Abstract
Wastewater containing methanesulfonic acid (MSA) mainly originates from the production process of metal detergent, which also contributes significantly to environmental pollution. This study investigates the extraction of MSA from wastewater using trioctylamine (TOA). A thorough investigation was carried out into the factors that affect extraction efficiency, such as the type of extractant, phase ratio (O/A), temperature, stirring speed, and extraction time. An extraction efficiency of 96.1% was achieved using TOA at 25 °C, 1400 r·min− 1, and an extraction time of 30 min. Various techniques including FT-IR, XPS, and high-resolution ESI-MS were employed to investigate the extraction mechanism. The results of different techniques revealed that the complexation between TOA and MSA occurred through ionic and hydrogen bonding interactions. Moreover, TOA was successfully regenerated through back-extraction with sodium hydroxide. The proposed extraction system is advantageous for eco-friendly engineering applications.
Similar content being viewed by others
Introduction
Organic sulfonic acids are widely employed in a variety of sectors, including petroleum chemistry, printing and dyeing, electroplating, and pharmaceuticals. Examples of these include alkyl sulfonic acid, benzene sulfonic acid, and naphthalene sulfonic acid1,2,3,4. The large-scale production in these sectors generates significant quantities of organic sulfonic acid wastewater, which is characterized by its complex composition, high organic content, strong toxicity, and high acidity5,6. One of the most commonly used organic sulfonic acids is methanesulfonic acid (MSA). It is a strong Lewis acid with a pKa of − 1.9, low molecular weight (96.1 g·mol− 1), and a simple structure. MSA is widely used as a salting agent in electro-coating and resin-curing procedures and as a homogeneous catalyst in esterification and alkylation reactions7. Metal salts have a significantly better solubility in MSA than in sulfuric and hydrochloric acids, which makes it very beneficial for a variety of industrial processes8,9.
Several strategies have been developed for treating high concentrations of organic sulfonic acids in wastewaters, including adsorption10,11, reverse osmosis12, advanced oxidation13, bioanalysis14, electrolysis15, and solvent extraction16. However, the expenses of adsorption, electrolysis, or advanced oxidation procedures are prohibitively high due to the normally high content of MSA in these wastewaters, which is between 60 and 80 g·L− 1. Moreover, the high MSA concentration blocks the membrane pores, rendering membrane separation techniques inefficient. Therefore, the best choice for treating wastewater with a high MSA amount is solvent extraction. The benefits of this approach include a quick reaction rate, great selectivity, efficient separation, and low cost17. The extraction of organic compounds is frequently employed as a pretreatment step for biochemical processes in the case of high-concentration organic wastes. With this method, biodegradability is improved and valuable byproducts can be recovered, which has significant advantages for the environment and the economy18,19.
The chemical extraction of different organic acids from aqueous solutions has been the subject of numerous studies in recent years. For example, Kalpana Rewatkar et al.20 used tri-n-butyl phosphate and TOA with hexanol or octanol as diluents for the extraction of gallic acid. Moreover, they investigated the thermodynamics of the extraction process. They found that both enthalpy and entropy were negative, indicating that the formation of the extraction complex increased the orderliness of the system. Lower temperatures favored the extraction because the reaction was exothermic. Furthermore, Pallavi Dandekar et al.21 investigated various polar extractants, including 1-octanol, iso-butanol, toluene, xylenes mix, petroleum ether, and benzene, for the extraction of vanillic acid from wastewater. They demonstrated that the most effective extractant was a neutral solvent that had polarity similar to the organic acid.
Based on Lewis acid-base theory22, Lewis bases are predicted to extract organic acids with great selectivity and good extraction efficiency. For example, Hasan Uslu et al.23 used secondary amines to extract picric acid and found that the extraction rate perc increased with the volume of the extractant while stirring speed demonstrated little effect. Furthermore, N. Thakre et al.24 studied the extraction of citric acid with different extractants and diluents and it was found that the extraction percent of citric acid reached 95.5% when TOA and ethyl acetate were employed. Moreover, the FT-IR analysis showed that the extraction process was driven by hydrogen bond formation. In conclusion, it has been shown that organic amines are useful in recycling organic acids from wastewater. However, there hasn’t been much focus on using them to recover methanesulfonic acid (MSA) from wastewater.
