Abstract
Unregulated, systemic inflammation negatively impacts health and production in dairy cows. Soluble mediators and platelets have been studied for their expansive role in mediating inflammation. Our objectives were to compare the plasma oxylipin and endocannabinoid profiles, and the platelet and plasma proteomic profiles of healthy cows to cows experiencing elevated systemic inflammation as indicated by plasma haptoglobin (Hp) concentrations. Postpartum cows at 3 DIM with plasma Hp concentrations \(\:\ge\:\) 0.50 g/L and no clinical disease were enrolled into the high-inflammation group (n = 8). Cows with plasma Hp concentrations \(\:\le\:\) 0.1 g/L and no clinical disease were enrolled into the low-inflammation group (n = 8). Targeted lipidomic analysis revealed differences in the plasma oxylipin and endocannabinoid profile between high- and low-inflammation cows. Cows in the high-inflammation group had increased plasma concentrations of the oxylipins 9(S)-HpOTrE, 9(S)-HOTrE, 13(S)-HpOTrE, and 9,10-EpOME, and the endocannabinoid anandamide. In-depth proteomic analysis of platelets between the high- and low-inflammation groups revealed significant differences in protein categories related to platelet granule release and cellular iron uptake. Proteomic outputs from plasma revealed 24 proteins to be different between high and low-inflammation groups, including proteins involved in autophagy and immune mediation. Together, our results indicate that cows experiencing an exacerbated systemic inflammatory response in the postpartum may have impaired disease resistance, and platelets could be contributors to their inflammatory state.
Similar content being viewed by others
Introduction
Unregulated, postpartum systemic inflammation can result in significant production losses and negative health outcomes in dairy cows1,2,3; therefore, identifying mediators of inflammation is of critical interest to the dairy industry. Soluble mediators, such as oxylipins, have been studied for their expansive role in mediating inflammation4,5,6,7. These oxidized polyunsaturated fatty acids (PUFA) have been described to exert both pro- and anti-inflammatory effects across numerous tissues and have begun to be characterized in dairy cows8,9,10. Notably, oxylipins derived from the oxidation of α-linolenic acid (ALA) and linoleic acid (LA), such as 9(S)-HOTrE, 9,10-DiHOME, and many others have been studied as mediators of inflammation in humans and mouse models. More recently, they have been identified in the plasma of dairy cows with systemic inflammation10,11,12.
In addition to oxylipins, studies have highlighted endocannabinoids as soluble mediators of inflammation13,14,15,16. The endocannabinoid system is a neuro-modulatory system comprising endogenous cannabinoids (endocannabinoids), cannabinoid receptors, and the enzymes that synthesize or degrade these soluble mediators17. Endocannabinoids are endogenous lipids derived from arachidonic acid that are capable of binding to cannabinoid receptors and are found in nearly all tissues. Cannabinoid receptor 1 (CB1) is primarily expressed in the central nervous system, and cannabinoid receptor 2 (CB2) is mainly expressed by immune cells and associated with several immune functions17,18,19. For example, one study utilizing an inflamed murine model reported a significant increase in the expression of CB2 receptors, while others have demonstrated the role of endocannabinoids to function as modulators of cytokine production and inflammatory cell migration20,21. Research on endocannabinoid function in inflammation has primarily occurred in humans and mouse models, although few studies have investigated the role of endocannabinoids in modulating adipose tissue metabolism and inflammation in postpartum dairy cows22,23. To our knowledge, no studies have investigated the differences in the circulating concentration of these mediators (anandamide and 2-arachidonoylglycerol) in dairy cows experiencing systemic inflammation.
In addition to soluble mediators, platelets have been recognized for their ability to contribute to inflammation or immune responses. Platelet activation occurs in response to acute endothelial injury and binding to agonists, such as exposed collagen, thrombin, adenosine diphosphate (ADP), or thromboxane A2 (TxA2)24,25. Platelet binding to agonists activates and releases storage granules known as dense granules, α-granules, and lysosomes, which act in autocrine and paracrine signaling24. It is unknown whether these activation mechanisms are always present during systemic inflammation. Instead, interactions of select oxylipins with platelets can promote their activation and granule release in the absence of vascular injury, as well as exposure to oxidative stress26,27,28. Anandamide (an endocannabinoid), for instance, is known to reduce platelet activation in vitro29.
Platelet α-granules contain transmembrane proteins such as P-selectin, CD40 ligand (also known as CD154), and inflammatory cytokines such as transforming growth factor-β (TGF-β) and interleukin-1β (IL-1β), which have been shown to contribute directly to inflammation29. Several studies have implicated platelets as inflammatory mediators by stimulating a proinflammatory response in leukocytes and helping induce the acute phase response30,31,32. However, research on the role of platelets in inflammation has been predominantly conducted in human and murine models of acute inflammation. Therefore, potentially characterizing the platelet proteome composition in dairy cows experiencing elevated markers of systemic inflammation in the early postpartum can potentially help with the understanding of their involvement in this condition.
This study aimed to compare the targeted plasma oxylipin and endocannabinoid profiles, and the platelet and plasma proteomes of cows experiencing elevated systemic inflammation in the early postpartum. We hypothesized that cows experiencing greater systemic inflammation would have increased circulating concentrations of proinflammatory oxylipins and endocannabinoids, and an increased abundance of proinflammatory-related proteins in platelets and plasma compared to healthy cows.
Results
Descriptive statistics
A total of 16 cows (8 per group) were categorized into the inflammation groups based on plasma Hp concentrations. The high-inflammation group had six primiparous and two multiparous cows, and a mean plasma Hp and fibrinogen concentrations of 0.71 \(\:\pm\:\) SEM 0.09 g/L and 0.73 \(\:\pm\:\) SEM 0.05 g/dL, respectively. The low-inflammation group was comprised of three primiparous and five multiparous cows, and a mean plasma Hp and fibrinogen concentrations of 0.03 \(\:\pm\:\) SEM 0.01 g/L and 0.44 \(\:\pm\:\) SEM 0.03 g/dL, respectively. No cows were enrolled in this study if they had received antibiotic treatment or were diagnosed with a clinical disease before or immediately following sampling. One cow from the low inflammation group was excluded from the platelet and plasma proteomic analyses due to insufficient sample volume.
There was a significant difference in the blood concentration of platelets isolated between the high- and low-inflammation groups. The high-inflammation group had a mean platelet count of 292 × 103\(\:\pm\:\) SEM 33 × 103 cells/µL, and the low-inflammation group had a mean platelet count of 173 × 103\(\:\pm\:\) SEM 15 × 103 cells/µL (P = 0.005).
