14,15.
The autonomic nervous system plays unique and pivotal roles in maintaining physiological homeostasis16,17,18,19,20,21,22,23,24. These roles are mainly exerted through their effects on the function of multiple organ systems. Aside from its well-known effects on cardiovascular and metabolic systems16,17, recent experimental research even showed the previously unexpected close connections of autonomic nervous system activity with inflammation, immune responses, and skeletal muscle physiology18,19. Consistent with its critical role in maintaining homeostasis, in addition to neurological and psychiatric disorders25,26,27, aberrant autonomic nervous system function has increasingly been identified in clinical and even the earlier subclinical states of multiple diseases, including cardiovascular, metabolic, and inflammatory diseases20,21,22,23,24,28,29. Emerging evidence indicates that autonomic nervous system function may alter during aging, for instance, in response to cellular and molecular-level stressors30,31,32. These alterations are postulated to contribute to the development of geriatric syndromes and, consequently, the accelerated health decline in late life though controversies still exist25,32,33,34,35.
Here, we conducted a longitudinal study with repeated measurements of heart rate variability (HRV), a measure of autonomic nervous system function, and functional capacity. We aimed to examine the longitudinal association of heart rate variability and its change with changes in functional capacity over time in older adults. We hypothesized an association of autonomic nervous system function and its change with functional capacity decline.
Methods
Study design and participants
Study participants were from the Healthy Aging Longitudinal Study in Taiwan (HALST), a prospective population-based cohort study that began in 2009 with the third wave follow-up examination still underway36. From 2021 to 2024, HALST participants aged 55 years and older were screened for eligibility to enter a study that examined the applicability, diagnostic, and prognostic values of digital health technologies in community-dwelling older adults (Supplementary Methods S1 in Supplementary Materials). After being recruited, each participant underwent a structured baseline examination assessing sociodemographic status, diseases, health status, and major lifestyle risk factors. Afterward, each participant underwent three follow-up examinations, which took place one month, three months, and six months after the baseline examination. A total of 233 participants entered this analysis (Supplementary Fig. S1).
An additional 322 HALST participants without arrhythmia or pacemakers underwent heart rate variability measurement during the HALST examinations between September 2012 and April 2015. A total of 309 individuals were also included in this analysis (Supplementary Fig. S1).
The study was conducted according to the guidelines of the Declaration of Helsinki. All participants provided written informed consent. This study was approved by the institutional review boards of the National Health Research Institutes.
Measurement of autonomic nervous system function
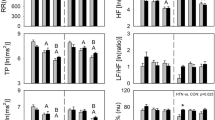
The main exposure in this study was autonomic nervous system function, which was assessed by measuring heart rate variability during both the baseline and follow-up examinations according to standardized procedures37. Participants received a 5-minute resting single lead (lead I) electrocardiogram while breathing spontaneously and normally in a room that was maintained at a comfortable temperature with dim light after a 15-minute rest. The electrocardiogram signals were then processed by an HRV monitor (8Z05, Wegene Technology Inc., Taiwan)38,39,40. Both time-___domain (standard deviation of all NN intervals [SDNN] [in milliseconds])) and frequency-___domain (powers [in milliseconds squared] in low-frequency [LF] [0.04–0.15 Hz], high-frequency [HF] [0.15–0.4 Hz] ranges, LF/HF ratio and the total power [in milliseconds squared] [TP] ( < = 0.4 Hz)) measures were used for analysis37. Resting heart rate (HR) was also calculated from the electrocardiogram signals.
Measurement of functional capacity
The main outcome of this study was functional capacity, which was assessed by conducting chair-rising tests during both the baseline and follow-up examinations according to standardized procedures. During the chair-rising tests, participants were asked to stand up (from a straight-backed chair) and sit down as fast as possible five times while keeping their arms across the chest4. The time taken to complete this task, the chair rise time, was measured by a trained examiner using a handheld stopwatch that measured to the nearest hundredth of a second (s) and was recorded. This objective physical performance test represents a composite measure of muscle strength, muscle power, and balance2,41,42.
