Abstract
Spinal manipulative therapy (SMT) has been shown to significantly alleviate pain in patients with lumbar disc herniation (LDH), with its effects closely associated with brain function modulation. This study investigates the neural biomarkers linked to pain relief efficacy following a complete SMT treatment cycle in LDH patients. A total of 59 LDH patients were randomized into two groups: SMT treatment (Group 1, n = 28) and sham treatment (ST) (Group 2, n = 31). A matched healthy control group (Group 3, n = 28) was also included. Functional magnetic resonance imaging (fMRI) was performed on LDH patients at two time points (TPs)—before (TP1) and after (TP2) treatment—while healthy controls were scanned once. Clinical assessments were conducted using the Visual Analogue Scale (VAS) and the Japanese Orthopaedic Association (JOA) scale. Post-treatment results indicated significant improvements in both VAS and JOA scores for Group 1, while the improvement was limited to VAS scores for Group 2. Graph properties analysis revealed notable differences in brain network connectivity between LDH patients and healthy controls, particularly between the left precentral gyrus (left PreCG) and left inferior frontal gyrus, opercular part (left IFGoperc). Enhanced functional connectivity (FC) was observed in Group 1, notably between the right angular gyrus (right ANG) and the left middle orbital gyrus (left ORBmid), with right ANG showing a significant positive correlation with clinical scores. This study identifies the sensorimotor network—salience network are significantly activated in chronic pain among LDH patients. The default mode network—dorsal attention network may serve as key neural biomarkers for the efficacy of SMT treatment in alleviating pain in LDH.
Similar content being viewed by others
Introduction
Lumbar disc herniation (LDH), primarily manifesting as chronic low back pain1,2, boasts a lifetime prevalence rate of up to 70%3,4 with the highest disability rate5. Research indicates that the risk of developing this condition escalates with age6,7, and in recent years, there has been an increasing incidence among younger populations8,9. Furthermore, a significant proportion of LDH patients commonly experience comorbidities involving both sensory pain perception and pain-related emotions, such as anxiety and depression10,11. Consequently, this imposes a substantial burden on societal healthcare systems12. Typically, only 10%-20% of patients with lumbar disc protrusion ultimately require surgical intervention, which is recommended only when conservative treatment proves ineffective13,14. Consequently, both physicians and patients are inclined to seek safe and effective physical therapy methods. Spinal manipulative therapy (SMT) serves as a supplementary therapy for LDH, applying a controllable force to the patient’s lumbar muscles and spine, effectively alleviating low back pain. With its strong comfort, high safety, and low cost, it has been widely used in China and validated by large-sample, multi-center randomized controlled trials15,16. Many scholars have proposed that the perception of chronic pain is closely related to the functional activation of the brain, and the processing of pain signals ultimately occurs in the brain’s highly integrated incoming signals17,18,19,20. Therefore, the brain’s functional regulation in patients with LDH, primarily manifesting as chronic low back pain, warrants in-depth exploration.
Multiple studies have confirmed changes in brain functional connectivity in patients with chronic low back pain due to LDH. For instance, Yu S21 found that midbrain periaqueductal gray matter dysfunction might mediate pain sensitization regulation in chronic low back pain. Mao CP22 noted that changes in the functional connectivity between the amygdala and the prefrontal cortex are related to the pathogenesis of pain. Yu R23 discovered that the periaqueductal gray plays a key role in the descending regulation of pain. Under the premise of manual therapy, Zhang B24 observed abnormalities in the paracentral lobule, supplementary motor area, post-/precentral gyrus, and anterior cingulate cortex in patients with chronic low back pain, and post-manual intervention activations in the insula, amygdala, hippocampus/parahippocampal gyrus, thalamus, and default mode network were noted. Isenburg K25 found that significant network connectivity increases post-manual therapy were related to pain reduction in chronic low back pain patients. However, current literature often observes brain function changes from a single indicator, leading to significant variability in similar studies. This is because the tasks of specific brain network connections can be compensated by other connections in the network or by the network itself26,27. Therefore, we need indicators that reflect higher-level network processes to more scientifically and comprehensively capture the brain’s complex processing of pain signals. In comparison, graph properties apply a robust mathematical quantification framework (such as clustering coefficients, path length, centrality, etc.) that describes and helps understand the efficiency, stability, and optimized state of brain networks28,29. It is noteworthy, however, that there is currently a scarcity of research on the graph properties of brain functions in LDH patients undergoing manual intervention for low back pain relief.
Our preliminary research30 confirmed that a single SMT intervention could significantly alleviate pain in LDH patients, and through low-frequency fluctuation and regional homogeneity analysis, it was discovered that the default mode network, prefrontal cortex, and primary somatosensory cortex areas are involved in regulating its clinical analgesic efficacy. This study provided an evidence base for further exploration into brain networks. Building on this preliminary work, the current study applies higher-level graph property algorithms to investigate key neural biomarkers effective in pain relief for SMT intervention matched with the clinical treatment cycle. Specifically: (1) We conducted SMT interventions corresponding to the clinical treatment cycle and added a ST group to validate the clinical efficacy of SMT. (2) We applied graph property algorithms to more scientifically and comprehensively capture the brain’s complex processing of pain signals. (3) We extracted brain regions significantly correlated with clinical questionnaires as regions of interest (ROIs) for targeted whole-brain functional connectivity analysis, aiming to elucidate key neural biomarkers in the efficacy of SMT intervention for low back pain relief in LDH patients.
Materials and methods
Participants
The Department of SMT at the Third Affiliated Hospital of Zhejiang Chinese Medical University recruited 105 patients who met the inclusion criteria. The sample size for this study satisfies the requirements for statistical reliability31,32. The recruitment period spanned from September 2021 to December 2023. The Institutional Review Board approved this research protocol at the Third Affiliated Hospital of Zhejiang Chinese Medical University, P.R. China. This protocol was registered on ClinicalTrials.gov (NCT06277739) on 26/02/2024. Furthermore, the study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants.