In this work, the MSA was extracted from metal detergent wastewater using solvent extraction (TOA as an extractant). The experimental conditions, extraction mechanism, and the recyclability of the extractant were systematically investigated. Regarding the extraction mechanism, high-resolution ESI-MS, FT-IR, and XPS were employed to investigate the complexation between MSA and TOA. Moreover, the feasibility of regenerating the amine extractant through back-extraction was confirmed. These experimental results provide important theoretical guidance for the industrial treatment of wastewater rich in MSA.
Experimental
Materials
Methyl sulfonic acid (MSA, > 99%), trioctylamine (TOA, > 98%), trialkylamine (N235, > 95%), sodium hydroxide (NaOH, > 99%), tributyl phosphate (TBP, > 98.5%), and methyl isobutyl ketone (MIBK, > 99%) were purchased from Shanghai McLin Biochemical Technology Co., Ltd. (China). Metal detergent wastewater was supplied by Lanzhou Lubricating Oil Research and Development Center (China). All chemicals were used without any further treatment. The MSA solution (1 mol/L) was prepared by dissolving 96.1 g of MSA in 600 mL of deionized water and stirring until the MSA was completely miscible. Following that, the mixture was diluted to a fixed volume of 1 L in a volumetric flask. Moreover, the organic phase solution was prepared by using TOA and N235 as extractants and MIBK and TBP as diluents. The TOA (20 mL) was added to 5 mL of MIBK. In this case, the TOA concentration of the organic phase was 0.65 g/mL, and it was named TOA + MIBK. Further, 20 mL of TOA was added to 5 mL of TBP. In this case, the TOA concentration of the organic phase was 0.64 g/mL, and it was denoted as TOA + TBP.
Methods
(1) Determination of the MSA concentration.
Ion chromatography was used to ascertain the amount of MSA present in wastewater25. The amount of MSA was quantified using ion chromatography (Metrohm 930, Switzerland) with a Hamilton PRP-X100-125/4.0 column. A pH 9 sodium carbonate-sodium bicarbonate buffer was employed as the mobile phase.
(2) Extraction.
In a typical experiment, 50 mL of metal detergent wastewater containing MSA was combined with specific volumes of extractant in a 200 mL conical flask. This mixture was equilibrated for 30 min at a fixed temperature using a thermostated oscillating water bath. Following equilibration, the mixture was allowed to settle for another 30 min, and the two phases were separated using a separation funnel.
The extraction percent (E) and distribution coefficient (D) were calculated using Eq. (1)26 and (2), respectively
Where C0, C1, and C2 represent the concentration of MSA in the solution before and after extraction (mg·L− 1), and the MSA concentration in the organic phase (mg·L− 1), respectively; V0 and V1 denote the volumes of MSA wastewater before and after extraction (L), respectively.
(3) Back extraction.
The organic phase (50 mL) was combined with a specific volume of 1 mol·L− 1 aqueous NaOH solution in a 200 mL conical flask. Following that, the mixture was equilibrated at 25 °C for 30 min using an oscillating water bath. After settling for an additional 30 min, the two phases were separated and the extractant was regenerated in the organic phase.
Instruments
FT-IR (Fourier Transform Infrared Spectrometer, Nicolet iS5, Thermo Fisher Scientific, USA) was employed to investigate the interactions between TOA and MSA in the organic phase. A single drop of the organic phase was placed on an ATR crystal, and the absorption spectrum was recorded in the range of 400 to 4000 cm− 1, with a resolution of 4 cm− 1 and a total scan time of 32 s. XPS (X-ray Photoelectron Spectroscopy) spectra were recorded on a K-Alpha XPS device (Thermo Fisher Scientific, USA) to analyze the elements in the organic phase and their oxidation states.
High-resolution ESI-MS (Electrospray Ionization Mass Spectrometry, Bruker solanX 70 FT-MS, USA) was used to determine the mass-to-charge ratio of organic molecules. The mobile phase for liquid chromatography separation was 90% acetonitrile containing 0.1% formic acid, and the chromatographic column was a BEH C18 with a 1.7 μm particle size and dimensions of 2.l × 50 mm (Waters).