Measurement of covariates
We measured sociodemographic status (age; sex), education, smoking status, physical activity, body mass index (BMI), chronic diseases (hypertension, diabetes mellitus, cardiovascular disease, stroke, lung disease, cancer, arthritis, chronic kidney disease, and Parkinson’s disease), and resting heart rate during the baseline examinations. (Supplementary Methods S1 in Supplementary Materials)
Statistical analyses
Participants’ characteristics were summarized using descriptive statistics. Categorical variables were summarized using numbers (percentage). Continuous variables were summarized using mean (standard deviation) if they were normally distributed and median (interquartile range) if they were nonnormally distributed.
To conduct subsequent analysis, all heart rate variability measures were natural log-transformed because of nonnormal distribution. Also, in order to facilitate effect size comparisons among different heart rate variability measures, we further standardized the natural log-transformed heart rate variability measures.
To investigate the longitudinal association between the exposure (HRV measures) and the outcome (chair rise time), both on continuous scales, we applied linear mixed models with participant-level random intercept and random slope to the longitudinal data. An unstructured covariance structure was assumed. With this model, the correlations between continuous outcomes measured in the same individual over time were properly accounted for. Time was defined as years (t) since the baseline (t0) examination. Assumptions of the linear mixed model, including the normality and functional form assumptions, were checked by examining the residual distributions.
We hypothesized an effect of baseline and time-varying HRV measures on chair rise time. For the baseline HRV measure k, we fitted a linear mixed model that included t, t2, and the term for the baseline HRV measure k (model 1). We included the t2 term in the model to allow for non-linear change with time. The coefficient estimates of the term for the baseline HRV measure k quantified the association of the differences in baseline HRV measure k with the differences in initial chair rise time (the intercept of the average true trajectory of change in chair rise time). Then, we added covariates that might confound the association between the baseline HRV measure k and chair rise time to the model (model 2). To quantify the association of the baseline HRV measure k with the rate of change of the chair rise time, we tested the interactions of baseline HRV measure k with t adjusting for covariates (model 3), which allow for different rates of change in chair rise time between those with differences in the baseline HRV measure k. In model 4, we tested the interactions of baseline HRV measure k with t2 adjusting for covariates, which allow for different change rates of rates of change in chair rise time between those with differences in the baseline HRV measure k. In models 3 and 4, we retained interaction terms that were statistically significant.
For the time-varying HRV measure k, we likewise fitted the following linear mixed models: model 1 included t, t2, and the term for the time-varying HRV measure k; model 2 additionally included covariates that might confound the association between the time-varying HRV measure k and chair rise time; model 3 tested the interactions of time-varying HRV measure k with t adjusting for covariates; and model 4 alternatively tested the interactions of time-varying HRV measure k with t2 adjusting for covariates. The coefficient estimates of the term for the time-varying HRV measure k in Model 2 quantified the association of the differences in HRV measure k at a time during follow-up with the differences in chair rise time at that time. The coefficient estimates of the time-varying HRV measure k x t interaction term in Model 3 and the time-varying HRV measure k x t2 interaction term in Model 4 quantified the association of the differences in HRV measure k with the differences in the rate of change in chair rise time and the differences in the change rates of rate of change in chair rise time, respectively.
To further isolate and test the effects of within-individual change in HRV measure k, we decomposed the time-varying HRV measure k at time t of subject i (HRVikt) into two parts: the HRV measure k at t0 (HRVikt0); and ΔHRVikt, where ΔHRVikt = HRVikt - HRVikt0. We then refitted the linear mixed models that included HRVkt0 and ΔHRVk. The coefficient estimates of the term ΔHRVk in Model 2 then quantified the association of the differences in within-individual change in HRV measure k from baseline to a time during follow-up with the differences in chair rise time at that time, controlling for baseline level of HRV measure k. The coefficient estimates of the ΔHRVk x t interaction term in Model 3 and the ΔHRVk x t2 interaction term in Model 4 quantified the association of the differences in within-individual change in HRV measure k with the differences in the rate of change in chair rise time and the differences in the change rates of rate of change in chair rise time.
In sensitivity analysis, we specifically examined the effects of hypertension on our findings by fitting the models that did and did not include hypertension. We also examined the possible impacts of medications by fitting models that additionally included β-blockers.
A two-sided P value of less than 0.05 was considered statistical significance. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the study population, consisting of 542 adults, are shown in Table 1. The mean (SD) age at baseline was 70.1 (7.0) years, and 46.5% were women. These 542 adults contributed to a mean of 2.3 functional capacity observations per person.
