Abstract
The gut microbiota alterations interact with the pathogenesis and progression of chronic kidney disease (CKD). Probiotics have received wide attention as a potential management in CKD. We investigated the effects of Lactobacillus paracasei N1115 (LP N1115) on intestinal microbiota and related short-chain fatty acids (SCFAs) in end stage kidney disease patients on peritoneal dialysis (PD) in a single-center, prospective, randomized, double-blind, placebo-controlled study. The patients were randomly allocated into two groups. The intervention group (n = 38, PR group) was given the probiotics (two bags) containing fructooligosaccharide (FOS) (additive amount > 80%), maltosaccharin, and LP N1115 (additive amount > 3 × 1010 CFU/bag) every day whereas the control group (n = 19, PL group) received placebo (two bags) containing only pregelatinized starch and lactose, both for 12 weeks. In addition to collecting fecal samples for 16S rRNA gene high-throughput sequencing and SCFAs analysis, gastrointestinal (GI) symptoms were also assessed at baseline and after the intervention. Probiotics administration caused significant changes in the composition of gut microbiota, as indicated by increased abundance of beneficial bacteria (Firmicutes), decreased Bacteroidetes, and opportunistic pathogens (Fusobacterium, Bilophila) (p < 0.05). However, there was no significant difference in intestinal microbial diversity. SCFAs levels increased in PR group although the change was not statistically significant between the two groups (P > 0.05). In addition, probiotics administration could effectively reduce GI symptoms, particularly in dyspepsia and constipation (p < 0.05). Together, the results suggest that probiotics administration caused significant changes in the composition of gut microbiota and also could effectively reduce GI symptoms, particularly in dyspepsia and constipation in PD patients. Trial registration: This study was registered with the Chinese Clinical Trial Registry (Trial registration number: ChiCTR-INR-17011718; Date of the first registration: 21/06/2017).
Similar content being viewed by others
Introduction
The gut microbiota has been shown to be closely related to human health. Many studies have reported on gut dysbiosis in chronic kidney disease (CKD), especially end stage kidney disease (ESKD)1,2. The gut microbiota and its metabolites also affect the pathogenesis and development of CKD and its complications3,4. Changes in intestinal dysbiosis can lead to imbalance in the synthesis of a range of metabolites, including short-chain fatty acids (SCFAs) and uremic toxins5. SCFAs exert multiple effects on human health such as the immune regulation, energy metabolism, lipid regulation, as well as improvement of intestinal barrier function6,7. The uremic toxins could resist removal by dialysis8, promote kidney fibrosis, and are important risk factors for cardiovascular complications and death in patients with CKD9,10. Therefore, more and more attention has been paid to the regulation of intestinal microbiota as a new target for the treatment of kidney diseases.
In recent years, the use of therapeutic agents including probiotics, prebiotics and synbiotics to regulate the gut microbiota has been studied as one of the therapies for CKD, aimed to delaying the progression of renal function, improving clinical symptoms and outcomes, and regulating the symbiotic intestinal environment3,11,12. However, most of the current studies on the therapeutic effects of prebiotics, probiotics and synbiotics have been conducted in non-dialysis patients with kidney disease, and few have targeted on dialysis patients, especially those on peritoneal dialysis (PD). Probiotics may play a role in inflammation, nutritional status, kidney function and quality of life13,14. However, the effects of probiotics on metabolites of intestinal microbiota in PD patients have rarely been studied.
Our previous studies have shown that the diversity, abundance and overall composition of intestinal microbiota in PD patients are different from those in the normal population, and the proportion of Bacteroides, Verrucomicrobium and Fusobacterium in PD patients is significantly increased, while the proportion of Prevotella, Bifidobacterium, Ruminococcus and Rosellais decreased4. Herein, we conducted a prospective, randomized, double-blind, placebo-controlled study of probiotics intervention therapy in long-term PD patients to explore the effects of probiotics on intestinal microbiota, SCFAs levels and its correlation with gastrointestinal(GI) symptoms in PD patients.
Subjects and methods
Participants
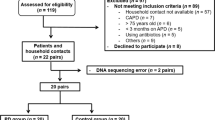
Patients who underwent continuous ambulatory peritoneal dialysis (CAPD) in the General Hospital of Ningxia Medical University between May 2020 and Dec 2022 were included. The inclusion criteria were as follows: (1) Stable CAPD treatment for > 3 months. (2) Age > 18 years. (3) Provided written informed consent. Exclusion criteria: (1) Unable to eat normally by mouth. (2) Confirmed the diagnosis of Infection of any organs or tissues within 1 month. (3) Used prebiotic, probiotics, synbiotics, hormones, immunosuppressants, antibiotics, antitumor drugs, metformin, or lactulose within 1 month. (4) GI endoscopy or surgery within 1 month. (5) Diagnosed as malignant tumors, autoimmune diseases, severe heart failure (grade III-IV), cognitive dysfunction and mental illness. (6) Diagnosed as inflammatory bowel disease, irritable bowel syndrome. (7) With a previous history of kidney transplantation, maintenance hemodialysis (HD) (> 3 months), and HD combined with PD. (8) Intolerance of probiotics supplements. (9) Poor compliance and the inability to adhere to dialysis prescriptions and execute sample collection instructions.