Results and discussion
Effect of an extraction system on the extraction performance of methanesulfonic acid
Figure 1 illustrates the effects of TOA, N235, TOA + MIBK (volume ratio of 4), and TOA + TBP (volume ratio of 4) on the extraction percent of simulated wastewater at 25 °C temperature, stirring speed of 1400 rpm, MSA concentration of 1 mol/L, extraction and standing time of 30 min. Moreover, the effects of phase ratio (the volume ratio of extractant to wastewater, O/A) in the range of 0.2 ~ 0.7 were studied. The extraction efficiencies ranged from 41 to 96.1% with trioctylamine (TOA), and from 32.5 to 87.6% with N235. Both N235 and TOA are tertiary amines with lone-pair electrons on the nitrogen atoms that can form complexes with Lewis acids27. The lower extraction efficiency of N235 (C27H57N) compared to TOA (C24H51N) could be attributed to the steric effect28, where larger substituents on the N atom create a greater shielding effect on the lone-pair electrons.
Next, the extraction performances of TOA + MIBK and TOA + TBP were explored. It is evident from Fig. 1 that the extraction efficiencies ranged from 42.1 to 96% for the TOA + MIBK system and from 43.5 to 96.7% for the TOA + TBP system. The molecular diameter is defined as the distance between the centers of mass of the two closest solvent molecules in a liquid phase16 and represents the molecular space of the solvent. Larger molecular diameter solvents are known to have poorer solvation abilities29. The molecular diameter of TBP is larger than that of MIBK. Moreover, TBP has more C-H bonds due to its alkyl substituents, which enhances its solubility in organic solvents. These two factors contributed to the higher extraction efficiency of the TOA + TBP in comparison to the TOA + MIBK system. Furthermore, there was a slight improvement in the extraction efficiency of TOA + TBP and TOA + MIBK when compared to pure TOA. TOA was chosen as the extractant due to the high cost and challenging regeneration of the two-component extraction systems.
Effect of temperature on the extraction efficiency of MSA
The extraction efficiency of MSA using TOA was investigated at various phase ratios (O/A), fixed stirring speed, and time, over a temperature range of 20 °C to 45 °C. Higher temperatures reduced the extraction performances of all the phase ratios tested (Fig. 2).
The extraction of MSA is a macroscopic process. Assuming that the entropy and enthalpy of an extraction reaction are constant over a certain temperature interval, the equilibrium constant (K) can be described using the Van’t Hoff Eq.
Since the temperature and pressure are constant during the extraction experiment, K can be replaced with the extraction distribution coefficient (D) using the formula16:
Where K represents the equilibrium constant of extraction, R denotes the gas constant (J/(mol⋅K)) and T signifies the temperature (K). α is a proportionality constant. Due to trioctylamine and organic acids complexed by ionic association or hydrogen bonding30,31. The complexation of trioctylamine with methanesulfonic acid may occur at a stoichiometric ratio of 1:1, thus α = 1.
By plotting the lnD against 1/T, the reaction enthalpy change (ΔH) and entropy change (ΔS) were calculated from the slope and intercept of the corresponding fitting line, respectively (Fig. 3). Furthermore, Gibbs free energy (ΔG) of extraction reaction was calculated by the Eq. (6). The values of ΔH, ΔS, and ΔG at 298 K are presented in Table 1.
The calculated ΔH for this reaction was found to be -30.6 kJ·mol− 1. ΔH < 0 indicates that the extraction of MSA using TOA is an exothermic process, resulting in lower extraction efficiencies at higher temperatures. The lowest temperature (20 °C) at which the maximum extraction efficiency was recorded also resulted in the solution emulsifying, which delayed phase separation and left part of the MSA in the aqueous phase.
The calculated ΔG was found to be -6.6 kJ·mol− 1. ΔG < 0 shows that the extraction reaction is an entropy-driven spontaneous process. This is consistent with the experimental results. Therefore, the optimal temperature for MSA extraction was determined to be 25 °C.
Effect of stirring speed on the extraction efficiency of MSA
The extraction of MSA (1 mol/L) using TOA at different stirring speeds at 25 °C for several phase ratios was explored. The extraction efficiency was significantly increased by improving the stirring speed (700–1000 rpm), followed by a gradual increase up to 1400 rpm (Fig. 4).