Baseline HRV measures and changes in chair rise time
There were statistically significant interactions of the baseline LF term with time (Model 3 in Table 2) and time2 (Model 4 in Table 2), where time represents years since baseline examination, in adjusted models. The point estimates of the coefficients were − 0.11 and − 0.02, respectively, meaning that one SD lower baseline LF was associated with a 0.11 s/year faster increase in chair rise time and a 0.02 s/year/year faster increase in the rate of change in chair rise time during the subsequent follow-up.
Unlike the slope, differences in any baseline HRV measure were not significantly associated with differences in the average true initial (baseline) status of chair rise time (Table 2).
Time-varying HRV measures and changes in chair rise time
A higher HF at a time during follow-up was significantly associated with a longer chair rise time at that time in the adjusted model (Models 2, 3 and 4 in Table 3). The point estimate of the coefficient was 0.18, meaning that one SD higher HF was associated with a 0.18 s longer chair rise time. We also observed a marginally significant association of a higher SDNN at a time during follow-up with a longer chair rise time at that time (Models 2, 3, and 4 in Table 3). Levels of LF, LF/HF ratio, and TP at a time during follow-up were not significantly associated with chair rise time at that time (Table 3).
In the analysis of within-individual change in HRV measures, we noted that a within-individual increase in HF from baseline to a time during follow-up was associated with a longer chair rise time at that time, controlling for baseline HF level and covariates (Models 2, 3 and 4 in Table 4). The point estimate of the coefficient reached 0.22, meaning that one SD increase in HF was associated with a 0.22s longer chair rise time. We also noted that a within-individual decrease in LF/HF ratio from baseline to a time during follow-up was associated with a longer chair rise time at that time (Table 4). The point estimate of the coefficient reached − 0.17, meaning that one SD decrease in LF/HF ratio was associated with a 0.17s longer chair rise time. Within-individual change in other HRV measures, including SDNN, LF, and TP, were not significantly associated with chair rise time (Table 4).
For each of the HRV measures, we failed to reject the null hypothesis that the population value of the time-varying HRV measure x time and the time-varying HRV measure x time2 interaction terms’ coefficients were 0 in both unadjusted (Supplementary Table S2) and adjusted analysis (Models 3 and 4 in Table 3). Likewise, we failed to reject the null hypothesis that the population value of the ΔHRVk x time and the ΔHRVk x time2 interaction terms’ coefficients were 0 in both unadjusted (Supplementary Table S3) and adjusted analysis (Models 3 and 4 in Table 4).
In sensitivity analysis, we noted that the coefficient estimates that quantify the associations of HRV measures with chair rise time in models that included hypertension were comparable to those in models that did not include hypertension (Supplementary Tables S4, S5 and S6).
Discussion
In this longitudinal study with repeated measurements of heart rate variability measures and functional capacity, we showed that heart rate variability was significantly associated with changes in functional capacity. First, a lower baseline LF was associated with a higher rate of gradual increase in chair rise time. Second, an increase in HF and a decrease in LF/HF ratio were each associated with an acute increase in chair rise time. These findings offer unique and valuable information about the relationship between autonomic nervous system activity and functional capacity.
The autonomic nervous system has two major components, namely the sympathetic and parasympathetic nervous systems. Its activity directly modulates the beat-to-beat variability in heart rate, with the sympathetic and parasympathetic nervous systems modulating the variability differently. Whereas the parasympathetic nervous system modulates the HF range variance (power), the sympathetic nervous system modulates the LF range variance (power) in heart rate. As such, autonomic nervous system function is frequently assessed non-invasively by measuring the heart rate variability in time or frequency domains. Whereas HF primarily reflexes the parasympathetic nervous system activity, the LF reflexes the sympathetic and parasympathetic nervous system activity. The LF/HF ratio indicates the sympathovagal balance and the sympathetic nervous system activity37. SDNN and TP reflex overall autonomic nervous system activity.
