Abstract
An induced cylinder and spherical power after implantation with an extended depth of focus (EDOF) and enhanced monofocal intraocular lens (IOL) could improve distance, intermediate (60 cm) and near (40 cm) visual acuity (VA). In this prospective study, forty eyes with Eyhance EDOF IOL (Johnson and Johnson, USA) and 40 eyes with Vivity EDOF IOL (Alcon Laboratories Inc. USA) were included. Induced cylinder (applied to non-dominant eye) in steps of + 0.25D were used and then VA was measured monocularly (only non-dominant eye). Similarly, induced sphere (applied to non-dominant eye) in steps of + 0.25D were used and then VA was measured monocularly (only non-dominant eye). The above methods were repeated for the dominant eye as well. Then, binocular defocus curve for each patient was obtained by inducing optimal sphere and cylinder (one at a time in front of the non-dominant eye only). In both IOL groups, induced cylinder and sphere independently led to significant improvement in near and distance vision (p < 0.05). Induced sphere binocularly caused a greater decrease (~ 0.1 LogMAR) in distance VA compared to induced cylinder but this was not clinically significant. Most patients accepted an induced cylinder of +1.0 to +1.5D in both IOL groups. Induced cylinder and sphere caused a favourable improvement in near and intermediate VA after surgery in both IOL groups without a significant drop in distance VA.
Similar content being viewed by others
Introduction
The goal of refractive cataract surgery is to provide optimum distance, intermediate and near vision to patients. Ophthalmologists have experimented with binocular optics to help patients achieve independence from spectacles across a range of distances1,2,3,4,5. Monofocal intraocular lenses (IOLs) correct for one single distance and provide good far vision6. In order to achieve spectacle independence, ophthalmologists have identified different strategies for providing patients with optimal vision at distance, intermediate and near. Advanced IOLs such as multifocal lenses split the incoming light to different foci to provide multifocality7. Changes in spherical aberration (SA) of the IOLs are used to improve the depth of focus and near vision8. Extended depth of focus (EDOF) and enhanced monofocal IOLs are newer generation IOLs that provide distance and intermediate vision using different mechanisms9. Monovision is a commonly used strategy that takes advantage of neuroadaptation to give distance and near vision using monofocal IOL implantation10. Conventionally, the dominant eye is targeted for distance and non-dominant eye for near vision11. Traditionally, full monovision aims at achieving an intended myopia of − 2.5D in the nondominant eye12.
One of the significant drawbacks of monovision is the relative reduction in stereoacuity making it unpopular13,14,15. Modified monovision or mini-monovision attempts to under correct the non-dominant eye by −0.75 to − 1.75 D12. The newer EDOF IOLs are more tolerant to residual refraction error16 and are often used in conjunction with mini or micro-monovision to provide a better tolerated depth of focus and stereo vision. In the Concerto study, micro-monovision method (− 0.50 to − 0.75 D residual myopia in the nondominant eye improved UDVA, spectacle independence and the satisfaction rate for near vision in patients implanted with EDOF IOLs17. Another study described the benefit of implementing mini-monovision in pseudophakic patients to improve spectacle independence and suggested it can be used with premium IOLs12. Various studies claimed to improve near vision by optimizing residual myopic astigmatism with monofocal IOL implantation18,19,20. This could be convenient option to provide patients with useful near vision. Near VA can be restored with uncorrected myopic astigmatism and a slight loss of distance VA in pseudophakic eyes implanted with monofocal IOLs. However, this has not been attempted with EDOF IOLs. These IOLs provide excellent intermediate vision but inadequate quality of vision for near distance21,22. This study aimed at studying the clinical impact of an uncorrected myopic astigmatism on near visual acuity (VA) of patients implanted with EDOF and enhanced monofocal IOLs.