However, at stirring speeds higher than 1400 rpm, the extraction efficiency slowly decreased. The distribution of MSA between the two phases is highly influenced by the degree of mixing. Lower stirring speeds often result in a slower mass transfer rate between the phases. Conversely, when the stirring speed exceeded 1400 rpm, bubbles formed in the extraction system, leading to emulsification. This made mixing and phase separation more difficult, causing a gradual decline in extraction efficiency. Therefore, 1400 rpm was chosen as the optimal stirring speed for MSA extraction with TOA.
Effect of extraction time on the extraction efficiency of MSA
The duration of the extraction is another important parameter for process optimization. In this study, the effect of extraction time on the extraction efficiency was investigated under the conditions of a temperature of 25 °C, a stirring speed of 1400 rpm, a phase ratio of 0.4, and MSA concentration of 1 mol/L. The results displayed in Fig. 5 demonstrate that the extraction efficiency increased significantly up to 20 min and showed negligible changes between 20 and 30 min. The lower extraction efficiencies at the initial stage could be attributed to the incomplete reaction between TOA and MSA, likely because of the slow mass transfer of both compounds. At the extraction time of 20 min, the extraction efficiency reached 81.8%, with the reaction being essentially complete within 30 min.
Effect of pH on the extraction efficiency of MSA
The pH value has a great effect on the extraction efficiency32. At different pH values, MSA was extracted under the conditions of extraction temperature 25 °C, stirring speed 1400 rpm, O/A = 0.4, extraction and standing time 30 min. The results shown in Fig. 6 indicate that extraction percent decreased as the pH increased.
As an aliphatic amine, TOA can participate in anion exchange under acidic conditions, and extract MSA from wastewater by forming complexes with MSA. In the lower pH range, MSA is mainly present in the aqueous phase in an undissociated form, which promotes the formation of a complex between TOA and MSA, and therefore, at lower pH values. The extraction percent is greater. With the increase in pH value, the MSA in the aqueous phase gradually dissociated, and the MSA involved in the complexation reaction decreased, thus the extraction percent decreased. In addition, under the condition of higher pH value, the content of various forms of MSA in the aqueous phase was lower, which affected the extraction process, resulting in the rapid decrease of the extraction percent and then gradually leveling off.
Extraction mechanism
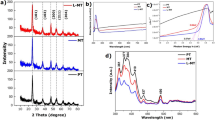
The interactions responsible for the complex formation between TOA and MSA were investigated using FT-IR, XPS, and high-resolution ESI-MS. FT-IR spectra of pure MSA, pure TOA, and TOA-MSA complex are shown in Fig. 7. The S = O stretching vibration of pure MSA, originally at 1103 cm− 1 33, shifted to 1160 cm− 1 upon complexation, indicating a strong interaction. Moreover, the S-OH stretching vibration around 900 cm− 1 disappeared upon the formation of the TOA-MSA complex. The C-N stretching vibration of pure TOA observed at 1098 cm− 1 34, shifted to a lower wavenumber of 1042 cm− 1 when interacting with MSA. These findings collectively suggest a redistribution of electron density between MSA and TOA upon complex formation and chemical reaction between the components.
The changes in FT-IR absorption peak positions and intensities were explained by conjugative and inductive effects. Two molecules interact via atoms or groups of atoms with opposite electron densities. In this case, the electron-rich N atom interacted with the electron-deficient S atom in the S = O group. This interaction altered the electron density and force constants of the corresponding bonds, which are reflected in the FT-IR spectra18. MSA acted as an electron acceptor from TOA, causing the vibrations from MSA to shift to higher frequencies, while those of TOA shifted to lower frequencies. However, when hydrogen bonding or ionic interactions stabilize the complex, these interactions cannot be distinguished by FT-IR alone, so additional techniques such as XPS and high-resolution ESI-MS are required to be employed.
The chemical composition and elemental states of both pure TOA and the TOA-MSA complex were further investigated using X-ray photoelectron spectroscopy (XPS). It is evident from Figs. 8 and 9 that pure TOA comprised primarily of C and N, while sulfur appeared in the TOA-MSA complex. The S 2p spectrum of the TOA-MSA complex (Fig. 9d) was deconvoluted into two peaks at 167.7 and 168.9 eV, which were attributed to the S 2p3/2 and S 2p1/2 peaks, respectively. These peaks were the result of high oxidation states of the sulfur atom, such as those in the sulfonic acid (–SO3H) group35. These findings support the observation of the FT-IR spectrum of the complex development between TOA and MSA.