The association of heart rate variability with late-life health, though possible, remains largely unclear, and controversies still exist25,35. Our data suggested that autonomic nervous system function is related to functional capacity declines, either gradually over a longer term or acutely. For the gradual decline in functional capacity, we demonstrated that a lower LF range variance in heart rate at baseline was associated with an accelerated rate of gradual decline in the chair-rising tests performance. This association seems to be unmasked after covariate adjustment (Supplementary Tables S1 and Table S2; Table 2), which may have controlled for negative confounding43. A lower SDNN reflects a lower overall autonomic nervous system activity. We observed a marginally insignificant interactions of the baseline SDNN term with time2 (Model 4 in Table 2). The point estimate of the coefficient was − 0.01, with 95% CI reaching − 0.03, which were comparable to those of the baseline LF. Our findings are consistent with the results of previous investigations. Koopman et al.44 observed that, among 822 adults aged 50 years and older, a lower SDNN tended to be cross-sectionally associated with lower grip strength, an objective measure of muscle strength. Chaves et al.33 showed that, among 389 women aged 65 years and older, a lower LF and SDNN were associated cross-sectionally with physical frailty with poor functional capacity. This association was replicated in another cross-sectional study of 276 women aged 65 years and older34. Interestingly, Ogliari et al.45 showed that, among 5042 older adults aged 70 years and older, a lower SDNN at baseline examination tended to be associated with greater odds of impairment in perceived (subjective) functional independence. Unlike LF, we did not observe a significant association of baseline LF/HF ratio with change in chair rise time. Although this finding still needs to be confirmed by future studies of a larger scale, it is generally consistent with previous studies showing that a lower LF at baseline was associated with a higher risk of adverse health outcomes, whereas a lower LF/HF ratio was not46,47.
On the other hand, for the acute decline in functional capacity, we noted that an increase in HF and a decrease in LF/HF ratio, reflecting an intercurrent increase in parasympathetic nervous system activity and a sympathovagal balance skewed toward the parasympathetic arm, was associated with an acute decline in the chair-rising tests performance. In agreement with the observed association of a decrease in LF/HF ratio with an acute decline in functional capacity, a lower LF/HF ratio was cross-sectionally associated with physical frailty with poor functional capacity33,34. Also, in agreement with the observed association of an increase in HF with an acute decline in functional capacity, Varadhan et al. noted that a lower level of a principal component of HRV measures consisting of a negatively weighted HF was associated with a greater odd of physical frailty with poor functional capacity34. Moreover, the association of a sympathovagal balance that is skewed toward the sympathetic arm with a preserved functional capacity is consistent with the important roles of autonomic nervous system activity plays in maintaining muscle function19. It is interesting to note that aging is associated with an increase in autonomic support of blood pressure that is correlated with an increase in muscle sympathetic nerve activity48.
There are several possible explanations for the observed association between autonomic nervous system function and functional capacity changes. First, the lower HRV at baseline could indicate poor health37,46,47, which could then contribute to a decline in functional capacity through multiple mechanisms. For instance, the lower HRV may be accompanied by impaired cardiac autonomic control, which could contribute to a decline in muscle strength and power by failing to maintain adequate organ perfusion16. The lower HRV at baseline may also be associated with subclinical chronic metabolic disorders, such as insulin resistance, adiposity, hyperglycemia, and inflammation21,22,23,28,29,49, which in turn could lead to a decline over time in muscle mass, strength, and power chronically. Second, the observed lower LF range variance in heart rate at baseline and the temporal increase in HF range variance and a decrease in LF/HF ratio during follow-up may be associated with biological aging processes that underly the functional capacity decline and physical frailty development during aging. Third, for the associations of the temporal increase in HF range variance and a decrease in LF/HF ratio with acute decline in the chair-rising tests performance, the possibility of impaired skeletal muscle function contributed by an altered autonomic nervous system activity could not be excluded19.
The results of this study could have clinical implications. First, changes in autonomic function with an increase in HF and a decrease in LF/HF ratio may serve as a risk marker of functional capacity decline with usefulness in risk assessment. Second, the association between autonomic function and the chair-rising test performance suggests the possibility that the chair-rising test might be used as a stress test during which the body was exposed to a specific stress. The body’s responses to this stress, including those involving autonomic function, might be a major determinant of the test performance. This possibility, once proved, may open up a direction of clinical application. For instance, the chair-rising test, currently used as an objective physical performance test, might be used as a test for autonomic function that could be easily applied in many clinical settings for screening or monitoring purposes, whereas the formal autonomic function test might be reserved for frail older adults who could not perform the task. Future research that examines this possibility would be valuable.