Results
Eighty eyes of 40 patients fulfilling the inclusion and exclusion criteria were included in the study. The mean age of the patients was 62.45 ± 9.07 years (range: 52 to 78 years). The male to female ratio was 7:3. All VA’s were recorded in LogMAR values. The mean spherical refractive error in the Eyhance (0.12 ± 0.35 D; range − 0.75 to 0.75 D) group was similar to Vivity (0.00 ± 0.37D; range − 1.25 D to 1.00 D) group (p = 0.11). The mean cylindrical refractive error was similar in the Vivity (−0.44 ± 0.39 D; range − 1.50 D to 0 D) and Eyhance (−0.31 ± 0.37; range: − 1 D to 0.75 D) group (p = 0.13).
The proportion of eyes (%) which reported best near VA for each induced cylinder under binocular condition in the two study groups is shown in (Table 1). The proportion of eyes (%) which reported best near VA for each induced sphere under binocular condition in the two study groups is shown in (Table 2). These proportions were significantly different between the two eye groups (p < 0.001). Similarly, the percentage distribution of eyes with optimal sphere for best near VA was significantly different between the two eye groups (p < 0.001). Thus, an optimal cylinder of + 1 D was accepted by the greatest number of eyes in the Eyhance and Vivity groups. Similarly, an optimal sphere of + 1 D was accepted by the greatest number of eyes in the Vivity group. In the Eyhance group, 45% of the eyes accepted an optimal sphere ranging from + 1 to + 1.5 D.
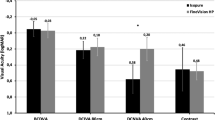
The change in the VA of eyes after the induction of positive cylinder (experiment 1) was significant (p < 0.05) across the range of defocus (Tables 3 and 4). The change in the VA after the induction of the positive sphere (experiment 1) was significant (p < 0.05) across the range of defocus except at the defocus of -1D sphere (Tables 5 and 6). Figure 1a and b (experiment 1) show the defocus curves (before and after induction of positive cylinder) in the Eyhance group and Vivity group, respectively, measured monocularly in the non-dominant eye. Similarly, Fig. 2a and b (experiment 2) show the defocus curves (before and after induction of positive cylinder) in the Eyhance group and Vivity group, respectively, measured binocularly. Overall, a decrease in VA was observed with defocus in both groups after induction of positive cylinder. With the induced cylinder, the distance VA marginally dropped while the intermediate and near VA were significantly better (p < 0.05). This trend was noted in the defocus curves of both the IOL groups when plotted monocularly and binocularly. In Fig. 3a and b (experiment 1), the defocus curves of the non-dominant eye before and after induction of positive cylinder and sphere (induced separately) in the Eyhance and Vivity IOL groups, respectively. As expected, the distance VA following induction of positive sphere was similar to the effect of positive cylinder. However, both intermediate and near VA were significantly improved (p < 0.05). A marginal drop in distance VA and improvement in near as well as intermediate VA was observed, when measured binocularly after induction of positive sphere and cylinder, in both Eyhance (Fig. 4a) and Vivity (Fig. 4b) IOL groups. Figure 4a and b show data from both experiments 2 and 3.
(a) Defocus curve derived from monocular testing of non-dominant eye (with induced cylinder) implanted with Eyhance intraocular lens; (b) Defocus curve derived from monocular testing of non-dominant (with induced cylinder) eye implanted with Vivity intraocular lens. Patients were made emmetropic for distance before the defocus curve was measured.
(a) Defocus curve derived from binocular testing with induced cylinder in the non-dominant eye implanted with Eyhance intraocular lens; (b) Defocus curve derived from binocular testing with induced cylinder in the non-dominant eye implanted with Vivity intraocular lens. Patients were made emmetropic for distance before the defocus curve was measured.
(a) Defocus curve derived from monocular testing of non-dominant eye (with induced sphere) implanted with Eyhance intraocular lens. Data from Fig. 1a is also included for comparison; (b) Defocus curve derived from monocular testing of non-dominant (with induced sphere) eye implanted with Vivity intraocular lens. Data from Fig. 1b is also included for comparison. Patients were made emmetropic for distance before the defocus curve was measured.