Study design
This study was a single-center, prospective, randomized, double-blind, placebo-controlled study, which has been approved by the Ethics Committee of Ningxia Medical University General Hospital (Approval number: 2016–254). The study was also conducted in accordance with the Declaration of Helsinki, and registered to clinical trials and the registration number was ChiCTR-INR-17011718 (Date of registration: 21/06/2017). All methods were performed in accordance with relevant guidelines and regulations.
Group allocation
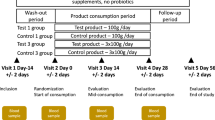
The randomization was conducted by SPSS23.0 software to allocate patients who met the inclusion criteria in a 2:1 ratio to the probiotics (PR) group and placebo (PL) group. Under the premise of increasing the number of patients in the PR group, the experimental group can be further analyzed according to the research results to obtain more meaningful information, which can provide more space for our research. The random allocation sequence was undertaken by a statistician who was not involved in the clinical trial, while the investigators enrolled participants, and assigned treatments. The PR group was given probiotics, while the PL group was given placebo for 12 weeks. Placebo only contains pregelatinized starch and lactose, which were the same in appearance, texture, and taste as probiotics. The experimental drug had been assigned and coded, and provided to the researcher. Researcher, patients, the information evaluator, and the statistical analyst were unaware of assignments to probiotics and placebo groups until the statistical analysis was completed.
Intervention strategy
The probiotics were N1115 ready-to-eat lactic acid bacteria from Junlebao Dairy Group Co., Ltd.. The ingredients included fructooligosaccharide (FOS) (additive amount > 80%), maltosaccharin, and LP N1115 (additive amount > 3 × 1010 CFU/bag), which should be stored in a cool and dry place. Lactobacillus paracasei N1115 (LP N1115) is a lactic acid bacteria, and FOS, as a prebiotic, can promote its proliferation. In combination with FOS, LP N1115 improves intestinal barrier function, preserves histological integrity, and decreases the release of inflammatory factors15. In order to avoid the loss of live bacteria, the probiotic powder should be blended with warm water or milk. Both groups of patients took 2 bags per day, with 20 ml of warm water below 40℃ taken during infusion for 12 weeks. During the intervention period, the subjects maintained their previous dietary habits, daily routines, and routine treatment, and were followed up regularly every two weeks to observe their compliance. Patients did not take lipid lowering medication, anti-anemia medication, phosphorus- or potassium-adsorbing agents, anti-microbial medication, and gastric acid lowering medication in the study period. The compliance of treatment was measured by the number of medications taken, and patients are followed up every 2 weeks to observe their compliance. Non-compliance was defined as patients who have not taken prescription drugs greater than 20%16.
Clinical data
The following information were collected: sex, age, primary disease, dialysis age, systolic blood pressure, diastolic blood pressure, peritoneal dialysis KT/V4, 24-h urine volume, ultra filtration volume, laboratory results (blood tests)17. Bioelectrical impedance analysis (BIA) (BCM, Fresenius) was used to assess the body composition and the volume status. SGA score17and Gastrointestinal symptom rating scale (GSRS) scores18 were employed to assess the nutrition and the symptom of digestive system (Supplementary Table 1). Clinical efficacy is evaluated based on the decrease in GSRS scores: The patient’s clinical symptoms have completely or largely disappeared with a decrease of more than 80% in GSRS scores was considered significant; the clinical symptoms of the patient were significantly reduced with a 60%−80% reduction in GSRS scores is considered effective; the patient’s clinical symptoms have not improved or even worsened, or a decrease of less than 60% in GSRS scores is considered ineffective.
Determination of fecal SCFAs
In this study, the content of SCFAs in feces was determined by gas chromatography-mass spectrometry (GC–MS). SCFAs include Acetic acid, Propionic acid, Butyric acid, Valeric acid, Caproic acid, Isobutyric acid and Isovaleric acid.
Study endpoint
Primary endpoint: The diversity, abundance, and composition of gut microbiota and the changes in fecal SCFAs levels in PD patients before and after 12 weeks of intervention. Secondary endpoint: Serological indices and GSRS scores of PD patients before and after intervention.