To our knowledge, this study represents the first attempt to test the temporally ordered association between the autonomic nervous system function and functional capacity. Other strengths include objective, standardized, and serially conducted measurements of the autonomic nervous system function and functional capacity. Despite these strengths, there are several limitations. First, this was an observational study. Causality inference still should be made with caution. Second, despite adjustment for multiple confounders, the possibility of biased estimates caused by residual confounding could not be eliminated. It is possible that other factors, such as severity of chronic diseases (e.g., diabetes mellitus, visceral adiposity), target organ damages, medications and time-varying covariates, may have confounded the observed associations. Although we attempted to adjust for medications that might have effects on the autonomic nervous system, we assessed medication use mainly based on self-report. The possibility of misclassification could not be excluded. Nevertheless, literature suggested that, unlike HRV at a single time point, HRV change is less likely to be affected by a wide range of confounding factors, including medication use50. In a sensitivity analysis, we further adjusted for the adjudicated β-blocker usage. The results were not materially different after this additional adjustment. Third, our study population consisted partly of older adults who regularly used mobile phones or tablets with easy access to internet connectivity. The generalizability of the study results to other populations remains to be confirmed. Fourth, to facilitate clinical translation, more research on the normative HRV change is necessary. This data would be valuable in defining physiological and pathological changes during aging. Fifth, HRV was assessed using a 5-minute resting single-lead electrocardiogram, and its change was assessed by repeated measurements performed three times during the six months follow-up period. Although it was assessed according to the guideline37, further investigations of HRV changes in which HRV is assessed by using longer-term, or even continuously51, measured heart rate signals are worthwhile. Finally, HRV measures were repeatedly assessed at the same time as the functional capacity. As such, the possibility of effect cause could not be totally excluded.
In conclusion, we showed that autonomic nervous system function, measured by HRV, was longitudinally associated with changes in functional capacity in older adults. A lower autonomic nervous system activity with a lower LF range variance in heart rate was associated with an accelerated rate of gradual decline in functional capacity. A change in autonomic nervous system activity with an increase in HF range variance in heart rate and a decrease in LF/HF ratio was associated with an acute decline in functional capacity. Changes in autonomic nervous system function may precede the decline in functional capacity in older adults and may serve as a risk marker of functional capacity decline in this population. The autonomic nervous system function may be associated with the mechanisms of the functional capacity change in older adults.
Data availability
The data that support the findings of this study are available from the National Health Research Institutes but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the National Health Research Institutes. Please contact the corresponding author for the data availability.
References
Guralnik, J. M., Patel, K. & Ferrucci, L. in In the Epidemiology of Aging. 91–117 (eds Newman, A. B., Jane, A. & Cauley) (Springer Netherlands, 2012).
Forman, D. E. et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with Cardiovascular Disease: A Scientific Statement for Healthcare professionals from the American Heart Association. Circulation 135, e894–e918. https://doi.org/10.1161/cir.0000000000000483 (2017).
Fried, T. R., McGraw, S., Agostini, J. V. & Tinetti, M. E. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J. Am. Geriatr. Soc. 56, 1839–1844. https://doi.org/10.1111/j.1532-5415.2008.01923.x (2008).
Guralnik, J. M., Ferrucci, L., Simonsick, E. M., Salive, M. E. & Wallace, R. B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl. J. Med. 332, 556–561. https://doi.org/10.1056/nejm199503023320902 (1995).
Cooper, R., Kuh, D. & Hardy, R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 341, c4467 (2010).
Cooper, R. et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 40, 14–23. https://doi.org/10.1093/ageing/afq117 (2011).
Celis-Morales, C. A. et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. Bmj 361, k1651. https://doi.org/10.1136/bmj.k1651 (2018).
Rantanen, T. et al. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J. Am. Geriatr. Soc. 51, 636–641. https://doi.org/10.1034/j.1600-0579.2003.00207.x (2003).
Kritchevsky, S. B. et al. Pathways, contributors, and correlates of functional limitation across specialties: Workshop Summary. J. Gerontol. Biol. Sci. Med. Sci. 74, 534–543. https://doi.org/10.1093/gerona/gly093 (2019).
Walston, J. et al. Moving Frailty toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. J. Am. Geriatr. Soc. 67, 1559–1564. https://doi.org/10.1111/jgs.15928 (2019).
Walston, J. et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in older adults. J. Am. Geriatr. Soc. 54, 991–1001. https://doi.org/10.1111/j.1532-5415.2006.00745.x (2006).
Ershler, W. B. & Keller, E. T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 51, 245–270. https://doi.org/10.1146/annurev.med.51.1.245 (2000).