(a) Defocus curve derived from binocular testing with induced sphere in the non-dominant eye implanted with Eyhance intraocular lens. Data from Fig. 2a is also included for comparison; (b) Defocus curve derived from binocular testing with induced sphere in the non-dominant eye implanted with Vivity intraocular lens. Data from Fig. 2b is also included for comparison. Patients were made emmetropic for distance before the defocus curve was measured.
Discussion
Techniques have used different optical aberrations and adjustments to enhance the depth of field1,3,4,5,19,23,24,25. In patients unwilling to pay or unsuitable for multifocal IOLs, monovision can be used by treating the dominant eye for distance and non-dominant eye for near vision. Similarly, residual astigmatism can be used to improve the depth of focus. In an emmetropic eye, rays of light from all axes focus on the retina to create a sharp image. In simple myopic astigmatism, only the astigmatic axis is defocused while rays of light from all other axes focus on the retina. The myopic axis helps focus near objects better. Thus, only the rays of light falling on the myopic astigmatic axis will focus on near objects while rays of light falling from other axes will focus for distance. This helps by compromising the distance vision to a lesser extent as compared to conventional monovision where all axes will focus for near since a spherical defocus is applicable across all axes. The flipside to this method would be that the near vision with cylindrical monovision may not be as sharp as that with spherical monovision due to the same reason. In our study, we evaluated the visual performance of an EDOF and an enhanced monofocal IOL, which have better tolerance to defocus and astigmatism than monofocal IOLs5. The authors aimed to identify if planning cylindrical monovision with EDOF and enhanced monofocal IOLs would help improve near vision without compromising the distance vision significantly.
We found that the change in the VA before and after the induction of positive cylinder and positive sphere was significant across the range of defocus regardless of the dominance of the eye. Though the distance vision was marginally lower with the induced positive cylinder and sphere, this decline may not be clinically significant binocularly. The defocus curves showed the changes in VA across the range of defocus when a patient was given an induced cylinder. Zero defocus represented distance vision, -1.5 D defocus represented intermediate vision and − 2.5 D represented near VA. Our results showed that the distance VA dropped marginally (monocularly) but intermediate and near VA improved with cylindrical monovision and these changes were similar between the Eyhance and Vivity group regardless of the dominance of an eye. Against the rule astigmatism benefitted greater number of patients than with the rule astigmatism in our study which was similar to the findings of an earlier study26. However, another study reported that with the rule astigmatism was beneficial for increasing the depth of focus19. The higher incidence of against the rule astigmatism benefiting near vision was probably a result of a higher incidence of against the rule astigmatism in elderly population compared to with the rule astigmatism. In a myopic astigmatism and presbyopia trial, it was claimed that low astigmatism improved the depth of focus5. Against the rule astigmatism was beneficial to near VA in a number of other studies4,27,28.
The Tecnis symphony EDOF IOL demonstrated excellent tolerance to residual refractive errors and that monocular and binocular UDVA did not greatly change with a mere difference of 0.05 LogMAR between a postoperative SE of ± 0.25 and ± 2.00 D29. The impact of induced astigmatism on the VA was evaluated in another study where four different types of multifocal lenses were compared30. In that study, highest tolerance with an EDOF IOL was observed, which retained good VA (0.7 decimal scale) even with an induced astigmatism of − 1.50D30. On the other hand, multifocal IOLs had a significant compromise of visual performance even with a small amount residual refractive error31. Targeting EDOF and enhanced monofocal IOLs for emmetropia can result in an improved intermediate VA but barely functional near vision and low degrees of ametropia may benefit near vision with such IOLs16. Enhancement of depth of focus with induced primary astigmatism was derived from the Theory of Sturm’s hypothesis. This theory stated that when there is a single point refracted through an astigmatic lens, there are two focal points separated by a focal interval4. If this extension of the focal range does not significantly deteriorate the retinal image quality (according to the definition of the depth of focus)32, it could result in an increased depth of focus. In the present study with induced cylinder or sphere, the spherical equivalent was not equal to zero and so the circle of least confusion did not fall on the retina leading to a mild compromise in distance vision.