The inclusion criteria were the following (referenced from the 2023 edition of the "Expert Consensus on the Integrated Traditional Chinese and Western Medicine Diagnosis and Treatment of Lumbar Disc Herniation," formulated by the "World Federation of Chinese Medicine Societies," a scholarly institution in China)33: conforms to the diagnostic criteria for lumbar disc herniation (the protrusion types on the central, paracentral, far lateral, foraminal, and subarticular are all applicable), with imaging findings consistent with neurological localization; ages 18 to 65, any gender; right-handed; non-acute phase with mild to moderate pain and functional impairment (duration of symptoms exceeding 2 weeks), VAS score > 434; JOA scores < 1535; has not taken analgesics, neurotropic nutrition drugs, or sedatives in the past month and has not undergone systemic treatment; individuals who have not undergone spinal manipulation or other physical therapies in the past month; voluntarily agrees to participate in this study and has signed an informed consent form.
Exclusion criteria were: Patients with concurrent internal medicine or gynecological conditions that can cause low back pain, such as nephritis, urinary stones, gynecological inflammations, uterine abnormalities, etc.; individuals with severe primary diseases affecting the cardiovascular system, cerebrovascular system, liver, kidneys, etc.; those with neurogenic functional disorders, psychiatric conditions, a history of head trauma, or a history of unconsciousness; individuals with primary sciatica or dry sciatica; those with lumbar spondylolisthesis; patients with lumbar tumors or tuberculosis; individuals with severe osteoporosis or localized skin lesions; painful conditions beyond the lumbar region; diseases characterized by structural alterations in the brain; those with impaired consciousness, severe visual or hearing impairments, speech disorders, and others unable to complete health assessments; individuals with dental implants, metal stents, or other elements that may interfere with MRI results; individuals with a fear of MRI or other reasons preventing MRI scans; and those diagnosed with lumbar disc herniation but without clinical symptoms.
We enrolled age- and gender- matched healthy controls (HCs) with no history of LDH (Group 3) from poster advertisements. The eligibility criteria were as follows: Right-handed; ages 18 to 65, any gender; no history of low back pain; no medication or relevant physical therapy received in the past month; understanding of the research process and willingness to sign the informed consent form.
In this study, the research team implemented several measures to ensure participant retention. However, during the course of the study, some participants were excluded or lost to follow-up. Specifically, six participants with LDH were excluded, and five were lost to follow-up; among HCs, three were excluded, and four were lost to follow-up (Fig. 1). For the participants included in the final analysis, we utilized the per-protocol (PP)36 approach commonly applied in clinical research.
Randomization and blinding
Randomization was conducted using a computer-generated randomization sequence, ensuring that allocation to either SMT or ST was concealed from the participants and the evaluators. The randomization process was monitored by an independent statistician to minimize potential biases. Participants were assigned to their respective groups based on sealed envelopes that contained the allocation sequence, which were opened only after the informed consent was obtained.
To ensure the integrity of the blinding method, both groups underwent identical treatment sessions in terms of time and environment. Evaluators assessing outcomes were blinded to group assignments; they were not involved in the treatment process and were trained to ensure consistent assessment protocols. The ST was designed to mimic the SMT procedure without delivering therapeutic effects, which further upheld the blinding integrity.
Intervention procedure
Group 1 received SMT treatment for 30 min, once every other day, totaling 14 sessions over a duration of 4 weeks. The treatment schedule for Group 2 was identical to that of Group 1.The Group 3 did not receive any intervention.
SMT treatment37: The participant was positioned laterally, facing the clinician, with the more afflicted side oriented upwards. The clinician passively flexed the participant’s hips and knees to induce lumbar flexion until the spinous processes of the affected lumbar vertebrae began to mobilize. Subsequently, the clinician passively rotated the participant’s torso away from the side they were lying on until rotation of the vertebrae above the suspected lesion was perceived. A rapid thrust was applied by the clinician at the shoulder (posteriorly directed force) and pelvis (anteriorly directed force), generating a rotational force couple at the segment in question. If a cavitation (i.e., an audible release) occurred, the treatment was considered complete. In the absence of cavitation, the participant was repositioned and the manipulation attempted again, with a maximum of two attempts per side. If no cavitation was produced after four attempts (i.e., two per side), the treatment was deemed complete.
Sham treatment37(ST): The laser manufacturing entity, MedX Health Corp, furnished a MedX 1100 apparatus which, whilst not discharging considerable quantities of luminous energy or warmth, seemed to be functional to both the participants and the medical practitioners involved. The administration of a placebo laser took place over the region afflicted with discomfort, situating the individuals under study in a manner akin to that specified for the procedures of spinal manipulation and spinal mobilization therapies.
Each session of SMT and ST endures approximately 30 min. Within our research cohort, no complications were reported in either group.
Assessment scales
Visual analogue scale (VAS)34
The VAS is a validated tool for measuring pain intensity. It consists of a horizontal line, typically 10 cm in length, anchored at both ends by descriptors such as “no pain” and “worst pain imaginable.” Patients were instructed to mark a point on the line that corresponded to their pain level. The distance from the “no pain” end to the mark made by the patient was measured in centimeters, providing a score ranging from 0 to 10, with higher scores indicating greater pain intensity. Before the intervention, all participants were trained on how to use the VAS effectively. Pain assessments using VAS were conducted at baseline (before treatment) and immediately after the final treatment session. Each participant’s VAS score was recorded by trained evaluators who ensured accurate documentation and clarified any uncertainties during the scoring process.