Safety assessment
The probiotics used in this study is Lactobacillus paracasei , which was included in the List of Bacteria that Can be Used in Food by the Ministry of Health of China in April 2010. LP N1115 is isolated from traditional homemade dairy products in Inner Mongolia, China. The food production license number of LP N1115 is SC13113012300078, and the product standard number is Q/HBYR 00,035. The manufacturer is Junlebao Dairy Group Co., Ltd.. LP N1115 has potential probiotic properties such as a high level of acid and bile salt resistance, and its fermented milk products have been patented19. It shows high similarity to the well- studied probiotic L. rhamnosus GG (LGG) and is considered safe for consumption20. Its efficacy and safety have also been confirmed in studies of patients with hepatitis B-related cirrhosis, pregnant women, healthy elderly, young children and other populations15,21,22,23.
Sequencing of intestinal microbiota
Stool samples were collected from the subjects before and after the intervention. Sterilized fecal specimen boxes were distributed to patients. After defecation, about 5–10 g of feces were taken from the middle of the stool and put into the specimen boxes. The collected feces should be kept away from urine and other substances. After the bacterial DNA was extracted from the fecal samples, the V3 ~ V4 region of each fecal sample was amplified by PCR using primers. And then, we commissioned Shanghai Mobio Biomedical Technology Co. Ltd. to perform high-throughput sequencing technology for the identification and typing of fecal microbiota. For more details about bacterial DNA extraction, PCR amplification and sequencing, see supplementary methods.
Bioinformatics and statistical analysis
Bioinformatics analysis sequencing data were spliced and filtered to obtain high-quality sequences after quality control. At 97% similarity level, they were clustered into operational taxonomic units (OTUs) for species classification. The representative sequences of each OTU were annotated according to SILVA database (SSU138), and the species composition information in the samples was obtained through taxonomy analysis. α diversity was used to analyze the richness and diversity of intestinal microbiota, included ACE, Chao, Shannon and Simpson index. Principal coordinate analysis (PCoA) based on Bray–Curtis, unweighted and weighted UniFrac distances was used to compare differences in community structure between groups. Permutation multivariate analysis of variance (PerMANOVA, also known as Adonis analysis) was used to statistically test the significance of differences in the overall bacterial population between groups. R software was used to analyze the difference in relative content of each bacterial group from phylum level to genus level. Mann–Whitney U test was used for comparison of data between two groups, and Kruskal–Wallis rank sum test was used for comparison of data between two or more groups. P < 0.05 indicated significant difference.
Statistical analysis
Sample size calculation: Due to the current challenge of estimating and testing energy efficiency and effect size in microbial community related research, the sample size cannot be estimated through calculation24. Based on previous studies on the gut microbiota of PD patients with a sample size of approximately 5–150, as well as factors such as previous research experience, time period, investigator dropout rate, and tolerance, a total of 57 PD patients were included in this study, including 38 in the PR group and 19 in the PL group.
SPSS23.0 software was used for statistical analysis: The measurement data of normal distribution were expressed by mean ± standard deviation, and the two groups comparison was performed by independent sample t test. Non normal distribution is represented by the median (upper and lower quartile). Wilcoxon rank sum test is used to compare two samples, and Kruskal Wallis test is used to compare multiple samples. The counting data is expressed as a percentage, and the Chi-square test or Fisher’s exact probability method is used for inter group comparison. P < 0.05 indicates significant differences.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Ningxia Medical University General Hospital (prot. no. 2016–254), and also registered to clinical trials and the registration number was ChiCTR-INR-17011718 (Date of registration: 21/06/2017).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Results
Disposition of patients
This double-blind, single-center, parallel-group, randomized controlled trial was conducted at General Hospital of Ningxia Medical University from May 2020 to Dec 2022. Fifty-seven hospitalized patients who provided informed consent and were screened were randomized into two groups: probiotics group (n = 38) and placebo group (n = 19). As indicated in Fig. 1., 2 patients from the probiotics group due to did not show up at the post-treatment measurements and 4 patients from the placebo group (2 patients due to did not show up at the post-treatment measurements and 2 patients due to discontinuation of the treatment) were excluded from the study. Therefore, 51 patients (probiotics group (n = 36), placebo group (n = 15)) completed the study.
Baseline information of subjects
A total of 51 PD patients were included in this study, including 25 males (49.0%), mean age 46.33 ± 13.26 years, median dialysis age 16 (9, 32) months. There were no differences in sex, age, dialysis age, primary disease, biochemical parameters, KT/V, 24-h urine volume, ultrafiltration volume, and SGA score between the PR group and PL group (Table 1).