The uncorrected myopic astigmatism improved near VA18. However, this gain was proportional to the loss in distance visual acuity in pseudophakic patients. The earlier studies were done with monofocal IOLs while our study was done with the Vivity and Eyhance (an EDOF and enhanced monofocal IOL, respectively). The EDOF lenses have better tolerance to defocus compared to monofocal and trifocal IOLs because of their design16,33,34. Their broad “landing zone” made them more forgiving to residual refractive error. Like any other study, our study also had a few limitations. The results of this study are in line with another study which reported that correcting astigmatism less than 0.5D did not result in degraded distance VA and potential benefits of fully correcting astigmatism in patients undergoing cataract surgery were limited35. Since the incision causes minimal changes in corneal aberrations, the current analyses clearly shows that the changes in the internal aberrations of an eye determined the VA of a patient at all reading distances36. First, the refractive tolerances were only tested with EDOF and enhanced monofocal IOLs. Ideally, at least three groups (other type of diffractive-refractive, EDOF or monofocal IOLs) should be tested to benefit from targeted ametropia. Another limitation of this study was that the quality of vision was not assessed, e.g., a subjective questionnaire and contrast sensitivity. It’s possible that patients with same VA may have different quality of vision. Future studies should address these limitations and prospectively evaluate the outcomes of residual refractive errors in patients with all types of non-monofocal IOLs.
Conclusions
Our study results suggest leaving a residual myopic astigmatism or spherical monovision while implanting the Vivity or Eyhance IOL in patients wanting better intermediate and near VA. These patients must be informed about the possible compromise on distance vision under these circumstances.
Methods
The study was approved by the Ethics’ Committee of Narayana Nethralaya eye hospital, India (Approval No: NNIEC/2023/05/06). The study was conducted in accordance with the tenets of the Declaration of Helsinki. A prospective observational study was commenced at our tertiary care eye hospital in Bengaluru, South India. A written informed consent was taken from all the patients. Eighty eyes of 40 patients having undergone uncomplicated cataract surgery a minimum of 5 weeks prior were included in the study. These patients were divided into two groups, those having undergone implantation (n = 20 patients) with Tecnis Eyhance (Johnson & Johnson Vision, Santa Ana, CA, USA) and those (n = 20 patients) with AcrySof IQ Vivity IOL (Alcon Laboratories, USA). Patients with either pre-existing retinal or ocular pathologies were excluded from the study. We also excluded patients who had an interocular difference of 0.2 LogMAR or greater in distance VA.
Patients were tested for distance (6 m), intermediate (60 cm) and near visual acuity (at 40 cm) monocularly and binocularly. Then, all patients underwent subjective refraction by a single experienced optometrist. Patients were made emmetropic for distance before every measurement. Assessments were done as described in an earlier study37. The dominance of the eyes was determined by the hole in the card test instructing the patient to fixate one letter at distance through a “hole” between his/her hands having their arms outstretched. Then, the patient’s eyes were occluded alternately and the patient was asked to report when the target was visible. The eye which could maintain fixation on the target while the fellow eye was occluded was the dominant eyes. There were 3 sets of assessments done for each patient:
-
Experiment 1 (monocular): First, the impact of magnitude and axis of induced myopic astigmatism (by placing a plus cylinder in the trial frame) on distance, intermediate and near VA was assessed. As per the standard protocol established for reporting IOL outcomes, the patients first underwent manifest refraction at distance which was in turn adjusted to infinity by adding −0.25D37. After making the patient emmetropic for distance, a plus cylinder at the patient’s accepted axis was placed in front of the non-dominant eye. With the manifest refraction in place, the plus cylinder (induced myopic astigmatism) was increased in steps of + 0.25D starting from +0.25D to +2.5D at the patient’s subjective acceptance axis. The VA of the same eye (non-dominant eye) for distance, intermediate (60 cm) and near (40 cm) were recorded. These astigmatic lenses were placed before the non-dominant eye on a trial frame at 12-mm vertex distance while the dominant eye was occluded. Distance, intermediate and near VA of the non-dominant eye were determined using standard ETDRS charts in LogMAR units38. No near-addition lenses were worn by subjects while measuring near or intermediate VA. The value of the cylindrical power placed in front of the non-dominant eye that helped achieve best near vision was considered as the optimal cylinder for this experiment. Then, all the above steps were repeated to assess the impact of induced sphere by placing a plus sphere in steps of + 0.25 to + 2.5 D in front the non-dominant eye after achieving emmetropia. The dominant eye was left occluded here as well. The value of the spherical power placed in front of the non-dominant eye that helped achieve best near vision was considered as the optimal sphere for this experiment. Then, all the above steps were repeated for the dominant eye while keeping the non-dominant eye occluded.