Japanese orthopaedic association (JOA) scale35
The JOA scale is a widely used tool for evaluating the functional status of patients with spinal conditions, including LDH. For this study, we specifically used the JOA back pain evaluation questionnaire, which is tailored to assess lumbar spine-related conditions. This JOA evaluates domains such as pain, lumbar function, walking ability, social life function, and mental health. The total score can range from 0 to 29, with higher scores indicating better functional status. Participants completed the JOA scale at baseline and after the treatment cycle. Evaluators provided clear instructions to participants to ensure accurate understanding of each question. Each score was recorded and cross-checked by independent evaluators to maintain consistency and accuracy in the assessment process.
Statistical analysis
The VAS and JOA scores were analyzed using means and standard deviations (M ± SD) for descriptive statistics. Additionally, paired t-tests were performed to compare scores at different time points within the same group, with a significance level set at P < 0.05 to indicate statistically significant differences.
MRI data acquisition
All participants in this study were recruited and assessed at the Massage Department of the Third Affiliated Hospital of Zhejiang Chinese Medical University. Those meeting the inclusion criteria underwent data collection using a Siemens Skyra 3.0 T superconducting MRI scanner at Zhejiang Hospital (MAGNETOM Skyra 3.0 T, Siemens, Germany). To eliminate confounding factors, clinical interventions for patients with LDH were uniformly administered at Zhejiang Hospital, with MRI data collection conducted within 1 h of completing the intervention.
For the LDH group, resting-state functional MRI (rs-fMRI) scans were conducted both before and after immediate treatment. The healthy control group underwent rs-fMRI scanning only at the time of enrollment. Typically, patients exhibit more pronounced pain sensations on the day of their medical consultation. Therefore, to ensure the timely acquisition and reliability of brain function data in response to pain stimuli, MRI scans were uniformly conducted on all study participants on the same day they were enrolled and had completed their baseline data assessment. The scanning sequences were as follows:
(1) T1 Structural Imaging Sequence: Gradient echo sequence 3D-T1W1. TR/TE: 2530 ms/2.89 ms. Flip angle (FA): 9°. Number of layers: 249. Layer thickness/Gap: 1.20 mm/1.00 mm. Voxel size: 1 × 1 × 1 mm3. Field of view (FOV): 256 × 256 mm2. Matrix size: 64 × 64. Resolution: 1 mm. Scan time: 5 min and 53 s.
(2) Rs-fMRI sequence: Echo-planar imaging (EPI) sequence. TR/TE: 2680 ms/30 ms. Number of layers: 43. Layer thickness/Gap: 3.00 mm/1.00 mm. Voxel size: 3 × 3 × 3 mm3. FOV: 192 × 192 mm2. Matrix size: 64 × 64. Scan time: 10 min and 53 s.
FMRI data processing
In this investigation, we refined and streamlined data preprocessing using the Graph Theoretical Network Analysis Toolbox (GRETNA-mater; https://www.nitrc.org/frs/downloadlink.php/10441) within the Matlab 2013b (Mathworks, Natick, MA, USA). The initial step involved transforming raw fMRI data into a format compatible with both GRETNA and SPM12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). To ensure signal consistency and facilitate participant adaptation, the first ten temporal points of the data were excluded.
We then conducted a thorough temporal correction to synchronize the time series across all slices. This was succeeded by a meticulous correction of head motion, employing a six-parameter rigid-body transformation to counteract any participant movement during the scanning process.
For image registration purposes, rs-fMRI scans were co-registered with high-resolution anatomical 3D-T1 scans. Subsequently, these scans were spatially standardized to align with the montreal neurological institute (MNI) template. This process involved the use of diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) for advanced non-linear warping of the 3D-T1 images, followed by the application of the same normalization parameters to the rs-fMRI scans.
We then performed spatial smoothing by applying a Gaussian kernel with a 3 mm full-width at half maximum to the normalized scans, thereby diminishing spatial noise and augmenting signal quality. To preserve the data’s integrity, we ensured that spatial movements and rotations were restricted to under 1.5 mm and 1.5° in any direction.
Finally, the data underwent detrending and filtering. Detrending, primarily aimed at removing noise caused by factors like scanner heating, was conducted using linear regression. Filtering was employed because the blood oxygenation level-dependent (BOLD) signal generated by spontaneous neuronal activity in the resting state is believed to be primarily concentrated in the low-frequency range. Therefore, high-frequency signals such as those caused by breathing and heartbeats were filtered out, while also avoiding low-frequency drift. The frequency band used in this study was 0.01–0.1 Hz.
Graph properties analysis
The establishment of the comprehensive whole-brain network rested upon the amalgamation of nodes (AAL regions) and links (functional connectivity between nodes). Initially, an exhaustive whole-brain parcellation unfolded within the native spatial ___domain, employing the anatomical automatic labeling (AAL 90) atlas (http://braph.org/download/aal90_atlas_atlas/). The AAL90 brain template is widely utilized for its standardization, ease of use, extensive application, research consensus, improved data interpretation, and adaptability in neuroimaging data processing38,39. Specifically, this meticulous process intricately delineated the cerebral expanse into 90 cortical and subcortical regions of interest (45 per hemisphere). Subsequently, adjacency matrices were ingeniously formulated through the utilization of GRETNA, wherein bivariate (Fisher’Z-transformed) correlations between the mean time-series—indicating the average signal across voxels—for each pair of nodes were computed, yielding a 90 × 90 undirected and unweighted correlation matrix.
Following this procedure, using GRETNA, we binarized the adjacency matrices across a varied spectrum of cost thresholds, thereby ensuring a stabilized number of edges at different connection densities. Our network analysis, which encompassed both brain network metrics and nodal metrics, was systematically conducted over a series of connection densities, ranging from 0.050 to 0.275, with increments of 0.02540. This careful gradation was intended to diminish the impact of threshold selection on our findings. Specifically, we utilized the calculated brain network measures to gauge the functional segregation and integration within the brain network. We opted for commonly used measures, including smallworld, betweenness centrality, and a combination of nodal with edge properties41.