Comparisons of clinical characteristics between two groups after 12 weeks intervention
Table 1 showed that the low-density lipoprotein in the PR group decreased significantly compared with the PL group. Fat tissue index (FTI), extracellular water/total body water (E/T) and hemoglobin (Hb) in the PR group increased significantly compared with the PL group after the 12-weeks intervention. Hb in the PR group was 118.26 ± 14.28 g/L compared with 109.14 ± 9.57 g/L in the PL group (p = 0.034) and low-density lipoprotein in PR group was 1.86 ± 0.60 (mmol/l) compared with 2.35 ± 0.64 (mmol/l) in the PL group (p = 0.017).
Comparison of gut microbiota composition between PR and PL group
There was no significant difference in ACE, Chao, Shannon, and Simpson indices between the PR and PL groups before and after intervention (P > 0.05) (Supplementary Fig. 1). PCoA analysis based on weighted UniFrac distance showed that there were differences in the bacterial diversity composition between the PR and PL groups both at baseline and after intervention, but there was no significant change in each group before and after intervention (Fig. 2). The Adonis analysis results also confirm this point (Supplementary Table 2).
At phylum level, Firmicutes, Bacteroidetes and Proteobacteria were the most dominant phyla in the four groups. Firmicutes, Bacteroidetes and Fusobacteriota showed significant differences between the PR group and the PL group after intervention. The abundance of Firmicutes in the PR group was significantly higher than that in the PL group, and the abundance of Bacteroidetes and Fusobacteriota was significantly lower than that in the PL group after intervention (Fig. 3). At the genus level, the most dominant bacteria genera in the four groups were Bacteroides,.the abundance of Fusobacterium and Bilophila in the PR group was significantly lower than that in the PL group after intervention (Fig. 4).
Intestinal microbiota composition of the probiotic group and placebo group at phylum levels. PLJ baseline of placebo group. PLF placebo group after intervention.PRJ Probiotics baseline. PRF after probiotic intervention. P1 Comparison between PRJ and PLJ. P2 Comparison between PRF and PLF. *: P < 0.05,**: P < 0.01 indicates statistically significant differences.
Intestinal microbiota composition of the probiotics group and placebo group at genus levels. PLJ baseline of placebo group. PLF placebo group after intervention.PRJ Probiotics baseline. PRF after probiotic intervention. P1 Comparison between PRJ and PLJ. P2 Comparison between PRF and PLF. *: P < 0.05, **: P < 0.01 indicates statistically significant differences.
Change of concentration of SCFAs before and after intervention in PR and PL group
There was no significant difference in the baseline concentration of SCFAs between the PR group and the PL group (P > 0.05) (Table 2).
The score of GI symptoms before and after intervention in the PR and PL group
There was no statistically significant difference in GSRS scores between the PR group and the PL group at baseline. After intervention, the scores of digestive disorders, constipation, and overall GSRS in PR group were significantly lower than those in PL group (Table 3). In addition, the GI symptoms of 30 PD patients (85.7%) in the PR group were significantly relieved, while 5 PD patients (33.3%) in the PL group were relieved, with statistically significant differences (Supplementary Table 3). Therefore, probiotics could significantly improve GI discomfort symptoms in PD patients, especially indigestion and constipation symptoms.
Discussion
Probiotics of lactic acid bacteria have been applied in the food industry25. However, the effect of probiotics on the nutrition in PD patients has seldom been reported. In this randomized, double-blind, placebo-controlled study, we found that intestinal microbiota diversity were not significantly affected after 12 weeks of intervention with LP N1115 in 51 PD patients, but the microbiota composition appears to have changed favourably. In addition, probiotics can significantly improve GI symptoms.