Fleg, J. L. et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112, 674–682. https://doi.org/10.1161/circulationaha.105.545459 (2005).
Barzilay, J. I. et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged > or = 70 years. Diabetes Care. 32, 736–738. https://doi.org/10.2337/dc08-1781 (2009).
Kalyani, R. R. et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J. Am. Geriatr. Soc. 60, 1701–1707. https://doi.org/10.1111/j.1532-5415.2012.04099.x (2012).
Rowell, L. B. Human Circulation: Regulation during Physical Stress (Oxford University Press, 1986).
Nonogaki, K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43, 533–549. https://doi.org/10.1007/s001250051341 (2000).
Pavlov, V. A., Chavan, S. S. & Tracey, K. J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 36, 783–812. https://doi.org/10.1146/annurev-immunol-042617-053158 (2018).
Khan, M. M. et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. U S A. 113, 746–750. https://doi.org/10.1073/pnas.1524272113 (2016).
Goldberger, J. J., Arora, R., Buckley, U. & Shivkumar, K. Autonomic nervous system dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 73, 1189–1206. https://doi.org/10.1016/j.jacc.2018.12.064 (2019).
Ribeiro, R. T., Lautt, W. W., Legare, D. J. & Macedo, M. P. Insulin resistance induced by sucrose feeding in rats is due to an impairment of the hepatic parasympathetic nerves. Diabetologia 48, 976–983. https://doi.org/10.1007/s00125-005-1714-6 (2005).
Gautam, D. et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell. Metab. 3, 449–461. https://doi.org/10.1016/j.cmet.2006.04.009 (2006).
Consolim-Colombo, F. M. et al. Galantamine alleviates inflammation and insulin resistance in patients with metabolic syndrome in a randomized trial. JCI Insight. 2. https://doi.org/10.1172/jci.insight.93340 (2017).
Koopman, F. A. et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U S A. 113, 8284–8289. https://doi.org/10.1073/pnas.1605635113 (2016).
Ebinger, J. E. et al. Blood pressure variability supersedes heart rate variability as a real-world measure of dementia risk. Sci. Rep. 14, 1838. https://doi.org/10.1038/s41598-024-52406-8 (2024).
Santos, C. Y., Machan, J. T., Wu, W. C. & Snyder, P. J. Autonomic cardiac function in preclinical Alzheimer’s Disease. J. Alzheimers Dis. 59, 1057–1065. https://doi.org/10.3233/jad-170217 (2017).
Choi, K. W. et al. Heart rate variability for treatment response between patients with major depressive disorder versus panic disorder: a 12-week follow-up study. J. Affect. Disord. 246, 157–165. https://doi.org/10.1016/j.jad.2018.12.048 (2019).
Carnethon, M. R. et al. The association among autonomic nervous system function, incident diabetes, and intervention arm in the diabetes Prevention Program. Diabetes Care. 29, 914–919. https://doi.org/10.2337/diacare.29.04.06.dc05-1729 (2006).
Carnethon, M. R., Jacobs, D. R. Jr., Sidney, S. & Liu, K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care. 26, 3035–3041. https://doi.org/10.2337/diacare.26.11.3035 (2003).
Umetani, K., Singer, D. H., McCraty, R. & Atkinson, M. Twenty-four hour time ___domain heart rate variability and heart rate: relations to age and gender over nine decades. J. Am. Coll. Cardiol. 31, 593–601. https://doi.org/10.1016/s0735-1097(97)00554-8 (1998).
Wagner, J. U. G. et al. Aging impairs the neurovascular interface in the heart. Science 381, 897–906. https://doi.org/10.1126/science.ade4961 (2023).
Fried, L. P. et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging. 1, 36–46. https://doi.org/10.1038/s43587-020-00017-z (2021).
Chaves, P. H. et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J. Am. Geriatr. Soc. 56, 1698–1703. https://doi.org/10.1111/j.1532-5415.2008.01858.x (2008).
Varadhan, R. et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J. Gerontol. Biol. Sci. Med. Sci. 64, 682–687. https://doi.org/10.1093/gerona/glp013 (2009).
Parvaneh, S. et al. Regulation of Cardiac Autonomic Nervous System Control across Frailty statuses: a systematic review. Gerontology 62, 3–15. https://doi.org/10.1159/000431285 (2015).