Furthermore, following complexation with MSA, the N1s binding energy of pure TOA, measured at 401.4 eV and corresponding to the C–N bond, shifted to 401.7 eV (Figs. 8c and 9c). This shift (0.3 eV) could be attributed to the interaction between the S–OH oxygen atom from the sulfonic acid group and the less electronegative N atom of TOA, which reduces the electron density around the nitrogen36,37. The nitrogen atom is in a more oxidized state as a result of this change toward higher binding energy. Moreover, the C 1s spectra showed no appreciable alterations during complexation (Fig. 9a), suggesting that the extraction mechanism did not entail modifications to the chemical state of carbon atoms as a result of interactions with the oxygen atoms of MSA.
As a soft ionization technique, high-resolution ESI-MS enables the analysis of m/z ratios in either positive or negative ionization mode without damaging the structure of the sample. The TOA ESI-MS spectrum acquired in positive ionization mode is displayed in Fig. 10. The spectrum demonstrates a single peak at m/z 354.5, corresponding to the protonated molecular ion of TOA ([C24H51N + H]+).
The ESI-MS spectrum of the TOA-MSA complex, acquired in negative ionization mode, is displayed in Fig. 11a. Similar to other organic acids38,39,40, TOA and MSA formed multiple complexes with distinct structures. Two distinct species with m/z (mass charge ratio) values of 448.9 and 544.4 were identified. These m/z values are speculated to represent 1:1 ([(R3N)·(RHSO3)-H]−) and 1:2 ([(R3N)·(RHSO3)2-H]−) complexes of TOA and MSA, as illustrated in Fig. 11b. The strong hydrogen bonding between methanesulfonic acid molecules led to the formation of MSA dimers bound to TOA41,42. The corresponding peak intensities in the MS spectrum indicated that the dimer was more prevalent than the monomer (1:1 complex).
From the thermodynamic perspective, the formation of larger complexes is facilitated by the “cavity effect”, where larger extraction complexes require less energy to form cavities within the organic solvents, thereby improving the extraction process43,44. Furthermore, the hydrophobic alkyl tails on the outside of the larger complexes encapsulate the hydrophilic groups and prevent them from hydrating, which improves the solubility of the complex in organic solvents.
3.7 Extractant regeneration
Recycling the extractant is important for reactive organic acid extraction to be used on a broad scale in practical applications This is typically achieved by adding a stronger base to deprotonate the organic acid and dissociate the extractant-acid complex. In this instance, 1 mol·L− 1 of sodium hydroxide was added to regenerate pure TOA in the organic phase and back-extract the acid into the aqueous phase.
Figure 12 shows the extraction efficiency of the regenerated TOA over six repeated cycles. The results reveal that the extraction efficiency decreased by only 1%. Moreover, the low aqueous solubility of TOA and the efficient phase separation during the many extraction stages account for the lack of discernible changes in the volume of the organic phase. This indicates that the extractant was successfully regenerated using the suggested back-extraction approach because the TOA amount in the organic phase did not change during the regeneration process.
Conclusion
This study demonstrated that MSA was successfully extracted from metal detergent wastewater using a TOA extractant. The extraction efficiency of MSA reached 96.1% under optimized conditions: a phase ratio(the volume ratio of TOA to wastewater) of 0.6, a temperature of 25 °C, a stirring speed of 1400 rpm, and an extraction time of 30 min. Furthermore, FT-IR, XPS, and high-resolution ESI-MS studies authenticated the development of two TOA-MSA complexes with 1:1 and 1:2 stoichiometries. This was achieved through proton transfer from the sulfonic acid group to TOA and the strong ionic association of the produced species. It was also revealed that MSA lost the hydrogen proton and TOA accepted the hydrogen proton to produce a complex compound. Moreover, the extractant was effectively regenerated through back-extraction, retaining its extraction efficiency throughout several cycles. This suggested extraction method holds considerable promise for engineering applications in recycling MSA from wastewater.
Data availability
All the data is available within the manuscript.
References
Chiang, C. H., Lin, C. C. & Hu, C. C. Effects of Thiourea and Allyl Thioura on the Electrodeposition and microstructures of copper from Methanesulfonic Acid baths. J. Electrochem. Soc. 168, 032505. https://doi.org/10.1149/1945-7111/abec56 (2021).