Targeted plasma lipidomic analysis
We targeted the identification of seven oxylipins and two endocannabinoids, all of which had detectable concentrations (Table 1). Our targets included oxylipins originating from the lipoxygenase (LOX) and cytochrome P450 (CYP450) pathways from ALA and LA, and endocannabinoids originating from arachidonic acid. These targets were selected based on results obtained by our group in a prior study10, with the aim of building up evidence for their association with an exacerbated systemic inflammatory state.
Results from the analysis of plasma oxylipins and endocannabinoid concentrations are shown in Table 2. Cows in the high-inflammation group had significantly greater plasma concentrations of 9(S)-HpOTrE, 9(S)-HOTrE, 13(S)-HpOTrE, and 9,10-EpOME compared to cows in the low-inflammation group. Additionally, cows in the high-inflammation group had greater plasma concentrations of the endocannabinoid AEA compared to cows in the low-inflammation. No other differences were identified between the inflammatory groups for the other analyzed compounds.
Label-free quantitative proteomic analysis
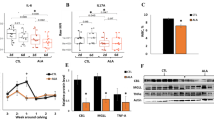
In total, 8,075 platelet proteins were detected. At a 1% false discovery rate (FDR), 2,074 unique proteins were identified with high confidence in the platelets of cows with varying degrees of systemic inflammation (Supplemental Table 1). A principal component analysis (PCA) revealed that the high-inflammation and low-inflammation groups clustered, indicating a significant difference in the proteomes between the treatment groups (Fig. 1A).
(panel A) PCA plot representing the differences in platelet protein expression between high- and low-inflammation cows. (panel B) Volcano plot representing the differentially abundant platelet proteins between cows with high inflammation compared to cows with low inflammation; increased (blue dots in the top right quadrant) and decreased (red dots in the top left quadrant) proteins are highlighted. The P-value (\(\:\le\:\) 0.05) is indicated on the Y-axis, and the fold change is indicated on the X-axis. (panel C) Gene Ontology (GO) Enrichment analysis displaying the biological processes for the differentially abundant proteins; bars indicate the fold enrichment for each biological process.
The relative abundances of 84 proteins were higher, and of 71 proteins were lower, in platelets isolated from cows in the high-inflammation group compared to the low-inflammation group (Fig. 1B, Supplemental Table 2).
The proteins identified in this study belonged to different classes, including those involved in molecular carrier activities, enzyme binding, nucleotide binding, the glucose metabolic process, chloride channel regulator activities, chloride channel inhibitor activities, and regulation of cell-cell adhesion mediated by integrin (Fig. 1C).The protein identified in platelets with the greatest fold change in the high-inflammation group was haptoglobin, with an approximately 4-fold increase in abundance compared to cows in the low-inflammation group (Fig. 2B, Supplemental Table 2). An additional protein identified in platelets from high-inflammation cows was the Unc-13 homolog D (UNC13D) protein, which had a 2-fold increase in abundance compared to low-inflammation cows. In contrast, transferrin receptor protein 1 (TFRC1) had a 5-fold lower abundance in high-inflammation cows compared to the low-inflammation group. Similarly, high-inflammation cows had a 3.5-fold decrease in the abundance of vesicle-associated membrane protein 8 (VAMP8) compared to cows in the low-inflammation group.
(panel A) Volcano plot representing the differentially abundant plasma proteins between cows with high inflammation compared to cows with low inflammation; increased (blue dots in the top right quadrant) and decreased (red dots in the top left quadrant) proteins are highlighted. The P-value (\(\:\le\:\) 0.05) is indicated on the Y-axis, and the fold change is indicated on the X-axis. (panel B) Venn diagrams representing the overlap between differentially abundant plasma and platelet proteins.
In total, 1,016 proteins were identified in the plasma proteome. At a 1% FDR, 331 unique proteins were identified with high confidence in the plasma from cows with varying degrees of systemic inflammation (Supplemental Fig. 1, Supplemental Table 3). Twenty-four differentially abundant proteins were identified between high- and low-inflammation groups (Fig. 2A, Supplemental Table 4). Cows classified into the high-inflammation group had four proteins with a higher relative abundance compared to low-inflammation cows. The protein endosome/lysosome-associated apoptosis and autophagy regulator family member 2 (ELAPOR2) had a 5-fold higher abundance in high-inflammation cows than in the low-inflammation group. There were 20 proteins with a lower relative abundance in high-inflammation cows compared to low-inflammation cows. Guanylate cyclase activator 2A (GUCA2A) is another protein identified in this study that had an approximate 3-fold decrease in abundance in the high-inflammation group compared to low-inflammation cows.
A comparison of differentially abundant proteins between the platelet and plasma samples is illustrated in Fig. 2B. When comparing the proteomes, there was no overlap of upregulated proteins. For downregulated proteins, conglutinin was the only protein with an overlap between the platelet and plasma proteomes. In both the platelet and plasma proteomes, conglutinin had an approximate 2-fold decreased abundance in high-inflammation cows compared to low-inflammation cows.
Discussion
This study investigated differences in the plasma oxylipin and endocannabinoid profiles and the platelet and plasma proteomes in postpartum dairy cows with varying degrees of systemic inflammation. To our knowledge, this is the first study that has analyzed the bovine platelet proteome and mapped out differences in platelet proteins between cows with high and low postpartum systemic inflammation. Our results indicate the potential for platelets to contribute to systemic inflammation through pathways involved in regulating molecular and biological functions and different signaling systems.
Recently, our group and others have reported differences in the oxylipin profile of postpartum dairy cows with varying degrees of systemic inflammation or clinical disease, and have suggested specific oxylipins as suitable biomarkers for these conditions9,10,33,34. Similarly, results from the current study also revealed differences in circulating plasma oxylipin profiles. Furthermore, our data indicates differences in the plasma endocannabinoid profile of postpartum dairy cows experiencing systemic inflammation. Of note, cows that were classified into the high-inflammation group had greater circulating concentrations of ALA-derived oxylipins, starting with 9(S)-HpOTrE, which is oxidized to 9(S)-HOTrE and 9-OxoOTrE upon further oxidation35. These oxylipins are derived from 5-lipoxygenase (5-LOX) activity on α-linolenic acid. Little is known about the function of these oxylipins; however, it has been proposed that they have anti-inflammatory effects36,37,38. For example, a study investigating the effects of ALA treatment on lipopolysaccharide (LPS)-stimulated human M1 macrophages reported a reduction in LPS-induced production of the proinflammatory cytokines interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α)38. Furthermore, after LPS stimulation, the group reported increased production of ALA-derived oxylipins by M1 macrophages. Therefore, it is possible that cows in the high-inflammation group have increased circulating concentrations of ALA-derived oxylipins to restore homeostasis and prevent a further inflammatory response.