In terms of nodal attributes, we calculated the frequency of different nodes appearing on the shortest paths between others, thereby reflecting their importance and influence within the network. To avert the potential issues of overly broad threshold ranges, which could lead to disconnected nodes and networks lacking small-world characteristics, we employed the network-based statistic (NBS)42 correction method. For this, the P-value for edges was set at 0.005, and for components at 0.05, undergoing 5000 iterations of correction. Post-NBS correction, we selected components in PNet with values lower than the corresponding PThrd, extracting these as differential brain regions for inter-group comparison.
We rigorously ensured that all participants demonstrated network attributes indicative of small-worldness, marked by a sigma value greater than 1 across all threshold levels43. This criterion was vital to confirm the presence of small-world network properties in each subject. Finally, we utilized the BrainNet Viewer (https://www.nitrc.org/projects/bnv/) for a visually articulate representation of the brain network, showcasing the strength of relationships between nodes and edges.
Clinical questionnaire correlation analysis
In patients with LDH (Group 1), after SMT treatment, the brain regions showing statistically significant differences are enhanced using betweenness centrality. Subsequently, Pearson44 partial correlation analysis is conducted between these regions and the clinical pain questionnaires. Statistical analyses are performed using Python 3.12.0 (https://www.python.org). The predetermined threshold for statistical significance is set at (P < 0.05), and a correlation coefficient (|r|> 0.3)45 is considered indicative of a significant correlation.
FC analysis of ROI
Extract brain regions significantly correlated with the clinical pain questionnaire as ROIs to further analyze the key brain networks involved in pain relief in LDH patients post-SMT treatment (TP 2). Specifically, we employ RESTplusV 1.8 (https://www.rfmri.org/REST) to extract and calculate the average time series of all voxels within the ROI. This time series is then correlated with the time series of the rest of the brain’s voxels using Pearson correlation analysis to determine FC values. These are subjected to Fisher’s Z transformation to achieve zFC, fulfilling the requirements for normal distribution in statistical analysis. SPM 12 is used to perform paired t-tests on Group 1 pre- and post-treatment. The statistical results are visualized using the xjview 9.5 (https://www.alivelearn.net/xjview/xjview-9-5-released/) toolkit, with the MNI reporting system used for coordinate localization and AAL 90 for brain region labeling. Finally, the BrainNet Viewer toolkit is employed to obtain a three-dimensional map of the entire brain. Brain regions showing statistically significant differences are defined as those with a cluster volume threshold (cluster) ≥ 50 voxels, a single voxel threshold of (P < 0.005) and False discovery rate (FDR)46 correction (P < 0.05) was executed to reduce the rate of Type I errors.
Results
Clinical data
In a prospective study, 59 LDH patients were randomized into two groups (Group 1, n = 28; Group 2, n = 31), with Group 1 receiving SMT and Group 2 undergoing ST. Additionally, a gender and age-matched healthy control group without LDH (Group 3, n = 28) was included. There were no statistically significant differences in gender distribution (M/F: Group 1: 15/13 vs Group 2: 16/15, χ2 = 0.23, P = 0.88; LDH: 31/28 vs HC: 14/14, χ2 = 0.49, P = 0.825), age (Group 1: 33.57 ± 7.31 vs Group 2: 34.23 ± 7.44, t = 0.0675, P = 0.9348; LDH: 33.92 ± 7.32 vs HC: 33.68 ± 7.41, t = 0.139, P = 0.889) or course of the disease (Group 1: 59.50 ± 11.44 vs Group 2: 57.34 ± 10.93, t = 1.05, P = 0.30) among the three groups (Table 1).
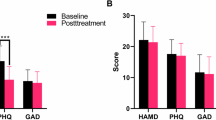
Post-treatment (TP 2), Group 1 showed a significant reduction in VAS scores compared to pre-treatment (TP 1) (P < 0.001) and a significant increase in JOA scores (P < 0.001). In Group 2, there was a significant reduction in VAS scores from pre-treatment (TP 1) (P = 0.0171), but the difference in JOA scores was not statistically significant (P = 0.0463) (Table 2).
Graph properties
In our study, we utilized graph property analysis to examine the frequency with which different nodes appeared on the shortest paths between nodes, reflecting their importance and influence within the network. The findings showed widespread differences in network connectivity between LDH patients and HCs, with the most significant difference in connection strength between left precentral gyrus (left PreCG) (MNI:-38, -5, 50) and left inferior frontal gyrus, opercular part (left IFGoperc) (MNI:-48, 12, 19) (Fig. 2). In Group 1, pre- and post-SMT treatment comparison revealed connectivity differences only between right angular gyrus (right ANG) (MNI:45, -59, 38) and left middle temporal gyrus (left MTG) (MNI:-55, -33, -2) (Fig. 3). For Group 2, pre- and post-ST comparison showed the largest difference in connection strength between right superior parietal gyrus (right SPG) (MNI:26, -59, 62) and left superior temporal-parietal junction (left TPOsup) (MNI:-39, 15, -20) (Fig. 4). Additionally, to verify the specific differences in brain network connections between SMT and ST, we also conducted graph property analysis, identifying two pairs of network connections with the most significant difference between left postcentral gyrus (left PoCG) (MNI: -42, -22, 48) and right hippocampus (right HIP) (MNI: 29, -19, -10) (Fig. 5).
To compare the differences in group-level graph properties both between different groups and in LDH patients pre- and post-treatment, we analyzed their small-world networks. This metric comprehensively combines aspects of local clustering and global integration (Fig. 6).