Losacco, G. L. et al. Expanding the range of sub/supercritical fluid chromatography: advantageous use of methanesulfonic acid in water-rich modifiers for peptide analysis. J. Chromatogr. A. 1642, 462048. https://doi.org/10.1016/j.chroma.2021.462048 (2021).
Koen, N., van Breda, S. V. & Loots, D. T. Metabolomics of colistin methanesulfonate treated Mycobacterium tuberculosis. Tuberculosis 111, 154–160. https://doi.org/10.1016/j.tube.2018.06.008 (2018).
Binnemans, K. & Jones, P. T. Methanesulfonic Acid (MSA) in Hydrometallurgy. J. Sustain. Metall. 9, 26–45. https://doi.org/10.1007/s40831-022-00641-6 (2023).
Thitame, P. V. & Shukla, S. R. Adsorptive removal of naphthalenesulfonic acids using wild almond shell activated carbon from aqueous solution. Env Prog Sustain. Energy. 36, 38–44. https://doi.org/10.1002/ep.12431 (2017).
Kong, K., Cheng, B., Liang, J., Guo, Y. & Wang, R. The aminated covalent organic polymers for reversible removal of concurrent perfluorooctane sulfonate and dichromate. Chem. Eng. J. 446, 137343. https://doi.org/10.1016/j.cej.2022.137343 (2022).
Gernon, M. D., Wu, M., Buszta, T. & Janney, P. Environmental benefits of methanesulfonic acid. Green. Chem. 1, 127–140. https://doi.org/10.1039/A900157C (1999).
Commarieu, A., Hoelderich, W., Laffitte, J. A. & Dupont, M. P. Fries rearrangement in methane sulfonic acid, an environmental friendly acid. J. Mol. Catal. A: Chem. 182–183, 137–141. https://doi.org/10.1016/S1381-1169(01)00506-4 (2002).
Li, H., Hu, Y., Li, L., Xie, Y. & Schaefer, H. F. Synthesis of methanesulfonic acid directly from methane: the cation mechanism or the radical mechanism? J. Phys. Chem. Lett. 12, 6486–6491. https://doi.org/10.1021/acs.jpclett.1c01619 (2021).
Wei, H. et al. Preparation of metakaolin-based geopolymer microspheres (MK@GMs) and efficient adsorption of F- from acidic wastewater. Sep. Purif. Technol. 310, 123159. https://doi.org/10.1016/j.seppur.2023.123159 (2023).
Jin, Z., Wang, X., Sun, Y., Ai, Y. & Wang, X. Adsorption of 4- n -Nonylphenol and Bisphenol-A on magnetic reduced graphene oxides: a combined experimental and theoretical studies. Environ. Sci. Technol. 49, 9168–9175. https://doi.org/10.1021/acs.est.5b02022 (2015).
Lin, S. H. & Juang, R. S. Removal of free and chelated Cu(II) ions from water by a nondispersive solvent extraction process. Water Res. 36, 3611–3619. https://doi.org/10.1016/S0043-1354(02)00074-X (2002).
Saravanan, A. et al. A detailed review on advanced oxidation process in treatment of wastewater: mechanism, challenges and future outlook. Chemosphere 308, 136524. https://doi.org/10.1016/j.chemosphere.2022.136524 (2022).
Huang, Z., Song, Y. D., Zhou, Y. X., He, X. W. & Xu, J. A study of removing 4-Toluene Sulfonic Acid in Wastewater Treatment by Aerobic Bio-fluidized Bed. AMM 522-524 (665-671). https://doi.org/10.4028/www.scientific.net/AMM.522-524.665 (2014).
Vidal, J., Villegas, L., Peralta-Hernández, J. M. & Salazar González, R. Removal of Acid Black 194 dye from water by electrocoagulation with aluminum anode. J. Environ. Sci. Health Part. A. 51, 289–296. https://doi.org/10.1080/10934529.2015.1109385 (2016).
Gai, H. et al. Extraction of 1-amino-2-Naphthol-4-Sulfonic acid from wastewater using trioctylamine (N, N-dioctyloctan-1-amine) in methyl isobutyl ketone. J. Clean. Prod. 201, 774–782. https://doi.org/10.1016/j.jclepro.2018.08.082 (2018).