-
Experiment 2 (binocular): This determined the impact of magnitude and axis of induced astigmatism (by placing the optimal plus cylinder in the trial frame) on distance, intermediate and near VA of pseudophakes tested binocularly. The plus cylinder was placed in front of the non-dominant eye only and the VA of the patient was tested by keeping both eyes open.
-
Experiment 3 (binocularly): This determined the impact of magnitude and axis of induced sphere (by placing a plus sphere in the trial frame) on distance, intermediate and near VA of pseudophakes tested binocularly. In this experiment, the plus sphere was placed in front of the non-dominant eye and the VA of the patient was tested by keeping both eyes open.
The axis at which the patients reported the most improvement in near VA for a given induced cylinder was considered as the patient acceptance axis.
The defocus curve is a method by which the best distance VA achieved by the patient is recorded for every + or – power placed in front of their eyes. The defocus (+ or – lenses) were placed in front of both the dominant and non-dominant eye before recording the VA. After making the patient emmetropic for distance, a baseline defocus curve was plotted using spherical powers between + 2 D to -5 D in steps of 0.5 D39. For each patient, the optimal cylinder and sphere was determined as explained in experiment 1. After identifying the optimal cylinder and optimal sphere for the non-dominant eye in experiment 1, the defocus curves were plotted after placing the optimal cylinder and optimal sphere (one at a time) in front of the non-dominant eye. Thus, three defocus curves were plotted: (1) baseline, (2) with optimal induced cylinder and (3) with optimal induced sphere. The optimal sphere or optimal cylinder was induced in the non-dominant eye only and the defocus was introduced binocularly. This was done to identify the impact of induced cylinder and sphere in front of the non-dominant eye on the defocus curves obtained via binocular testing of distance VA. Comparing the defocus curves could help establish the possible benefits and pitfalls experienced by the patient if such strategies were implemented. It was compared for a range of defocus from + 0.5 to -0.5 D for distance, -0.5 to -1.5 D for intermediate and near − 1.5 to -2.5 D for near vision.
Statistical analyses
The parameters were summarized as mean and standard deviation for normally distributed data. Kruskal-Wallis test was used to compare parameters between the two groups, if non-parametric distribution was obtained. Friedman test was used to analyse data within a group before and after induction of sphere or cylinder. A p-value less than 0.05 was considered statistically significant. MedCalc v20.015 (MedCalc Inc., Ostend, Belgium) software was used for all statistical analyses.
Data availability
The data for this study was collected from the research database of Narayana Nethralaya eye hospital in Bangalore, India with permission. Due to patient privacy protection, the availability of the data is restricted and not publicly accessible. For any data access requests, contact Dr. Naren Shetty at [email protected].
References
Rocha, K. M., Vabre, L., Chateau, N. & Krueger, R. R. Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. J. Cataract. Refract. Surg. 35, 1885–1892 (2009).
Huber, C. Planned myopic astigmatism as a substitute for accommodation in pseudophakia. J. Am. Intraocul. Implant. Soc. 7, 244–249 (1981).
Sawusch, M. R. & Guyton, D. L. Optimal astigmatism to enhance depth of focus after cataract surgery. Ophthalmology 98, 1025–1029 (1991).