Correlations of clinical questionnaire score with graph properties
Figure 7 illustrates significant differences in the brain regions right ANG and left MTG in Group 1, observed both pre- and post-SMT treatmen. These regions were scrutinized for their correlation with clinical scales, specifically the VAS and the JOA scores. The analysis revealed that the left MTG did not demonstrate a correlation with the scores from the clinical questionnaires. Conversely, the right ANG exhibited a positive correlation with the rate of change in VAS scores (P < 0.001, r = 0.71) and also showed a positive correlation with the rate of change in JOA scores (P = 0.0015, r = 0.57).
Analysis of the correlation between brain regions with significant differences before and after SMT treatment in Group 1 and the VAS and JOA clinical questionnaires. (a) Correlation between zFC value in the ANG_R at TP2 and the change rate of VAS and JOA scores between TP2 and TP1. (b) Correlation between zFC value in the MTG_L at TP2 and the change rate of VAS and JOA scores between TP2 and TP1.
Screen target brain areas for zFC analysis
To further elucidate the key brain networks involved in alleviating pain in LDH patients following SMT treatment, we identified right ANG—shown to be significantly correlated with the clinical pain questionnaire scores—as the ROI. In examining its zFC with the whole-brain network, we discovered an enhanced zFC between right ANG and the left middle orbital gyrus (left ORBmid) (MNI:-24, 39, -12), indicating a strengthened network connectivity associated with pain relief. Additionally, we observed a weakened zFC between right ANG and the right middle frontal gyrus (right MFG) (MNI:27, 33, 45), suggesting a potential shift in network dynamics following intervention (Fig. 8).
Discussion
In our study, we utilized rs-fMRI and graph property analysis to investigate the brain network topological characteristics of LDH patients with chronic low back pain following SMT. Our key findings revealed extensive differences in network connectivity between LDH patients and HCs, with the most pronounced difference in connection strength observed between left PreCG and left IFGoperc. Notably, after SMT treatment in Group 1, significant changes in connectivity were found only between right ANG and left MTG, with right ANG demonstrating a strong positive correlation with both the VAS and JOA scores. Furthermore, whole-brain network zFC analysis identified enhanced connectivity between right ANG and left ORBmid alongside reduced connectivity with right MFG. To further validate the effectiveness of SMT, we administered ST to another group of LDH patients (Group 2), who exhibited significantly lower clinical questionnaire scores. Their brain network characteristics also differed, with a primary disparity noted in the connectivity between right SPG and left TPOsup. These findings are consistent with previous studies that utilized similar neuroimaging methodologies and approaches, reinforcing the neurophysiological impact of SMT on pain modulation and emphasizing the critical role of right ANG in facilitating pain relief.
Brain functional characteristics associated with low back pain in LDH
We discovered that compared to HCs, patients with chronic low back pain due to LDH exhibit differences in graph attribute connectivity. Notably, the largest difference in connection strength was between left PreCG (MNI:-38, -5, 50) and left IFGoperc (MNI:-48, 12, 19). left PreCG is a key node in the sensorimotor network (SMN), and left IFGoperc is an important node in the salience network (SN). This suggests that, compared to HCs, LDH patients with long-term chronic low back pain exhibit significant activation in the "SMN-SN" neural circuit.
The SMN primarily coordinates and processes sensory input and motor output47,48, which aligns well with the pain perception and disability associated with LDH. Bagg MK49 even proposed that sensorimotor training could reduce pain intensity in patients with low back pain. The SN’s role is mainly to identify and filter out the most important and relevant stimuli or information7,50, playing a significant role in the processing of low back pain signals in LDH patients. Thus, the functions of these brain networks further corroborate the reliability of our findings. In similar research, Li T51 and Pijnenburg M52 have discovered activation and reorganization of the SMN in patients with chronic low back pain. Baumbach P53, using multimodal MRI, found that functional reorganization of the SN is a key pathological mechanism related to central nociception processing in chronic pain states. Furthermore, Tu Y’s54 findings about the functional connectivity of the SMN, SN, and medial prefrontal cortex being correlated with the symptoms of chronic low back pain align with our results. This convergence of findings underscores the significant role of these networks in the context of chronic pain and LDH.
Clinical superiority of SMT
To validate the clinical efficacy of SMT, we established ST group (Group 2) for comparison. The results indicated that after undergoing ST, LDH patients exhibited significantly lower clinical questionnaire scores compared to those receiving SMT treatment, and their brain network characteristics differed, primarily in the connectivity between right SPG (MNI:26, -59, 62) and left TPOsup (MNI:-39, 15, -20).
Regarding clinical efficacy, SMT has been demonstrated to be reliable through large-scale multicenter studies15,16. The key aspect of this technique is the application of leverage principles, which allow for precise displacement of the patient’s lumbar anatomy while reducing the physician’s exertion. In terms of brain function, right SPG is typically associated with pain perception and emotional regulation, likely involving cognitive and emotional responses to pain and primarily belonging to the prefrontal network55. Conversely, left TPOsup is generally related to language processing and social cognitive functions, playing a significant role in emotional regulation and social interactions, usually associated with the temporoparietal network56,57. In pain modulation, the interaction between right SPG and left TPOsup may occur through shared neural mechanisms, thereby regulating emotional and cognitive functions. Specifically, right SPG might influence emotional responses to pain, while left TPOsup could serve a complementary role in emotional regulation, together participating in the pain-relief process. Research has suggested that the analgesic mechanism of manual therapy for LDH-related low back pain involves the brain’s regulatory response, reducing expectations of pain and fear of movement58. Numerous studies have also demonstrated the positive guiding effect of the placebo response on patient treatment59,60. This explains why our study using ST could yield some clinical efficacy and brain network responses.