Hsu, C. C. et al. Cohort Profile: the healthy aging longitudinal study in Taiwan (HALST). Int. J. Epidemiol. 46 (j), 1106–1106. https://doi.org/10.1093/ije/dyw331 (2017).
Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996).
Tseng, T. H., Chen, H. C., Wang, L. Y. & Chien, M. Y. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: a randomized controlled trial. J. Clin. Sleep. Med. 16, 1483–1492. https://doi.org/10.5664/jcsm.8560 (2020).
Yang, C. C., Lai, C. W., Lai, H. Y. & Kuo, T. B. Relationship between electroencephalogram slow-wave magnitude and heart rate variability during sleep in humans. Neurosci. Lett. 329, 213–216. https://doi.org/10.1016/s0304-3940(02)00661-4 (2002).
Huang, W. L. et al. Gender differences in personality and heart-rate variability. Psychiatry Res. 209, 652–657. https://doi.org/10.1016/j.psychres.2013.01.031 (2013).
Schenkman, M., Hughes, M. A., Samsa, G. & Studenski, S. The relative importance of strength and balance in chair rise by functionally impaired older individuals. J. Am. Geriatr. Soc. 44, 1441–1446. https://doi.org/10.1111/j.1532-5415.1996.tb04068.x (1996).
Hardy, R. et al. Is chair rise performance a useful measure of leg power? Aging Clin. Exp. Res. 22, 412–418. https://doi.org/10.1007/bf03324942 (2010).
Vittinghoff, E., Glidden, D. V., Shiboski, S. C. & McCulloch, C. E. Regression Methods in Biostatistics Linear, Logistic, Survival, and Repeated Measures Models 2 edn (Springer, 2011).
Koopman, J. J. et al. Heart rate variability, but not heart rate, is associated with handgrip strength and mortality in older africans at very low cardiovascular risk: a population-based study. Int. J. Cardiol. 187, 559–561. https://doi.org/10.1016/j.ijcard.2015.03.383 (2015).
Ogliari, G. et al. Resting heart rate, heart rate variability and functional decline in old age. Cmaj 187, E442–e449. https://doi.org/10.1503/cmaj.150462 (2015).
Tsuji, H. et al. Reduced heart rate variability and mortality risk in an elderly cohort. Framingham Heart Study Circulation. 90, 878–883. https://doi.org/10.1161/01.cir.90.2.878 (1994).
Liao, D., Carnethon, M., Evans, G. W., Cascio, W. E. & Heiss, G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes 51, 3524–3531. https://doi.org/10.2337/diabetes.51.12.3524 (2002).
Jones, P. P., Christou, D. D., Jordan, J. & Seals, D. R. Baroreflex buffering is reduced with age in healthy men. Circulation 107, 1770–1774. https://doi.org/10.1161/01.Cir.0000057811.86187.88 (2003).
Carnethon, M. R., Golden, S. H., Folsom, A. R., Haskell, W. & Liao, D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the atherosclerosis risk in communities study, 1987–1998. Circulation 107, 2190–2195. https://doi.org/10.1161/01.cir.0000066324.74807.95 (2003).
Jandackova, V. K., Scholes, S., Britton, A. & Steptoe, A. Are changes in Heart Rate Variability in Middle-aged and older people normative or caused by pathological conditions? Findings from a large Population-based longitudinal cohort study. J. Am. Heart Assoc. 5. https://doi.org/10.1161/jaha.115.002365 (2016).
Walter, J. R., Xu, S. & Rogers, J. A. From lab to life: how wearable devices can improve health equity. Nat. Commun. 15. https://doi.org/10.1038/s41467-023-44634-9 (2024).
Acknowledgements
We thank all participating men and women in the HALST study. We also thank Dr. Hung-Yi Chiou and all members of the HALST study group.
Author information
Authors and Affiliations
Contributions
I.C.W., Y.Y.L. and P.F.C. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. I.C.W. conceived and designed the study. I.C.W., Y.Y.L. W.T.T., P.F.C. contributed to the data acquisition. I.C.W., P.F.C. contributed to the data analysis. I.C.W. contributed to data interpretation. I.C.W., Y.Y.L. and P.F.C. drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content and approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, IC., Lu, YY., Tseng, WT. et al. Autonomic function and change in functional capacity in older adults: a longitudinal investigation. Sci Rep 14, 29104 (2024). https://doi.org/10.1038/s41598-024-80659-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80659-w