The effect of probiotics on intestinal ecology in PD patients is still unclear. Herein, we explored the effects of probiotics on intestinal microbiome and SCFAs. Specifically, there was no significant difference in intestinal microbial diversity and distribution between PL group and PR group after the intervention. However, the abundances of Firmicutes in the PR group were significantly higher, and the abundances of Bacteroidetes, Fusobacterium and Bilophilawere significantly lower than those in the PL group. Among them, Firmicutes are the dominant bacteria in the human gut and have strong resistance26, which have many beneficial strains, and most of the butyric acid in SCFAs is produced by it. Butyric acid is the only SCFA associated with GI health including mucosal immunity, immunity and integrity, which also plays an important role in metabolic health including body weight regulation and glucose homeostasis27. Bacteroides produce SCFAs such as succinate, acetate, butyrate, and occasionally propionate, which have many beneficial influences on the host. However, some Bacteroidesspp. can be opportunistic pathogens in scenarios of GI disease, trauma, cancer, or GI surgery, and cause infection, most commonly intra-abdominal infection28. The role of Bacteroidesin CKD is not fully understood29. Fusobacterium necrophorum and Fusobacterium nucleatum are the main strains in the genus Fusobacterium, both of which are opportunistic pathogens. F. necrophorumcan cause angina, peritonitis, liver abscess, birth canal infection and severe cases may appear bacteremia30,31. Bilophilais also an opportunistic pathogen that produces hydrogen sulfide, which has been linked to bowel disease. Additionaly, it has been shown to inhibit metabolic homeostasis, and reduce the production of butyrate by the microbiome32. In addition, there was no significant difference in the concentration of SCFAs between the PR and PL groups after intervention. Although the SCFAs producing microbiota increased after intervention, the type and amount of increase were relatively small, which may be the reason why the level of metabolic product SCFAs did not change significantly. In addition, no significant difference in SCFAs levels between the PR group and PL group after intervention could not completely rule out the influence of dietary confounders. Although there was no significant difference in SCFAs between the two groups at baseline, the two groups maintained the same diet as before during the intervention. We did not keep detailed dietary records intake during the study, and consumption of prebiotic fibers influences microbiota composition and levels of SCFA33. These results show that LP N1115 have a certain effect on intestinal microbiota, increasing the production of potential beneficial microbiota and reducing the harmful microbiota.
Anemia is a common and serious complication of chronic renal failure that reduces patients’ quality of life, and increases the risk of death34. Previous studies of the effects of probiotics on Hb levels in HD patients have produced inconsistent results35,36. In this study, Hb did not increased in the PR group but decreased significantly in the PL group after intervention, and there was a significant difference between the two groups, indicating that probiotics may be beneficial to decrease the fluctuation of Hb level and avoid Hb reduction in PD patients. Considering that inflammation is one of the main factors affecting anemia and Hb fluctuations in patients with CKD37. The previous study has found that LP N1115 could reduces the release of inflammatory factors15. In our study, we found no significant difference in C-reactive protein (CRP) levels between the two groups before and after intervention. However, the relatively more significant decrease in CRP levels in the probiotic group after the intervention might explain the results of the changes in Hb. In line with the results of this study, probiotics have a positive effect on lowering low-density lipoprotein in patients with CKD and PD11,14, showing that the use of probiotics have beneficial effects on lipid. The probiotics in this study contained an added amount of > 80% FOS, which acts as a prebiotic and might interact with the bile acid metabolism in the intestine to inhibit cholesterol reabsorption38. In addition, body composition analysis of patients found that FTI and ECW/TBW ratio in the PR group was higher than that in the PL group. As mentioned before, the changes of intestinal microbiota composition lead to the increase of Firmicutes/Bacteroidetes (F/B) ratio and F/B is usually observed with obesity39, which explains the increase of FTI value. Some previous studies have indicated that high FTI in PD patients confers survival advantages40,41. That is similar to the reverse epidemiology in HD patients42. Whereas others have shown FTI is associated with an increased risk of death and poor quality life in PD patients43,44. The effects of FTI on PD patients is not exactly clear. As well known, increased ECW/TBW represents fluid overload and associated with mortality and cardiovascular events45. Probiotics has been shown in animal experiments to increase water intake46, which can directly lead to changes in body composition including ECW/TBW47. At the same time, probiotics promote water absorption by increasing the expression of aquaporins in the colon48. Few studies have reported on the effects of probiotics therapy on body composition in PD patients, and the possible effects of probiotics on ECW/TBW needs further studies. In addition, it should be noted here that none of the subjects took lipid lowering medication, anti-anemia medication, phosphorus- or potassium-adsorbing agents, anti-microbial medication, and gastric acid lowering medication in the study period.
The present study showed that 56.9% PD patients developed GI symptoms. Consistent with the findings of Yi C et al.49, dyspepsia and constipation are the most common GI symptoms in PD patients. Probiotics have also been shown to improve the GI symptoms in HD patients50. However, a randomized controlled trial in patients with CKD showed that there was no significant changes in the GSRS after synbiotic therapy51. It is worth noting that our findings provide evidence for the beneficial effects of probiotics supplementation on GI health in PD patients, and speculate that this may be related to probiotics affecting the composition of the gut microbiota. Even though constipation is notably rarer in PD than in HD patients, its clinical impact is relatively more serious. Improvements in the constipation score might prevent frequent complications, including peritonitis episodes or mechanical complications, reducing the rate of hospitalizations and improving quality of life for this group of patients52.