Brain functional characteristics of SMT in treating low back pain in patients with LDH
In this study, we found that after SMT treatment in Group 1, connectivity differences emerged between right ANG (MNI:45, -59, 38) and left MTG (MNI:-55, -33, -2), with only right ANG showing a significant positive correlation with VAS and JOA clinical questionnaire scores. Consequently, we further extracted right ANG as a ROI for whole-brain network FC analysis and found an enhanced FC between right ANG and left ORBmid (MNI:-24, 39, -12) and a weakened FC with right MFG (MNI:27, 33, 45). In the realm of brain networks, right ANG is an important node of the default mode network (DMN), while left ORBmid and right MFG, to which it connects, are key nodes of the dorsal attention network (DAN). Therefore, we infer that the "DMN-DAN" might be key neural biomarkers for the effectiveness of SMT treatment in alleviating pain in LDH.
The DMN primarily functions in regulating pain perception and cognition, processing pain memory, and making decisions related to pain responses61,62,63. Meanwhile, the DAN focuses on localizing the source of pain, processing pain stimuli, and coordinating with other brain networks64,65. Numerous studies have illustrated that the DMN and DAN often function in conjunction to process tasks66,67,68, which aligns closely with the concept of brain functional network remodeling69. This idea is congruent with our results suggesting that the "DMN-DAN" might be pivotal neural biomarkers for the effectiveness of SMT treatment in alleviating pain in LDH patients. Furthermore, research by Chen70 and Tan71 has indicated that spinal manipulative therapy can alter the functionality of the DMN in LDH patients to provide pain relief. Another study revealed significant activation of the DMN-DAN in acupuncture’s modulation of non-acute sciatica, potentially linked to the importance of the right inferior parietal lobule (right IPL)—which is located near right ANG and also belongs to the DMN—in introspection and emotional responses related to pain72. Presently, there is a lack of direct evidence to affirm that manual therapy can regulate the DAN to alleviate low back pain, underscoring the need for additional research in this ___domain. Our study, by identifying brain regions significantly correlated with clinical outcomes through large-scale brain network screening and then conducting precise ROI analysis on a smaller scale, offers substantial value and reference for future research in this ___domain. This approach allows for a more nuanced understanding of how specific brain networks may be influenced by therapeutic interventions like SMT, potentially leading to more targeted and effective treatments for conditions like LDH.
In summary, our study indicates that the SMN and SN are significantly activated in patients with LDH suffering from chronic pain. Furthermore, the DMN and DAN may serve as key neural biomarkers for the effectiveness of SMT treatment in alleviating LDH-related pain. These findings contribute to the selection of new, safe, and effective treatment modalities for LDH patients and provide important evidence for the neuroscientific explanation of pain relief in LDH. This enhanced understanding of the neural underpinnings of pain and the impact of specific treatments can lead to more targeted and effective therapeutic strategies for LDH, ultimately improving patient outcomes and quality of life.
Limitations
This study has several limitations. Firstly, our clinical questionnaires focused primarily on pain perception, yet in reality, many patients with chronic pain experience negative emotions such as anxiety or depression. In future research, we plan to consider the impact of negative emotions on pain and observe their interactive effects. This approach will offer a more holistic understanding of the patient’s experience and potentially reveal additional insights into how emotional states interact with pain perception and response. Secondly, in our study, we did not conduct repeated rs-fMRI measurements in the healthy control group and the LDH group at the same time interval post-treatment. This means we cannot fully exclude the possibility that the healthy group might exhibit time-dependent brain network effects that differ from those in the LDH group. Future research should include repeated brain network measurements at the same time intervals in the healthy control group to validate the reliability of our findings. Such an approach will help in understanding whether the observed brain network changes are specific to LDH patients and the effects of SMT treatment, or if they are part of normal temporal variations in brain network activity.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- LDH:
-
Lumbar disc herniation
- HCs:
-
Healthy controls
- SMT:
-
Spinal manipulative therapy
- ST:
-
Sham treatment
- fMRI:
-
Functional magnetic resonance imaging
- rs-fMRI:
-
Resting-state functional MRI
- TPs:
-
Time points
- JOA:
-
Japanese Orthopaedic Association
- VAS:
-
Visual Analog Scale
- HCs:
-
Healthy controls
- PP:
-
Per-protocol
- FC:
-
Functional connectivity
- ROIs:
-
Regions of interest
- FA:
-
Flip angle
- FOV:
-
Field of view
- EPI:
-
Echo-planar imaging
- GRETNA:
-
Graph theoretical network analysis toolbox
- MNI:
-
Montreal Neurological Institute
- DARTEL:
-
Diffeomorphic anatomical registration through exponentiated lie algebra
- AAL:
-
Anatomical automatic labeling
- BOLD:
-
Blood oxygenation level-dependent
- NBS:
-
Network-Based Statistic
- FDR:
-
False discovery rate
- SMN:
-
Sensorimotor network
- SN:
-
Salience network
- DMN:
-
Default mode network
- DAN:
-
Dorsal attention network
- left PreCG:
-
Left precentral gyrus
- left IFGoperc:
-
Left inferior frontal gyrus, opercular part
- right ANG:
-
Right angular gyrus
- left ORBmid:
-
Left middle orbital gyrus
- left MTG:
-
Left middle temporal gyrus
- right SPG:
-
Right superior parietal gyrus
- left TPOsup:
-
Left superior temporal-parietal junction
- left PoCG:
-
Left postcentral gyrus
- right HIP:
-
Right hippocampus
- right MFG:
-
Right middle frontal gyrus
- right IPL:
-
Right inferior parietal lobule
References
Zhang, A. S. et al. Lumbar Disc Herniation: Diagnosis and Management. Am J Med. 136(7), 645–651 (2023).
Knezevic, N. N. et al. Low back pain. Lancet. 398(10294), 78–92 (2021).
Himstead, A. S. et al. Trends in Diagnosis and Treatment of Sacroiliac Joint Pathology Over the Past 10 Years: Review of Scientific Evidence for New Devices for Sacroiliac Joint Fusion. Cureus. 13(6), e15415 (2021).