Julião, D. et al. Desulfurization of diesel by extraction coupled with Mo-catalyzed sulfoxidation in polyethylene glycol-based deep eutectic solvents. J. Mol. Liq. 309, 113093. https://doi.org/10.1016/j.molliq.2020.113093 (2020).
Gai, H. et al. Designing Ionic liquids with Dual Lewis Basic sites to efficiently separate Phenolic compounds from low-temperature coal tar. ACS Sustainable Chem. Eng. 6, 10841–10850. https://doi.org/10.1021/acssuschemeng.8b02119 (2018).
Li, S., Zhuang, J., Zhi, T., Chen, H. & Zhang, L. Combination of complex extraction with reverse osmosis for the treatment of fumaric acid industrial wastewater. Desalination 234, 362–369. https://doi.org/10.1016/j.desal.2007.09.105 (2008).
Rewatkar, K., Shende, D. Z. & Wasewar, K. L. Effect of temperature on reactive extraction of gallic acid using Tri- n -butyl phosphate, Tri- n -octylamine and aliquat 336. J. Chem. Eng. Data. 61, 3217–3224. https://doi.org/10.1021/acs.jced.6b00310 (2016).
Dandekar, P. & Wasewar, K. L. Experimental investigation on extractive separation of vanillic acid. Chem. Data Collections. 30, 100564. https://doi.org/10.1016/j.cdc.2020.100564 (2020).
Hu, H., Yang, M. & Dang, J. Treatment of strong acid dye wastewater by solvent extraction. Sep. Purif. Technol. 42, 129–136. https://doi.org/10.1016/j.seppur.2004.07.002 (2005).
Uslu, H., Datta, D. & Bamufleh, H. S. Extraction of Picric Acid from Wastewater by a secondary Amine (Amberlite LA2) in Octan-1-ol: equilibrium, Kinetics, thermodynamics, and Molecular Dynamics Simulation. Ind. Eng. Chem. Res. 55, 3659–3667. https://doi.org/10.1021/acs.iecr.5b04750 (2016).
Thakre, N. et al. Modeling and optimization of reactive extraction of citric acid. J. Chem. Eng. Data. 61, 2614–2623. https://doi.org/10.1021/acs.jced.6b00274 (2016).
Bruzzoniti, M. C., De Carlo, R. M. & Sarzanini, C. Determination of sulfonic acids and alkylsulfates by ion chromatography in water. Talanta 75, 734–739 (2008). https://www.sciencedirect.com/science/article/pii/S0039914007008673
Jiao, P. et al. The recovery of gallic acid from wastewater by extraction with tributyl phosphate/4-methyl-2-pentanone/n-hexane, tributyl phosphate/n-octanol/n-hexane and n-hexanol. RSC Adv. 6, 93626–93639. https://doi.org/10.1039/C6RA13470J (2016).
Sarma, B. et al. Highly active primary amine ligated Ru(II)-arene complexes as selective catalysts for solvent-free N-alkylation of Anilines. Mol. Catal. 548, 113440. https://doi.org/10.1016/j.mcat.2023.113440 (2023).
Banert, K. & Seifert, J. Steric hindrance classified: treatment of isothiocyanatoallene with secondary amines bearing bulky substituents to generate 2-aminothiazoles. Org. Chem. Front. 6, 3517–3522. https://doi.org/10.1039/C9QO00312F (2019).
Li, X., Kersten, S. R. A. & Schuur, B. Extraction of acetic acid, glycolaldehyde and acetol from aqueous solutions mimicking pyrolysis oil cuts using ionic liquids. Sep. Purif. Technol. 175, 498–505. https://doi.org/10.1016/j.seppur.2016.10.023 (2017).
Tamada, J. A., Kertes, A. S. & King, C. J. Extraction of carboxylic acids with amine extractants. 1. Equilibria and law of mass action modeling. Ind. Eng. Chem. Res. 29, 1319–1326. https://doi.org/10.1021/ie00103a035 (1990).
Prochazka, J., Heyberger, A., Bizek, V., Kousova, M. & Volaufova, E. Amine extraction of hydroxycarboxylic acids. 2. Comparison of Equilibria for Lactic, Malic, and citric acids. Ind. Eng. Chem. Res. 33, 1565–1573. https://doi.org/10.1021/ie00030a016 (1994).