There are some limitations to our study. First, the relatively small sample size of this study may have amplified individual differences. Second, this study was limited to PD patients in our hospital, and there were also factors such as geography, ethnicity, and living habits. Therefore, the generalizability of the current results may be limited. Third, the follow-up time was relatively short, treatment time may bring different microbiological findings, such as the difference in SCFAs changes may be more significant. Finally, the gut microbiota metabolites that our study focused on were SCFAs, it would have been informative if we evaluated other gut-derived uremic toxins such as indoxyl sulfate (IS) and p-cresol sulfate (PCS). This study is a preliminary exploration of intestinal microecological preparations in the treatment of PD population and their effects on fecal microbiota and SCFAs. In the future, we will increase the sample size to further explore how probiotics, especially their metabolic behavior, play a role in PD patients and its influencing factors.
In summary, the twelve-week probiotics supplementation, as investigated in the current study, had no significant effects on the primary outcome of SCFAs and bacterial diversity but altered the composition of the gut microbiota in PD patients. Specifically, the structure of the intestinal microbiota was characterized by an increase in the proportions of SCAFs-producing bacteria (Firmicutes) and a reduction in harmful bacteria (Bacteroidetes, Fusobacterium, Bilophila), which might be beneficial to gut health in PD patients. In addition, the probiotics could significantly alleviate GI complications in PD patients, including dyspepsia and constipation. Also, probiotics have certain benefits on the clinical characteristics of PD patients. Although our findings added new evidence for the clinical application of probiotics to remodel the disturbed gut microbiota, and to alleviate the GI complications, more studies are required to further explore the underlying mechanisms and verify the effectiveness and safety of probiotics.
Data availability
The raw sequence data of the 16S rRNA gene V3-V4 regions and relevant information are available in NCBI Sequence Read Archive database under accession number PRJNA1193117 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1193117?reviewer = ffklaplutmfo7jdf77s6u61cs)and PRJNA704190 (http://dataview.ncbi.nlm.nih.gov/object/PRJNA704190?reviewer = ofesdl3lcssb4q0oto9hir9m78). Other datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhao, J. et al. Specific alterations in gut microbiota in patients with chronic kidney disease: an updated systematic review. Ren. Fail. 43, 102–112. https://doi.org/10.1080/0886022X.2020.1864404 (2021).
Jiang, N. et al. Clinical characteristics associated with the properties of gut microbiota in peritoneal dialysis patients. Perit. Dial. Int. 41, 298–306. https://doi.org/10.1177/0896860820976983 (2021).
Tian, N., Li, L., Ng, J. K. & Li, P. K. The potential benefits and controversies of probiotics use in patients at different stages of chronic kidney disease. Nutrients 14, 4044. https://doi.org/10.3390/nu14194044 (2022).
Tian, N. et al. Relationship between gut microbiota and nutritional status in patients on peritoneal dialysis. Sci. Rep. 13, 1572. https://doi.org/10.1038/s41598-023-27919-3 (2023).
Devlin, A. S. et al. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe 20, 709–715. https://doi.org/10.1016/j.chom.2016.10.021 (2016).
Markowiak-Kopeć, P. & Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 12, 1107. https://doi.org/10.3390/nu12041107 (2020).
Tingö, L. et al. Potential modulation of inflammation by probiotic and omega-3 supplementation in elderly with chronic low-grade inflammation-A randomized, placebo-controlled trial. Nutrients 14, 3998. https://doi.org/10.3390/nu14193998 (2022).
Tanaka, H., Sirich, T. L. & Meyer, T. W. Uremic solutes produced by colon microbes. Blood Purif. 40, 306–311. https://doi.org/10.1159/000441578 (2015).
Lano, G., Burtey, S. & Sallée, M. Indoxyl, Sulfate, a uremic endotheliotoxin. Toxins 12, 229. https://doi.org/10.3390/toxins12040229 (2020).
Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455. https://doi.org/10.1161/CIRCRESAHA.116.305360 (2015).
Zheng, H. J. et al. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 61, 577–598. https://doi.org/10.1080/10408398.2020.1740645 (2021).
McFarlane, C., Ramos, C. I., Johnson, D. W. & Campbell, K. L. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J. Ren. Nutr. 29, 209–220. https://doi.org/10.1053/j.jrn.2018.08.008 (2019).
Jia, L., Jia, Q., Yang, J., Jia, R. & Zhang, H. Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Press. Res. 43, 1623–1635. https://doi.org/10.1159/000494677 (2018).
Pan, Y. et al. Effects of probiotics on malnutrition and health-related quality of life in patients undergoing peritoneal dialysis: a randomized controlled trial. J. Ren. Nutr. 31, 199–205. https://doi.org/10.1053/j.jrn.2020.04.008 (2021).
Hu, Y. C. et al. Effects of lactobacillus paracasei N1115 on gut microbial imbalance and liver function in patients with hepatitis B-related cirrhosis. World J. Gastroenterol. 30, 1556–1571. https://doi.org/10.3748/wjg.v30.i11.1556 (2024).