In the present study, the plasma concentrations of 9(S)-HpOTrE and its derivative compound, 9(S)-HOTrE, differed between treatment groups, where cows in the high-inflammation group had higher plasma concentrations compared to low-inflammation cows. Recent research has shown 9(S)-HpOTrE to have a significant anti-inflammatory effect on LPS-stimulated murine RAW 264.7 macrophages11. Kumar et al. reported that stimulated macrophages had a 52.7% reduction in nitric oxide (NO) concentrations in the presence of 9(S)-HpOTrE. Furthermore, the group reported significantly reduced production of inducible nitric oxide synthase (iNOS), TNF-α, and IL-1β by inhibiting nuclear factor-κB (NF-κB) translocation to the nucleus in murine macrophage in vivo samples. 9(S)-HOTrE has been described to exert an anti-inflammatory effect by activating peroxisome proliferator-activated receptor alpha (PPARα), which represses NF-κB signaling and thus limits proinflammatory cytokine production39,40,41. The noted increase in the circulating plasma concentrations of ALA-derived oxylipins identified in this study (9(S)-HpOTrE, 9(S)-HOTrE, 9-OxoOTrE, and 13(S)-HpOTrE) may indicate that high-inflammation cows were attempting to restore homeostasis as we observed increased concentrations of these anti-inflammatory oxylipins.
High-inflammation cows had increased concentrations of 9,10-EpOME compared to low-inflammation cows. This oxylipin is derived from linoleic acid oxidation by cytochrome P450 and is generally considered pro-inflammatory42,43. For example, 9,10-EpOME has been shown to exert proinflammatory effects on porcine endothelial cells by activating NF-κB and activator protein-1 (AP-1) transcription factors and inducing oxidative stress44. 9,10-EpOME, as well as its derivative 9,10-DiHOME, have previously been identified in the plasma of dairy cows; however, the ability of these oxylipins to contribute to negative health outcomes, such as systemic inflammation or clinical disease, is still unknown9,10. The oxylipins identified in this study may indeed contribute to the inflammatory response in postpartum dairy cows, though more research is needed to fully understand their biological relevance.
Endocannabinoids are part of a versatile system involved in numerous processes, including inflammation45. To our knowledge, no studies have been published describing the circulating endocannabinoid profile of dairy cows experiencing systemic inflammation, as characterized by plasma Hp concentrations. Our current study revealed an increase in plasma concentrations of AEA in high-inflammation cows compared to low-inflammation cows. AEA is an endocannabinoid synthesized by the cleavage of the membrane precursor N-arachidonoyl phosphatidylethanolamine (NAPE) by phospholipase D (NAPE-PLD)46. Although no research has elucidated the function of AEA in dairy cows, several studies have demonstrated the ability of AEA to exert an anti-inflammatory effect in human and murine cells47,48,49. For example, pro-inflammatory stimulation of human and murine aortic smooth muscle cells resulted in the increased production of AEA, suppressed the production of proinflammatory cytokines, and significantly reduced leukocyte infiltration47,48.
During periods of increased inflammation, dairy cows experience elevated circulating concentrations of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-650,51,52. The cows in the high-inflammation group may have had elevated plasma concentrations of AEA as an anti-inflammatory response that functions in an attempt to restore homeostasis.
Numerous studies have been published linking activated platelets to immune or inflammatory responses. However, no studies have investigated the role of platelets in dairy cows experiencing systemic inflammation. As such, this study was designed to investigate the platelet proteome in postpartum dairy cows with systemic inflammation as a manner to gain some insights. Notably, platelets isolated from cows categorized into the high-inflammation group had an increased abundance of Hp compared to the low-inflammation cows. Hp is a positive acute-phase protein produced primarily by the liver in response to inflammation53. However, a study that evaluated the mRNA abundance expression of Hp in LPS-challenged mice reported induced Hp gene expression in extrahepatic tissues such as the spleen, thymus, heart, kidney, lung, and epididymis54. Very few studies have identified haptoglobin as a differentially abundant protein present in platelets, and none have described the origin of this protein in platelets55. Cows were enrolled in this study based on their plasma Hp concentrations at 3 DIM, however the difference in abundance of platelet Hp is still unknown. It remains to be evaluated if platelets can secrete Hp or if the higher abundance of this protein results from Hp binding to receptors on activated platelets. While previous studies have reported Hp gene expression in extrahepatic tissues, no studies have reported Hp expression in platelets. Interestingly, a study completed by Khan et al. (2022) revealed that the presence of Hp in plasma prevented platelet aggregation by inhibiting cyclooxygenase TxA2 formation in human platelets56. The results from Khan et al., as well as those in the present study, indicate a relationship between platelets and Hp that is not yet fully understood in dairy cows; therefore, future studies should focus on elucidating the possible role of platelets in Hp secretion.
Several other differentially abundant platelet proteins identified in dairy cows with increased inflammation are of interest, namely, the downregulated protein TFRC1. TFRC1 is a well-known transmembrane protein found in nearly all cells and is responsible for binding iron-bound transferrin and mediating cellular iron uptake57,58,59. Numerous studies have demonstrated the relationship between serum iron concentrations and inflammation in dairy cows60,61,62,63. In the referenced studies, cows experiencing inflammation, as indicated by elevated Hp concentrations, had significantly decreased serum iron concentrations compared to control cows. The importance of iron concentrations during periods of elevated inflammation was further corroborated by our findings, where the platelets from high-inflammation cows had a decreased abundance of TFRC1, which occurs in response to inflammation64,65.
The methods utilized in this study produced highly purified platelet samples, as was evident by the strong clustering of treatment groups in the platelet PCA plot and the lack of overlap in the Venn Diagrams that compare the differentially abundant platelet and plasma proteins. Surprisingly, the greatest variation within the platelet proteome, as indicated by the platelet PCA plot, was the variation between individual cows in the low-inflammation group. The high- and low-inflammation categorizations are an attribute of the data that contributes to the second greatest amount of variation, as indicated by the distinct clustering of high- and low-inflammation data points. Splits in the clustering of data points in PCA plots occur when there is a \(\:\ge\:\)20% difference between groups, thus indicating genuine increased variability of proteomics data in the low-inflammation cows compared to the high-inflammation cows. Due to the study design and sample size, we cannot determine the cause of this split in the low-inflammation group, though minute differences in plasma Hp concentration, or differences in protein extraction efficiency, are plausible explanations. Among the nearly 200 differentially abundant proteins identified in the bovine platelet and plasma proteomes, conglutinin was the only differentially abundant protein identified across both datasets. Conglutinin is a collectin protein and an important mediator of immune and inflammatory responses66,67. Collectins, including conglutinin, are mediators of the innate immune response. They function as soluble pattern-recognition receptors that recognize surface carbohydrates on pathogens and organize their removal by neutralization, agglutination, and opsonization66,68. Additionally, conglutinin uniquely binds the complement protein iC3b, demonstrating its involvement in immune and inflammatory processes68. An early study demonstrated a negative correlation between plasma conglutinin levels and disease incidence in cattle, where cows with lower plasma concentrations of conglutinin had a higher incidence of disease69. Conglutinin binds to sugar residues on microbial surfaces to subsequently induce oxidative burst in phagocytes and eliminate pathogens67. In our study, cows with high inflammation had a decreased abundance of conglutinin in their plasma and platelets, which may contribute to an impaired innate immune response that predisposes cows to disease and inflammation.