Song, Y. et al. Treatment of cyanide-bearing wastewater by the N263-TBP synergistic extraction system. Chemosphere 291, 133052. https://doi.org/10.1016/j.chemosphere.2021.133052 (2022).
Masoud, M. S., Ramadan, A. M., Soayed, A. A. & Ammar, S. M. S. Solvatochromic responses and pH effects on the electronic spectra of some azo derivatives of 1-amino-2-hydroxy-4-naphthalenesulfonic acid. J. Iran. CHEM. SOC. 13, 931–943. https://doi.org/10.1007/s13738-016-0809-y (2016).
Chen, F. et al. Selective extraction of nitric and acetic acids from etching waste acid using N235 and MIBK mixtures. Sep. Purif. Technol. 169, 50–58. https://doi.org/10.1016/j.seppur.2016.06.008 (2016).
Nasef, M. M. & Saidi, H. Surface studies of radiation grafted sulfonic acid membranes: XPS and SEM analysis. Appl. Surf. Sci. 252, 3073–3084. https://doi.org/10.1016/j.apsusc.2005.05.013 (2006).
Yang, J. P., Hu, H. P., Cheng, Z. Y., Qiu, X. J. & Wang, C. X. Structural insights into the coordination and selective extraction of copper(II) by tertiary amine ligands derived from 2-aminomethylpyridine. Polyhedron 128, 76–84. https://doi.org/10.1016/j.poly.2017.02.037 (2017).
Sun, X. et al. The inner synergistic effect of bifunctional ionic liquid extractant for solvent extraction. Talanta 81, 1877–1883. https://doi.org/10.1016/j.talanta.2010.03.041 (2010).
Kunimoto, M. et al. Spectroscopic and computational analyses of liquid–liquid interfacial reaction mechanism of Boric Acid Esterification with 2,2,4-Trimethyl-1,3-pentanediol in Boron extraction processes. J. Phys. Chem. C. 122, 10423–10429. https://doi.org/10.1021/acs.jpcc.8b01086 (2018).
Ren, X. et al. Utilization of the dilute acidic sulfate effluent as resources by coupling solvent extraction–oxidation–hydrolysis. J. Hazard. Mater. 299, 702–710. https://doi.org/10.1016/j.jhazmat.2015.08.003 (2015).
Kakku, S. et al. Reactive extraction of Gluconic Acid using trioctylamine in different diluents. Chem. Eng. Technol. 45, 417–424. https://doi.org/10.1002/ceat.202100373 (2022).
Chen, J., Yang, X. J., Xie, Q. Y. & Wu, J. G. Solvent extraction of gold from Alkaline Aurocyanide solutions by Cationic Surfactants. Progress Chem. 21, 1583 (2009).
Zhang, Q. et al. Highly Effective Removal of Metal Cyanide Complexes and Recovery of Palladium Using Quaternary-Ammonium-Functionalized MOFs. Molecules 23, (2086). https://doi.org/10.3390/molecules23082086 (2018).
Jiang, J., Zhou, W., Gao, H., Wu, J. & Xu, G. Solvent extraction and stripping of gold(I) cyanide in the tetradecyldimethylbenzylammonium chloride system. Hydrometallurgy 70, 73–81. https://doi.org/10.1016/S0304-386X(03)00047-1 (2003).
Jiang, Z. et al. Mechanism of boric acid extraction by trioctylamine and tartaric acid. Sep. Purif. Technol. 331, 125597. https://doi.org/10.1016/j.seppur.2023.125597 (2024).
Acknowledgements
This project is supported by the National Natural Science Foundation of China (52364055), Department of Education of Gansu Province: Major cultivation project of scientific research innovation platform in university (2024CXPT-14), National Key R&D Program of China (2015BAE04B01).
Author information
Authors and Affiliations
Contributions
J .Z.(Conceptualization, Data curation, Writing – original draft); Y. J.(Methodology, Investigation, Writing – original draft, Writing – review and editing); C. j. W.(Visualization, Writing – review and editing); H. r. L. (Formal analysis, Writing – review and editing); Y.f. L.(Resources, Validation).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, J., Jia, Y., Wei, C. et al. Reactive extraction of methanesulfonic acid from wastewater using trioctylamine. Sci Rep 14, 30029 (2024). https://doi.org/10.1038/s41598-024-81916-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81916-8