Urits, I. et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 23(3), 23 (2019).
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan 10;385(9963):117–71.
Dammers R, Koehler PJ. Lumbar disc herniation: level increases with age. Surg Neurol. 2002 Sep-Oct;58(3–4):209–12; discussion 212–3.
Kim, J. et al. Altered attentional control over the salience network in complex regional pain syndrome. Sci Rep. 8(1), 7466 (2018).
Dang L, Chen Z, Liu X, et al. Lumbar Disk Herniation in Children and Adolescents: The Significance of Configurations of the Lumbar Spine. Neurosurgery. 2015 Dec;77(6):954–9; discussion 959.
Dang, L. & Liu, Z. A review of current treatment for lumbar disc herniation in children and adolescents. Eur Spine J. 19(2), 205–214 (2010).
Mu, W. et al. Analysis of the depression and anxiety status and related risk factors in patients with lumbar disc herniation. Pak J Med Sci. 35(3), 658–662 (2019).
Heikkinen, J. et al. Depressive disorders, anxiety disorders and subjective mental health in common musculoskeletal diseases: A review. Maturitas. 127, 18–25 (2019).
Lee, C. H. et al. Health Care Burden of Spinal Diseases in the Republic of Korea: Analysis of a Nationwide Database From 2012 Through 2016. Neurospine. 15(1), 66–76 (2018).
Fjeld OR, Grøvle L, Helgeland J, et al. Complications, reoperations, readmissions, and length of hospital stay in 34 639 surgical cases of lumbar disc herniation. Bone Joint J. 2019 Apr;101-B(4):470–477.
Geere JH, Swamy GN, Hunter PR, et al. Incidence and risk factors for five-year recurrent disc herniation after primary single-level lumbar discectomy. Bone Joint J. 2023 Mar 1;105-B(3):315–322.
Wen, Y. et al. A spinal manipulative therapy altered brain activity in patients with lumbar disc herniation: A resting-state functional magnetic resonance imaging study. Front Neurosci. 7(16), 974792 (2022).
Yang, Y. C. et al. The Changes of Brain Function After Spinal Manipulation Therapy in Patients with Chronic Low Back Pain: A Rest BOLD fMRI Study. Neuropsychiatr Dis Treat. 5(18), 187–199 (2022).
Mercer Lindsay N, Chen C, Gilam G, et al. Brain circuits for pain and its treatment. Sci Transl Med. 2021 Nov 10;13(619):eabj7360.
Kuner, R. & Kuner, T. Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol Rev. 101(1), 213–258 (2021).
Perez-Nievas, B. G. From pain to brain. Nat Neurosci. 26(4), 534 (2023).
Tan, L. L., Oswald, M. J. & Kuner, R. Neurobiology of brain oscillations in acute and chronic pain. Trends Neurosci. 44(8), 629–642 (2021).
Yu, S. et al. Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. Neuroimage. 218, 116969 (2020).
Mao, C. P. et al. Altered Amygdala-prefrontal Connectivity in Chronic Nonspecific Low Back Pain: Resting-state fMRI and Dynamic Causal Modelling Study. Neuroscience. 1(482), 18–29 (2022).
Yu, R. et al. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 23(6), 100–108 (2014).
Zhang, B. et al. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth. 123(2), e303–e311 (2019).
Isenburg, K. et al. Increased Salience Network Connectivity Following Manual Therapy is Associated with Reduced Pain in Chronic Low Back Pain Patients. J Pain. 22(5), 545–555 (2021).
Xia, J., Chen, N. & Qiu, A. Multi-level and joint attention networks on brain functional connectivity for cross-cognitive prediction. Med Image Anal. 90, 102921 (2023).
Sporns, O. From simple graphs to the connectome: networks in neuroimaging. Neuroimage. 62(2), 881–886 (2012).
Ciano, G. et al. On Inductive-Transductive Learning With Graph Neural Networks. IEEE Trans Pattern Anal Mach Intell. 44(2), 758–769 (2022).
Bouritsas, G. et al. Improving Graph Neural Network Expressivity via Subgraph Isomorphism Counting. IEEE Trans Pattern Anal Mach Intell. 45(1), 657–668 (2023).
Zhou, X. C. et al. Lever positioning manipulation alters real-time brain activity in patients with lumbar disc herniation: An amplitude of low-frequency fluctuation and regional homogeneity study. Psychiatry Res Neuroimaging. 334, 111674 (2023).
Desmond, J. E. & Glover, G. H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 118(2), 115–128 (2002).
Mayer, E. A. et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 21(6), 579–596 (2009).
Cui, X. & Liang, Q. Expert consensus on the diagnosis and treatment of lumbar disc herniation using integrated traditional Chinese and Western medicine. World Chinese Medicine 18(07), 945–952 (2023).
Lee, J. S. et al. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. 10(10), 1128–1130 (2003).
Haro H, Ebata S, Inoue G, et al. Japanese Orthopaedic Association (JOA) clinical practice guidelines on the management of lumbar disc herniation, third edition - secondary publication. J Orthop Sci. 2022 Jan;27(1):31–78.
Sedgwick, P. Per protocol analysis. BMJ. 7(340), c1825 (2010).
Thomas, J. S. et al. Effect of Spinal Manipulative and Mobilization Therapies in Young Adults With Mild to Moderate Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Netw Open. 3(8), e2012589 (2020).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15(1), 273–289 (2002).
Rolls, E. T. et al. Automated anatomical labelling atlas 3. Neuroimage. 1(206), 116189 (2020).
Bullmore, E. T. & Bassett, D. S. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 7, 113–140 (2011).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 52(3), 1059–1069 (2010).
Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: identifying differences in brain networks. Neuroimage. 53(4), 1197–1207 (2010).