Plasma proteomics data revealed a 5-fold increase in the abundance of the ELAPOR2 protein in high-inflammation cows compared to low-inflammation cows. This protein’s function is poorly understood, though it is predicted to be involved in cellular autophagy. The Human Protein Atlas has reported RNA expression of ELAPOR2 in numerous tissues, including the brain, the respiratory system, bone marrow, lymphoid tissues, and the gastrointestinal tract70,71. While this protein is not functionally characterized, it is assumed to have the capacity to interact with numerous cells and tissues. Therefore, future studies are needed to investigate the function of ELAPOR2 and how it could be involved in regulating autophagy and the inflammatory response in postpartum dairy cows.
This is a first-of-its-kind study in which numerous potential mediators of inflammation were evaluated in postpartum dairy cows classified in high or low inflammation. The results from this study have revealed differences in the profiles of plasma oxylipins as well as endocannabinoids, thus highlighting lipid mediators that warrant further investigation for their biological function and ability to impact inflammation in dairy cows. The results from this study also provide evidence that can be used to support future studies that investigate the ability of select oxylipins to be biomarkers of inflammation in dairy cows. Furthermore, our group aimed to critically evaluate bovine platelets, which have previously been reported to mediate inflammation in human and mouse models. Interestingly, we have identified differences in the platelet protein profile of cows with varying degrees of inflammation. Namely, we have found that platelets isolated from cows with high inflammation had an increased abundance of haptoglobin and a decreased abundance of transferrin-receptor protein 1. While Hp is a well-known inflammatory biomarker, many other proteins identified in this study have not yet been linked to systemic inflammation in dairy cows. Our group also identified differences in the plasma proteome between high- and low-inflammation cows. Notably, high-inflammation cows had an increased abundance of ELAPOR2 in their plasma and a decreased abundance of conglutinin in both platelets and plasma. The results from this study can provide the framework for future research that aims to understand the functional differences in dairy cows suffering from systemic inflammation.
Methods
Study design and study population
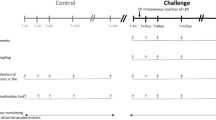
A cross-sectional study was conducted in a commercial dairy herd in northern Indiana, milking on average 5,000 Holstein cows. Primiparous (n = 9) and multiparous (n = 7) Holstein cows were enrolled in the study. Cows were housed in freestall barns bedded with recycled sand and were milked twice daily.
Sample collection
This study was approved by Purdue University Institutional Animal Care and Use Committee, and all experiments were performed in accordance with ARRIVE guidelines. The research group visited the farm daily at the same time (0900 h). Blood was collected from cows free from clinical diseases at 3 days in milk (DIM) via coccygeal venipuncture using a 20-gauge x 2.54 cm needle into vacuum tubes containing spray-dried lithium heparin and K2 EDTA (Vacuette®, 6 mL; Greiner Bio-One, Kremsmünster, AUT). After collection, tubes were gently inverted to allow proper anticoagulant mixing. Blood tubes were placed in a thermo-conductive, passive-temperature-regulating module (CoolRack; BioCision, San Rafael, CA) and kept inside an ice cooler. All blood samples were transported back to Purdue University (West Lafayette, IN) within 2 h after collection for processing. Samples were centrifuged at 2,000 x g and 4 °C for 10 min. Plasma collected from lithium heparin tubes was flash-frozen in liquid nitrogen before being stored at −80 °C until further analysis. To prevent ex vivo peroxidation, butylated hydroxytoluene 89,910 (Cayman Chemical, Ann Arbor, MI) was added to EDTA plasma aliquots (10 µL/1mL of plasma), after which samples were flash-frozen in liquid nitrogen and stored at −80 °C until further analysis.
A blood sample was collected by jugular venipuncture using a 14-gauge x 2.54 cm hypodermic needle and two 35 mL syringes prefilled with 4% sodium citrate (Haemonetics, Boston, MA), maintaining an anticoagulant to blood ratio of 1:6. To prevent platelet activation, blood was allowed to flow from the needle for 3 s before attaching the syringes. Blood was then slowly drawn into the syringe. Syringes were stored at room temperature and transported to Purdue University within 2 h after collection for platelet isolation. All cows sampled in this study remained clinically healthy up until 21 DIM.
Platelet isolation
The 4% sodium citrate anticoagulated blood was gently transferred to 50 mL conical tubes and centrifuged at room temperature at 400 x g for 10 min with no break. After centrifugation, the top 2/3 of plasma (platelet-rich plasma (PRP)) was collected and diluted 1:1 in PBS without Ca2+ or Mg2+ (Gibco, Billings, MT) and supplemented with 1 µM PGE1 (Sigma-Aldrich, St. Louis, MO) to prevent platelet activation. Diluted PRP was carefully layered onto Ficoll (Ficoll-Paque™ PREMIUM; Cytiva, Marlborough, MA) while maintaining a 1:2 ratio of Ficoll to dilute PRP, then centrifuged at 400 x g for 30 min with no break to deplete the diluted PRP of white and red blood cells. Following gradient centrifugation, the platelet-rich supernatant was collected while avoiding the cellular interface containing white blood cells and centrifuged at 200 x g for 10 min with no break to pellet any contaminating white blood cells. The supernatant was again collected, supplemented with 1 µM PGE1, and centrifuged at 400 x g for 10 min to pellet platelets. The platelet pellet was resuspended in CGS buffer, pH 6.5 (Biochemazone, Alberta, CAN), supplemented with 1 µM PGE1, and gently transferred to a 2.0 mL microcentrifuge tube for cell counting and washing.
To obtain complete cell counts, a small volume of the resuspended platelets was aliquoted and submitted to the Purdue University College of Veterinary Medicine Clinical Pathology laboratory for analysis on an ADVIA 2120i Hematology System (Siemens Diagnostics, Munich, Germany). Results from the complete cell counts were used to quantify platelets and ensure the purity of platelet samples. After submission for complete cell counts, platelets were centrifuged at 400 x g for 10 min with no break to pellet the cells. The supernatant was removed, and the surface of the platelet pellets was washed three times by adding CGS buffer to the microcentrifuge tube without disturbing the platelet pellet. After washing, all supernatants were removed and platelet pellets were flash-frozen in liquid nitrogen and stored at −80 °C until further analysis.