Dimenäs, E. et al. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand. J. Gastroenterol. 28, 681–687. https://doi.org/10.3109/00365529309098272 (1993).
Auguste, B. L. & Bargman, J. M. Peritoneal dialysis prescription and adequacy in clinical practice: core curriculum 2023. Am. J. Kidney Dis. 81, 100–109. https://doi.org/10.1053/j.ajkd.2022.07.004 (2023).
Svedlund, J., Sjödin, I. & Dotevall, G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 33, 129–134. https://doi.org/10.1007/BF01535722 (1988).
Wang, S. et al. Whole genome sequence of the probiotic strain lactobacillus paracasei N1115, isolated from traditional chinese fermented milk. Genome Announc. https://doi.org/10.1128/genomeA.00059-14 (2014).
Sun, Y., Chen, S., Ren, F. & Li, Y. Lactobacillus paracasei N1115 attenuates obesity in high-fat diet-induced obese mice. Food Sci. Nutr. 11, 418–427. https://doi.org/10.1002/fsn3.3073 (2023).
Dang, C. et al. In vitro intervention of lactobacillus paracasei N1115 can alter fecal microbiota and their SCFAs metabolism of pregnant women with constipation and diarrhea. Curr. Microbiol. 79, 212. https://doi.org/10.1007/s00284-022-02906-5 (2022).
Pu, F. et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial[J]. Clin. Interv. Aging 12, 1223–1231. https://doi.org/10.2147/CIA.S141518 (2017).
Li, P. et al. Effect of lacticaseibacillus paracasei N1115 on immunomodulatory and gut microbial composition in young children: a randomized, placebo-controlled study. Nutrients https://doi.org/10.3390/nu15081970 (2023).
Knight, R. et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 16, 410–422. https://doi.org/10.1038/s41579-018-0029-9 (2018).
Bai, M. et al. Probiotic lactobacillus casei zhang improved the properties of stirred yogurt. Food Biosci. 37, 100718. https://doi.org/10.1016/j.fbio.2020.100718 (2020).
Browne, H. P. et al. Host adaptation in gut firmicutes is associated with sporulation loss and altered transmission cycle. Genome. Biol. 22, 204. https://doi.org/10.1186/s13059-021-02428-6 (2021).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455. https://doi.org/10.3920/BM2020.0057 (2020).
Shin, J. H. et al. Bacteroides and related species: The keystone taxa of the human gut microbiota[J]. Anaerobe 85, 102819. https://doi.org/10.1016/j.anaerobe.2024.102819 (2024).
De, A. M. et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 9, e99006. https://doi.org/10.1371/journal.pone.0099006 (2014).
Engevik, M. A. et al. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. mBio. 12, e02706-e2720. https://doi.org/10.1128/mBio.02706-20 (2021).
Chen, Y. et al. Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics 10, 323–339. https://doi.org/10.7150/thno.38870 (2020).
Natividad, J. M. et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 9, 2802. https://doi.org/10.1038/s41467-018-05249-7 (2018).
Cantu-Jungles, T. M., Rasmussen, H. E. & Hamaker, B. R. Potential of prebiotic butyrogenic fibers in parkinson’s disease. Front. Neurol. 10, 663. https://doi.org/10.3389/fneur.2019.00663 (2019).
KDOQI; National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am. J. Kidney. Dis. 47, S11–S145. https://doi.org/10.1053/j.ajkd.2006.03.010 (2006).
Haghighat, N. et al. The effect of synbiotic and probiotic supplementation on mental health parameters in patients undergoing hemodialysis: a double-blind, randomized, placebo-controlled trial. Indian J. Nephrol. 31, 149–156. https://doi.org/10.4103/ijn.IJN_341_19 (2021).
Shariaty, Z., Mahmoodi, S. G., Farajollahi, M., Amerian, M. & Behnam, P. N. The effects of probiotic supplement on hemoglobin in chronic renal failure patients under hemodialysis: A randomized clinical trial. J. Res. Med. Sci. 22, 74. https://doi.org/10.4103/jrms.JRMS_614_16 (2017).
Mora-Gutiérrez, J. M., Ferrer-Nadal, A. & García-Fernández, N. Effect of pentoxifylline on anaemia control in haemodialysis patients: retrospective observational case-control study[J]. Nefrologia 33, 524–531. https://doi.org/10.3265/Nefrologia.pre2013.Apr.11654 (2013).
O’Connor, S., Chouinard-Castonguay, S., Gagnon, C. & Rudkowska, I. Prebiotics in the management of components of the metabolic syndrome. Maturitas 104, 11–18. https://doi.org/10.1016/j.maturitas.2017.07.005 (2017).