Huang, Y. et al. Small-world properties of the whole-brain functional networks in patients with obstructive sleep apnea-hypopnea syndrome. Sleep Med. 62, 53–58 (2019).
Pearson K. On further methods of determining correlation[M]. Dulau and Company, 1907.
Lynch, C. P. et al. Patient Health Questionnaire-9 Is a Valid Assessment for Depression in Minimally Invasive Lumbar Discectomy. Neurospine. 18(2), 369–376 (2021).
Glickman, M. E., Rao, S. R. & Schultz, M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 67(8), 850–857 (2014).
Cassady, K. et al. Network segregation varies with neural distinctiveness in sensorimotor cortex. Neuroimage. 15(212), 116663 (2020).
Standage, D. I. et al. Whole-brain dynamics of human sensorimotor adaptation. Cereb Cortex. 33(8), 4761–4778 (2023).
Bagg, M. K. et al. Effect of Graded Sensorimotor Retraining on Pain Intensity in Patients With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA. 328(5), 430–439 (2022).
Cushnie, A. K., Tang, W. & Heilbronner, S. R. Connecting Circuits with Networks in Addiction Neuroscience: A Salience Network Perspective. Int J Mol Sci. 24(10), 9083 (2023).
Li, T., Zhang, S. & Kurata, J. Suppressed descending pain modulatory and enhanced sensorimotor networks in patients with chronic low back pain. J Anesth. 32(6), 831–843 (2018).
Pijnenburg, M. et al. Resting-State Functional Connectivity of the Sensorimotor Network in Individuals with Nonspecific Low Back Pain and the Association with the Sit-to-Stand-to-Sit Task. Brain Connect. 5(5), 303–311 (2015).
Baumbach, P. et al. Functional connectivity and neurotransmitter impairments of the salience brain network in chronic low back pain patients: a combined resting-state functional magnetic resonance imaging and 1 H-MRS study. Pain. 163(12), 2337–2347 (2022).
Tu Y, Jung M, Gollub RL, et aj. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain. 2019 Jun;160(6):1308–1318.
Loayza, F. R. et al. Right parietal dominance in spatial egocentric discrimination. Neuroimage. 55(2), 635–643 (2011).
De Benedictis, A. et al. Anatomo-functional study of the temporo-parieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J Anat. 225(2), 132–151 (2014).
Lettieri, G. et al. Emotionotopy in the human right temporo-parietal cortex. Nat Commun. 10(1), 5568 (2019).
Ellingsen, D. M. et al. Brain Mechanisms of Anticipated Painful Movements and Their Modulation by Manual Therapy in Chronic Low Back Pain. J Pain. 19(11), 1352–1365 (2018).
Vase, L. & Wartolowska, K. Pain, placebo, and test of treatment efficacy: a narrative review. Br J Anaesth. 123(2), e254–e262 (2019).
Benedetti, F. Placebo analgesia. Neurol Sci. 27(Suppl 2), S100–S102 (2006).
Kaefer, K. et al. Replay, the default mode network and the cascaded memory systems model. Nat Rev Neurosci. 23(10), 628–640 (2022).
Menon, V. 20 years of the default mode network: A review and synthesis. Neuron. 111(16), 2469–2487 (2023).
Smallwood, J. et al. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci. 22(8), 503–513 (2021).
Kobayashi, K. et al. Relationship between media multitasking and functional connectivity in the dorsal attention network. Sci Rep. 10(1), 17992 (2020).
Majerus, S. et al. The Dorsal Attention Network Reflects Both Encoding Load and Top-down Control during Working Memory. J Cogn Neurosci. 30(2), 144–159 (2018).
Dixon, M. L. et al. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage. 15(147), 632–649 (2017).
Esposito, R. et al. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 12(1), 127–141 (2018).
Lee, D., Park, J. Y. & Kim, W. J. Altered functional connectivity of the default mode and dorsal attention network in subjective cognitive decline. J Psychiatr Res. 159, 165–171 (2023).
Isenburg, K. et al. Functional network reconfiguration supporting memory-guided attention. Cereb Cortex. 33(12), 7702–7713 (2023).
Chen, X. M. et al. Traditional Chinese Manual Therapy (Tuina) reshape the function of default mode network in patients with lumbar disc herniation. Front Neurosci. 15(17), 1125677 (2023).
Tan, W. et al. Spinal Manipulative Therapy Alters Brain Activity in Patients With Chronic Low Back Pain: A Longitudinal Brain fMRI Study. Front Integr Neurosci. 19(14), 534595 (2020).
Liu, C. H. et al. Changes in resting-state functional connectivity in nonacute sciatica with acupuncture modulation: A preliminary study. Brain Behav. 10(2), e01494 (2020).
Acknowledgements
The authors thank Min Lin, Cai-yun Mou, and Li-hao Zhai for their help in the consultation of this neuroimaging trial.
Funding
This study was supported by the General Program of National Natural Science Foundation of China (Grant No. 82274672), the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (Grant No. 2022C03123), the Scientific research project of Zhejiang Provincial Department of Education (No. Y202351266).
Author information
Authors and Affiliations
Contributions
Contributed to the study design: ZXC, CLH, WS, HSW, LZZ, LLJ. Contributed to the data acquisition: ZXC,TY, HHJ, LJ. Contributed to the data analysis and interpretation: ZXC, WKZ, WZC, XYX, YZH. Wrote the manuscript: ZXC, CLH, WS, LZZ, LLJ, YZH, TY, HHJ. Approved the final manuscript: all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Xc., Wu, S., Wang, Kz. et al. Default mode network and dorsal attentional network connectivity changes as neural markers of spinal manipulative therapy in lumbar disc herniation. Sci Rep 14, 29541 (2024). https://doi.org/10.1038/s41598-024-81126-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81126-2