Systemic inflammation classification
Haptoglobin is a well-documented biomarker of systemic inflammation in dairy cows, and thus, our classification of cows was validated by measuring plasma Hp concentrations. Plasma samples collected from lithium heparin tubes were assessed for Hp concentrations using a commercially available assay (Cow Haptoglobin ELISA, Hapt-11; Life Diagnostics, West Chester, PA). Intra- and inter-assay coefficients of variation were 7.11% and 8.50%, respectively. Cows were classified as low or high inflammation based on their plasma Hp concentration on DIM 3. Cows with Hp concentrations \(\:\le\:\) 0.1 g/L were classified into the low inflammation group (n = 8), and cows with Hp concentrations \(\:\ge\:\) 0.50 g/L were classified into the high inflammation group (n= 8). Plasma Hp concentration thresholds were chosen based on previously established thresholds72. Plasma samples and platelet pellets from high and low-inflammation cows were submitted for lipidomic and proteomic analyses.
Targeted LC-MS/MS lipidomic analysis of oxylipins
EDTA-collected plasma for targeted oxylipin lipidomics was prepared for LC-MS/MS as described in Grantz et al. (2024) with slight modifications. All reagents used for plasma lipid extraction were HPLC-grade. Briefly, 500 µL of plasma was mixed with 1.5 mL of 75:25 (v/v) acetone to acetonitrile (ACN) and 10 µL of an internal standard mixture containing the oxylipins of interest (catalog numbers: 20666, 21393, 22280, and 22638; Cayman Chemical, Ann Arbor, MI). The oxylipins of interest were selected based on results obtained by our group in a prior study10 to further evaluate their concentrations in cows experiencing systemic inflammation. Samples were vortexed for 10 min and centrifuged at 3220 x g for 10 min. Supernatants were transferred to a microcentrifuge tube and evaporated in a SpeedVac Savant SPD2030 (Thermo Fisher Scientific Inc., Waltham, MA). Dried extracts were reconstituted in 50% ACN before the targeted lipidomic analysis was completed.
An Agilent 1290 Infinity II liquid chromatography (LC) system coupled to an Agilent 6495 C series QQQ mass spectrometer (MS/MS) was used to analyze samples. (Agilent Technologies, Santa Clara, CA). An Acquity UPLC BEH C18 2.1 mm x 100 mm, 1.8 μm column was used for LC separation (Waters Corp., Milford, MA). The buffers were water + 0.1% formic acid (FA) (A) and ACN + 0.1% FA (B). The linear LC gradient was as follows: time 0 min, 20% B; time 1 min, 20% B; time 28 min, 100% B; time 30 min, 100% B; time 30.1 min, 20% B; time 33 min, 10% B. The flow rate was 0.3 mL/min, and the column was heated to 40 °C. Multiple reaction monitoring was used for MS analysis, according to Table 3. Data were acquired in negative electrospray ionization (ESI) mode. The jet stream ESI interface had a gas temperature of 225 °C, gas flow rate of 17 L/min, nebulizer pressure of 50 psi, sheath gas temperature of 275 °C, sheath gas flow rate of 12 L/min, capillary voltage of 3000 V in negative mode, and nozzle voltage of 1500 V, ifunnel high-pressure RF was 200 V and low-pressure RF was 110 V. The multiplier voltage (ΔEMV) was 300 V. Raw data was processed with Agilent Masshunter Quantitative analysis software (version 12) (Agilent Technologies, Santa Clara, CA). Oxylipin quantification was achieved by constructing standard curves by completing five serial dilutions of the internal standard mix in water with 0.1% FA. Concentration ranges were defined before this study using bovine plasma test samples.
Targeted LC-MS/MS lipidomic analysis of endocannabinoids
Lithium heparin collected plasma for targeted endocannabinoid lipidomics was prepared for LC-MS/MS by mixing 500 µL of plasma with 4.5 mL of 10X methyl tert-butyl ether (MtBE) and 20 µL of a 1:10 diluted internal standard mixture for the endocannabinoids of interest (catalog numbers: 10007270, 62160; Cayman Chemical, Ann Arbor, MI). Samples were vortexed for 10 min, and the supernatant was transferred to a microcentrifuge tube and evaporated in a SpeedVac Savant SPD2030. Dried extracts were reconstituted in 50% ACN before the targeted lipidomic analysis was completed.
An Agilent 1260 Infinity II Binary liquid chromatography (LC) system coupled to an Agilent 6495 C Series QQQ mass spectrometer (MS/MS) was used to analyze samples (Agilent Technologies, Santa Clara, CA). An Agilent Eclipse Plus C18 2.1 mm x 50 mm, 1.8 μm column was used for LC separation (Waters Corp., Milford, MA). The buffers were water + 0.1% FA (A) and acetonitrile + 0.1% FA (B). The linear LC gradient was as follows: time 0 min, 30% B; time 1 min, 30% B; time 9 min, 100% B; time 11 min, 100% B; time 11.1 min, 30% B; time 16 min, 30% B. The flow rate was 0.3 mL/min. Multiple reaction monitoring was used for MS analysis, according to Table 4. Data were acquired in positive electrospray ionization (ESI) mode. The jet stream ESI interface had a gas temperature of 150 °C, gas flow rate of 17 L/min, nebulizer pressure of 20 psi, sheath gas temperature of 200 °C, sheath gas flow rate of 10 L/min, capillary voltage of 3500 V in positive mode, and nozzle voltage of 1000 V, ifunnel high-pressure RF was 200 V and low-pressure RF was 110 V. The ΔEMV voltage was 400 V. Raw data was processed with Agilent Masshunter Quantitative analysis software (version 12). Endocannabinoid quantification was achieved using the response ratio value to calculate the absolute concentration of the unknown.
Platelet sample preparation for LC-MS/MS proteomic analysis
Platelet pellets selected for proteomics were prepared for LC-MS/MS as described by Piersma et al. (2013) with some modifications73. Cell pellets were lysed in 1x lysis buffer (5% SDS in 50mM ammonium bicarbonate buffer (AmBIC) using a Precellys Evolution homogenizer and Precellys tough micro-organism lysing kits (Bertin Technologies, Montigny-le-Bretonneux, France). After lysis, the total protein concentration for each sample was assessed in duplicate using a colorimetric bicinchoninic acid (BCA) assay (Pierce™). Samples were concentrated to obtain the desired quantity of protein for further analysis using a vacuum centrifuge (SpeedVac Savant SPD2030, Thermo Fisher Scientific Inc., Waltham, MA).