Stojanov, S., Berlec, A. & Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8, 1715. https://doi.org/10.3390/microorganisms8111715 (2020).
Paudel, K., Visser, A., Burke, S., Samad, N. & Fan, S. L. Can bioimpedance measurements of lean and fat tissue mass replace subjective global assessments in peritoneal dialysis patients?. J. Ren. Nutr. 25, 480–487. https://doi.org/10.1053/j.jrn.2015.05.003 (2015).
Parthasarathy, R., Oei, E. & Fan, S. L. Clinical value of body composition monitor to evaluate lean and fat tissue mass in peritoneal dialysis. Eur. J. Clin. Nutr. 73, 1520–1528. https://doi.org/10.1038/s41430-019-0391-3 (2019).
Kalantar-Zadeh, K., Block, G., Humphreys, M. H. & Kopple, J. D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 63, 793–808. https://doi.org/10.1046/j.1523-1755.2003.00803.x (2003).
Verger, C. et al. Association of prescription with body composition and patient outcomes in incident peritoneal dialysis patients. Front. Med. 8, 737165. https://doi.org/10.3389/fmed.2021.737165 (2021).
Kim, S. M. et al. Associations among body composition parameters and quality of life in peritoneal dialysis patients. Sci. Rep. 12, 19192. https://doi.org/10.1038/s41598-022-19715-2 (2022).
Wang, Y. & Gu, Z. Effect of bioimpedance-defined overhydration parameters on mortality and cardiovascular events in patients undergoing dialysis: a systematic review and meta-analysis. J. Int. Med. Res. 49, 675875305. https://doi.org/10.1177/03000605211031063 (2021).
Wu, Y. et al. Probiotics (Lactobacillus plantarum HNU082) supplementation relieves ulcerative colitis by affecting intestinal barrier functions, immunity-related gene expression, gut microbiota, and metabolic pathways in mice. Microbiol. Spectr. 10, e165122. https://doi.org/10.1128/spectrum.01651-22 (2022).
Zhang, J., Zhang, N., Du, S., Liu, S. & Ma, G. Effects of water restriction and water repl-enishment on the content of body water with bioelectrical impedance among young adults in baoding, China: a randomized controlled trial (RCT). Nutrients 13, 553. https://doi.org/10.3390/nu13020553 (2021).
Kopacz, K. & Phadtare, S. Probiotics for the prevention of antibiotic-associated diarrhea. Healthcare 10, 1450. https://doi.org/10.3390/healthcare10081450 (2022).
Yi, C., Wang, X., Ye, H., Lin, J. & Yang, X. Patient-reported gastrointestinal symptoms in patients with peritoneal dialysis: the prevalence, influence factors and association with quality of life. BMC Nephrol. 23, 99. https://doi.org/10.1186/s12882-022-02723-9 (2022).
Viramontes-Hörner, D. et al. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J. Ren. Nutr. 25, 284–291. https://doi.org/10.1053/j.jrn.2014.09.008 (2015).
Rossi, M. et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): q randomized trial. Clin. J. Am. Soc. Nephrol. 11, 223–231. https://doi.org/10.2215/CJN.05240515 (2016).
Kosmadakis, G., Albaret, J., Da, C. C. E., Somda, F. & Aguilera, D. Constipation in peritoneal dialysis patients. Perit. Dial. Int. 39, 399–404. https://doi.org/10.3747/pdi.2018.00169 (2019).
Acknowledgements
We appreciate the help and support from all participants in the study.
Funding
This work was supported by the Natural Science Foundation of China (NSFC) (No. 81960144, 82360153), Ningxia Natural Science Foundation (Key Project, 2022AAC02062), Key R&D Projects in Ningxia Autonomous Region (2022BEG03120), and the Natural Science Foundation Project of The Second Affiliated Hospital of Xi’an Medical University (23KY0122).
Author information
Authors and Affiliations
Contributions
N.T., M.H.C. and P.K.T.L. designed the study. N.T., S.N.Z. and Y.Y.Y. drafted the manuscript. N.T., Y.Y.Y., R.C., N.C. and M.T.W. followed up the cohort. L.W., H.X.Z. and Y.W. participated in the data collection. L.N. dealed the sample. H.Y.R. analyzed the sequence data. N.T., Y.Y.Y. and S.N.Z. performed the statistical analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, S., Yan, Y., Chu, R. et al. Probiotic treatment induces changes in intestinal microbiota but does not alter SCFA levels in peritoneal dialysis patients—a randomized, placebo-controlled trial. Sci Rep 14, 31413 (2024). https://doi.org/10.1038/s41598-024-83056-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83056-5