After determining total protein concentrations for all samples, 4x LDS sample buffer was added to 30 µg aliquots of platelet lysate and heated at 95 °C for 10 min to denature proteins. The protein was loaded into a 1 mm x 12-well NuPAGE™ 12% Bis-Tris gel NP0322BOX (Thermo Fisher Scientific Inc., Waltham, MA) and ran at 200 V for 5 min using MOPS Running Buffer (Thermo Fisher Inc., Waltham, MA). Once the run was complete, gels were placed on a clean glass plate and separated by sample ___location into whole gel lanes using a clean X-acto knife. Gel slices were placed in deionized water and washed on a rocker. Gel slices were digested using Trypsin/LysC (Pierce™, 186352; Lot: UC370715) (1 µg enzyme/sample), which was allowed to soak into the dried gel cubes for 16 h at 37 °C. Following protein digestion, peptides were subjected to C18 peptide cleanup. Gel slices were proceeded to extract peptides as described in Swart et al. (2018). Briefly, peptides were extracted before reduction and alkylation using 1:1 acetonitrile (ACN)/50 mM ABC for 5 min. The supernatant was removed, and the gel slices were vortexed in 100% ACN until they looked dehydrated.
Plasma preparation for LC-MS/MS proteomic analysis
Before protein extraction, EDTA plasma samples were thawed slowly on ice and centrifuged briefly with a bench-top microcentrifuge to precipitate cells, debris, and aggregated proteins at the bottom of the tube. Plasma samples were depleted of albumin using AlbuSorb™ Plus Albumin and IgG depletion Kit (APK285-20, BioTech Support Group, Monmouth Junction, NJ), following the manufacturer’s instructions. Before digestion, each sample’s total protein concentration was assessed in duplicate using a colorimetric BCA assay (Pierce™). Plasma samples underwent in-solution digestion. Briefly, 50 µg of albumin-depleted protein was denatured in a denaturation buffer containing 50 mM AmBIC, 2 M thiourea, and 6 M urea. Proteins were then reduced in DTT (5 mM) for 30 min at room temperature before alkylation with iodoacetamide (IAM) for 30 min in the dark. Trypsin/LysC was added to the samples 1:100 (1 µg trypsin/sample) and incubated with high humidity for 16 h at 37 °C. The digested peptides were quenched by adding trifluoroacetic acid (TFA) to approximately 1% of the final TFA concentration and a pH < 3 and dried in a vacuum centrifuge.
Label-free quantitative LC-MS/MS proteomic analysis
Dried peptides obtained from platelet and plasma proteins were reconstituted in 3% ACN and 0.1% formic acid (FA) before being analyzed by HPLC-MS/MS using a Lumos Orbitrap MS (Thermo Fisher Inc., Waltham, MA) equipped with an Ultimate 3000 HPLC (Thermo Fisher Inc., Waltham, MA) and an Aurora™ Ultimate analytical column (IonOpticks, Victoria, AUS). Approximately 1 µg of peptides were loaded on a trap column (150 mm long x 75 μm id, 3 μm particle size, 100 Å C18 medium) and separated on a 25 cm nanoflow UHPLC IonOpticks C18 column. A 130 min elution gradient was constructed by mixing mobile phase solvent A (0.1% FA in water) with solvent B (80% ACN, 0.1% FA in water). 2% of solvent B was initially used and increased to 12% at 1.6 min, 25% at 80 min, 35% at 100 min, 45% at 105 min, and 95% at 120 min, at which point the gradient was held for 5 min before reverting to 2% at 125.1 min, and 5% at 130 min. An injection of 1 µg of sample peptide was performed for each sample. All the data were acquired using Orbitrap Fusion Lumos Tribrid mass spectrometer (ThermoFisher Scientific). Orbitrap mass analyzer and data were collected using a higher-energy collisional dissociation) fragmentation scheme. For MS scans, the scan range was from 375 to 1500 m/z at a resolution of 120000, the automatic gain control (AGC) target was set at 4 × 105, maximum injection time 50 ms, dynamic exclusion 60 s, and intensity threshold 5.0 × 103.
MS data for platelet and plasma proteomics were acquired in data-dependent mode with a cycle time of 3 s/scan. MS/MS data were collected at a resolution of 7500. The integrity and performance of the mass spectrometer was monitored by HeLa standards before and after the experimental runs.
Proteomics data analysis
Raw platelet and plasma proteomics data were processed using the Proteome Discoverer (version 3.1, Thermofisher Scientific) software using bovine proteome (Uniprot). An in-house list of common contaminants was added to the search. Sequest HT tool was used to assign the peptides, allowing a maximum of two missed tryptic cleavages, a minimum peptide length of six, a precursor mass tolerance of 10 ppm, and a fragment mass tolerance of 0.02 Da. Carbamidomethylation of cysteines and oxidation of methionine were specified as static and dynamic modifications, respectively. A false discovery rate of high confidence validated peptide spectral matches was used for the downstream analysis. Label-free quantification based on MS1 precursor ion intensity was performed in Proteome Discoverer with a minimum quan value threshold set to 0.001 for unique peptides; the ‘3 Top N’ peptides were used for area calculation. The normalized protein abundances were calculated among the measured samples, and values were Log transformed and imputed following a normal distribution pattern. R-studio and Perseus were used to generate the graphs, and Adobe Illustrator has been used to present them.
Statistical analysis
Statistical analyses for plasma lipidomics were performed on SAS v9.4 (SAS Institute, Cary, NC). Plasma oxylipin and endocannabinoid concentrations were initially assessed for normality using the UNIVARIATE procedure by visual assessment of Q-Q plots and Shapiro-Wilk tests. Data not normally distributed underwent square root transformations. All compounds, except for 9,10-DiHOME, required a square root transformation. Mixed linear regression models were built using the MIXED procedure and used to assess differences in oxylipin or endocannabinoid concentrations between high-inflammation and low-inflammation cows. Parity was included as a fixed effect in the models. Studentized residuals were visually assessed for normality. Hp concentrations and platelet counts were assessed using the paired, two-tailed, Student’s T-test. Platelet and plasma proteins with at least P\(\:\le\:\) 0.05 and a \(\:\ge\:\) 1.2-fold change were accepted as significant. Differentially abundant proteins were visualized according to their corresponding volcano plots.
Data availability
ata is provided within the manuscript and in supplementary information files. The proteomics dataset is deposited in MassIVE (https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp; ID: MSV000096534) and can be accessed upon reasonable request to the corresponding author.
14. References
Huzzey, J. M. et al. Short communication: Haptoglobin as an early indicator of metritis. J. Dairy. Sci. 92, 621–625 (2009).
Huzzey, J. M., Mann, S., Nydam, D. V., Grant, R. J. & Overton, T. R. Associations of peripartum markers of stress and inflammation with milk yield and reproductive performance in Holstein dairy cows. Prev. Vet. Med. 120, 291–297 (2015).
Bradford, B. J., Yuan, K., Farney, J. K., Mamedova, L. K. & Carpenter, A. J. Invited review: inflammation during the transition to lactation: new adventures with an old flame. J. Dairy. Sci. 98, 6631–6650 (2015).
Tam, V. C. et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 154, 213–227 (2013).
Dennis, E. A. & Norris, P. C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15, 511–523 (2015).
Misheva, M., Johnson, J. & McCullagh, J. Role of Oxylipins in the inflammatory-related diseases NAFLD, obesity, and type 2 diabetes. Metabolites 12, 1238 (2022).
Villain, E. et al. Integrated analysis of whole blood oxylipin and cytokine responses after bacterial, viral, and T cell stimulation reveals new immune networks. iScience 26, 107422 (2023).
Sordillo, L. M. Symposium review: Oxylipids and the regulation of bovine mammary inflammatory responses1. Journal of Dairy Science 101, 5629–5641 (2018).
Putman, A. K., Gandy, J. C., Contreras, G. A. & Sordillo, L. M. Oxylipids are associated with higher disease risk in postpartum cows. J. Dairy. Sci. 105, 2531–2543 (2022).
Grantz, J. M. et al. Plasma oxylipin profile of postpartum dairy cows categorized into different systemic inflammatory grades in the first week after parturition. JDS Commun. https://doi.org/10.3168/jdsc.2023-0410 (2024).
Kumar, N. et al. 15-Lipoxygenase metabolites of α-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 6, 31649 (2016).
Bergmann, C. B. et al. sEH-derived metabolites of linoleic acid drive pathologic inflammation while impairing key innate immune cell function in burn injury. Proc. Natl. Acad. Sci. U S A. 119, e2120691119 (2022).
Berdyshev, E. V. et al. Influence of fatty acid ethanolamides and ∆9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur. J. Pharmacol. 330, 231–240 (1997).
Chang, Y. H., Lee, S. T. & Lin, W. W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J. Cell. Biochem. 81, 715–723 (2001).
Eisenstein, T. K., Meissler, J. J., Wilson, Q., Gaughan, J. P. & Adler, M. W. Anandamide and ∆9-Tetrahydrocannabinol directly inhibit cells of the Immune System via CB2 receptors. J. Neuroimmunol. 189, 17–22 (2007).
Solorzano, C. et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. U S A. 106, 20966–20971 (2009).
Lu, H. C. & Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry. 79, 516–525 (2016).
Chiurchiù, V., Battistini, L. & Maccarrone, M. Endocannabinoid signalling in innate and adaptive immunity. Immunology 144, 352–364 (2015).
Hillard, C. J. Circulating endocannabinoids: from Whence do they come and where are they going? Neuropsychopharmacol 43, 155–172 (2018).
Klein, T. W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 5, 400–411 (2005).
Nomura, D. K. et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334, 809–813 (2011).
Zachut, M. et al. Characterization of the endocannabinoid system in subcutaneous adipose tissue in periparturient dairy cows and its association to metabolic profiles. PLOS ONE. 13, e0205996 (2018).
Kra, G. et al. Effects of omega-3 supplementation on components of the endocannabinoid system and metabolic and inflammatory responses in adipose and liver of peripartum dairy cows. J. Anim. Sci. Biotechnol. 13, 114 (2022).
Stalker, T. J., Newman, D. K., Ma, P., Wannemacher, K. M. & Brass, L. F. Platelet signaling. Handb. Exp. Pharmacol. 59–85. https://doi.org/10.1007/978-3-642-29423-5_3 (2012).
Yun, S. H., Sim, E. H., Goh, R. Y., Park, J. I. & Han, J. Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed Res Int 2016, 9060143, (2016).
Sambrano, G. R. et al. Cathepsin G activates protease-activated Receptor-4 in human platelets *. J. Biol. Chem. 275, 6819–6823 (2000).
Yeung, J., Hawley, M. & Holinstat, M. The expansive role of oxylipins on platelet biology. J. Mol. Med. (Berl). 95, 575–588 (2017).
Masselli, E. et al. ROS in platelet Biology: functional aspects and methodological insights. Int. J. Mol. Sci. 21, 4866 (2020).
De Angelis, V. et al. Endocannabinoids control platelet activation and limit aggregate formation under Flow. PLoS One. 9, e108282 (2014).
Morrell, C. N., Aggrey, A. A., Chapman, L. M. & Modjeski, K. L. Emerging roles for platelets as immune and inflammatory cells. Blood 123, 2759–2767 (2014).
Aggrey, A. A., Srivastava, K., Ture, S., Field, D. J. & Morrell, C. N. Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria. J. Immunol. 190, 4685–4691 (2013).
Sonmez, O. & Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2, 311–314 (2017).
Raphael, W., Halbert, L., Contreras, G. A. & Sordillo, L. M. Association between polyunsaturated fatty acid-derived oxylipid biosynthesis and leukocyte inflammatory marker expression in periparturient dairy cows. J. Dairy Sci. 97, 3615–3625 (2014).
Mavangira, V. & Sordillo, L. M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 116, 4–14 (2018).
Cambiaggi, L., Chakravarty, A., Noureddine, N. & Hersberger, M. The role of α-Linolenic acid and its Oxylipins in Human Cardiovascular diseases. Int. J. Mol. Sci. 24, 6110 (2023).
Gabbs, M., Leng, S., Devassy, J. G., Monirujjaman, M. & Aukema, H. M. advances in our understanding of Oxylipins Derived from Dietary PUFAs12. Adv. Nutr. 6, 513–540 (2015).
Caligiuri, S. P. B. et al. Dietary linoleic acid and α-Linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in Diet-Induced obese rats. J. Nutr. 143, 1421–1431 (2013).
Pauls, S. D. et al. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 57, 121–129 (2018).
van der Krieken, S. E. et al. Large-scale screening of natural products transactivating peroxisome proliferator-activated receptor α identifies 9S-Hydroxy-10E,12Z,15Z-Octadecatrienoic acid and Cymarin as potential compounds capable of increasing apolipoprotein A-I transcription in Human Liver cells. Lipids 53, 1021–1030 (2018).
Youssef, J. & Badr, M. Role of Peroxisome Proliferator-Activated Receptors in Inflammation Control. J Biomed Biotechnol 2004, 156–166 (2004).
Decara, J. et al. Peroxisome proliferator-activated receptors: experimental targeting for the treatment of Inflammatory Bowel diseases. Front. Pharmacol. 11, 730–748 (2020).
Hildreth, K., Kodani, S. D., Hammock, B. D. & Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: a review of recent studies. J. Nutr. Biochem. 86, 108484 (2020).
Gilroy, D. W. et al. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. U S A. 113, E3240–E3249 (2016).
Viswanathan, S. et al. Involvement of CYP 2C9 in mediating the Proinflammatory effects of Linoleic Acid in Vascular endothelial cells. J. Am. Coll. Nutr. 22, 502–510 (2003).
Witkamp, R. Fatty acids, endocannabinoids and inflammation. Eur. J. Pharmacol. 785, 96–107 (2016).
Okamoto, Y., Morishita, J., Tsuboi, K., Tonai, T. & Ueda, N. Molecular characterization of a phospholipase D Generating Anandamide and its congeners *. J. Biol. Chem. 279, 5298–5305 (2004).
Pflüger-Müller, B. et al. The endocannabinoid anandamide has an anti-inflammatory effect on CCL2 expression in vascular smooth muscle cells. Basic. Res. Cardiol. 115, 34 (2020).
Hegde, V. L. et al. Attenuation of experimental autoimmune Hepatitis by Exogenous and Endogenous cannabinoids: involvement of Regulatory T cells. Mol. Pharmacol. 74, 20–33 (2008).
Karsak, M. et al. Attenuation of allergic contact Dermatitis through the Endocannabinoid System. Science 316, 1494–1497 (2007).
Kasimanickam, R. K. et al. Associations among serum pro- and anti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reproductive Biology Endocrinol. 11, 103 (2013).
Brodzki, P., Kostro, K., Krakowski, L. & Marczuk, J. Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet. Res. Commun. 39, 143–149 (2015).
LeBlanc, S. J. Relationship of peripartum inflammation with reproductive health in dairy cows* *Presented as part of the Joint Physiology and Endocrinology/Animal Health Symposium: Determinants and Consequences of Systemic Inflammation During the Dairy Cow Transition Period held at the ADSA Annual Meeting, June 2022. JDS Communications 4, 230–234 (2023).
Wang, Y., Kinzie, E., Berger, F. G., Lim, S. K. & Baumann, H. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep. 6, 379–385 (2001).
Kalmovarin, N. et al. Extrahepatic expression of plasma protein genes during inflammation. Inflammation 15, 369–379 (1991).
Cremer, S. E. et al. The canine activated platelet secretome (CAPS): a translational model of thrombin-evoked platelet activation response. Res. Pract. Thromb. Haemost. 5, 55–68 (2020).
Khan, N., Kurnik-Łucka, M., Latacz, G., Gil, K. & Saeed, S. A. The inhibitory effect of human plasma albumin and haptoglobin on platelet aggregation and 5-HT release. Folia Med. Cracov. 62, 5–18 (2022).
Wang, S. et al. Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 105, 2071–2082 (2020).
Gammella, E., Buratti, P., Cairo, G. & Recalcati, S. The transferrin receptor: the cellular iron gate. Metallomics 9, 1367–1375 (2017).
Candelaria, P. V., Leoh, L. S., Penichet, M. L. & Daniels-Wells, T. R. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front. Immunol. 12, 607–628 (2021).
Baydar, E. & Dabak, M. Serum iron as an indicator of acute inflammation in cattle. J. Dairy. Sci. 97, 222–228 (2014).
Tsukano, K. & Suzuki, K. Serum iron concentration is a useful biomarker for assessing the level of inflammation that causes systemic symptoms in bovine acute mastitis similar to plasma haptoglobin. J. Vet. Med. Sci. 82, 1440–1444 (2020).
Tsukano, K., Fukuda, T., Ikeda, K., Sato, K. & Suzuki, K. Serum iron concentration is candidate inflammatory marker for respiratory diseases in beef cows. J. Vet. Med. Sci. 83, 824–828 (2021).
Tsukano, K. et al. Serum iron concentration as a marker of inflammation in young cows that underwent dehorning operation. J. Vet. Med. Sci. 81, 626–628 (2019).
Recalcati, S., Taramelli, D., Conte, D. & Cairo, G. Nitric oxide–mediated induction of Ferritin Synthesis in J774 macrophages by Inflammatory cytokines: Role of Selective Iron Regulatory Protein-2 downregulation. Blood 91, 1059–1066 (1998).
Praticò, D. et al. Iron-dependent human platelet activation and hydroxyl radical formation. Circulation 99, 3118–3124 (1999).
Paterson, J. M. et al. Atomic-resolution crystal structures of the immune protein conglutinin from cow reveal specific interactions of its binding site with N-acetylglucosamine. J. Biol. Chem. 294, 17155–17165 (2019).
Dec, M., Wernicki, A., Puchalski, A., Urban-Chmiel, R. & Waśko, A. The effect of conglutinin on production of reactive oxygen species in bovine granulocytes. J. Vet. Sci. 13, 33–38 (2012).
Murugaiah, V., Tsolaki, A. G. & Kishore, U. Collectins: Innate Immune Pattern Recognition molecules. Lectin Host De?F. Against Microb. Infections. 1204, 75–127 (2020).
Holmskov, U., Jensenius, J. C., Tornøe, I. & Løvendahl, P. The plasma levels of coglutinin are heritable in cattle and low levels predispose to infection. Immunology 93, 431–436 (1998).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
The Human Protein Atlas. KIAA1324L Protein Expression Summary.
Kerwin, A. L. et al. Transition cow nutrition and management strategies of dairy herds in the northeastern United States: Part II-Associations of metabolic- and inflammation-related analytes with health, milk yield, and reproduction. J. Dairy. Sci. 105, 5349–5369 (2022).
Piersma, S. R. et al. Whole gel processing procedure for GeLC-MS/MS based proteomics. Proteome Sci. 11, 17 (2013).
Acknowledgements
The authors thank the participating farm for allowing us to collect samples from their cows and the Purdue University College of Veterinary Medicine Clinical Pathology laboratory for assessing our platelet samples.
Author information
Authors and Affiliations
Contributions
Contributions were as follows: JMG, conceptualization, data collection, data analysis, and manuscript preparation; VPTK, data collection, data analysis, and manuscript editing; AHJ, data collection, data analysis, and manuscript editing; CA, data collection and manuscript editing; NT, data collection and manuscript editing; LAG, data collection and manuscript editing; RCN, conceptualization, data collection, data analysis, and manuscript editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Grantz, J.M., Thirumalaikumar, V.P., Jannasch, A.H. et al. The platelet and plasma proteome and targeted lipidome in postpartum dairy cows with elevated systemic inflammation. Sci Rep 14, 31240 (2024). https://doi.org/10.1038/s41598-024-82553-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82553-x