Nagpal, K. M., Desai, C., Trivedi, R. H. & Vasavada, A. R. Is pseudophakic astigmatism a desirable goal? Indian J. Ophthalmol. 48, 213–216 (2000).
Savage, H., Rothstein, M., Davuluri, G., El Ghormli, L. & Zaetta, D. M. Myopic astigmatism and presbyopia trial. Am. J. Ophthalmol. 135, 628–632 (2003).
Cao, K. et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv. Ophthalmol. 64, 647–658 (2019).
Kim, B. H., Hyon, J. Y. & Kim, M. K. Effects of bifocal versus trifocal diffractive intraocular lens implantation on visual quality after cataract surgery. Korean J. Ophthalmol. 33, 333–342 (2019).
Hervella, L., Villegas, E. A., Robles, C. & Artal, P. Spherical aberration customization to extend the depth of focus with a clinical adaptive optics visual simulator. J. Refract. Surg. 36, 223–229 (2020).
Kanclerz, P., Toto, F., Grzybowski, A. & Alio, J. L. Extended depth-of-field intraocular lenses: An update. Asia Pac. J. Ophthalmol. (Phila) 9, 194–202 (2020).
J Evans, B. Monovision: a review. Ophthalmic Physiol. Opt. 27, 417–439 (2007).
Xiao, J., Jiang, C. & Zhang, M. Pseudophakic monovision is an important surgical approach to being spectacle-free. Indian J. Ophthalmol. 59, 481–485 (2011).
Goldberg, D. G., Goldberg, M. H., Shah, R., Meagher, J. N. & Ailani, H. Pseudophakic mini-monovision: high patient satisfaction, reduced spectacle dependence, and low cost. BMC Ophthalmol. 18, 293 (2018).
Finkelman, Y. M., Ng, J. Q. & Barrett, G. D. Patient satisfaction and visual function after pseudophakic monovision. J. Cataract. Refract. Surg. 35, 998–1002 (2009).
Marques, F. F. et al. Evaluation of visual performance and patient satisfaction with pseudophakic monovision technique. Arq. Bras. Oftalmol. 72, 164–168 (2009).
Ito, M., Shimizu, K., Amano, R. & Handa, T. Assessment of visual performance in pseudophakic monovision. J. Cataract. Refract. Surg. 35, 710–714 (2009).
Son, H. S., Kim, S. H., Auffarth, G. U. & Choi, C. Y. Prospective comparative study of tolerance to refractive errors after implantation of extended depth of focus and monofocal intraocular lenses with identical aspheric platform in Korean population. BMC Ophthalmol. 19, 187 (2019).
Cochener, B. & Concerto Study, G. Clinical outcomes of a new extended range of vision intraocular lens: International multicenter concerto study. J. Cataract. Refract. Surg. 42, 1268–1275 (2016).
Singh, A., Pesala, V., Garg, P. & Bharadwaj, S. R. Relation between uncorrected astigmatism and visual acuity in pseudophakia. Optom. Vis. Sci. 90, 378–384 (2013).
Leube, A., Ohlendorf, A. & &Wahl, S. The influence of induced astigmatism on the depth of focus. Optom. Vis. Sci. 93, 1228–1234 (2016).
Bradbury, J. A., Hillman, J. S. & Cassells-Brown, A. Optimal postoperative refraction for good unaided near and distance vision with monofocal intraocular lenses. Br. J. Ophthalmol. 76, 300–302 (1992).
Bohm, M., Petermann, K., Hemkeppler, E. & Kohnen, T. Defocus curves of 4 presbyopia-correcting IOL designs: Diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J. Cataract. Refract. Surg. 45, 1625–1636 (2019).
Alio, J. L. & Presbyopic lenses evidence, masquerade news, and fake news. Asia Pac. J. Ophthalmol. (Phila) 8, 273–274 (2019).
Huber, C. Myopic astigmatism as a substitute for accommodation in pseudophakia. Dev. Ophthalmol. 5, 17–26 (1981).
Rocha, K. M., Soriano, E. S., Chamon, W., Chalita, M. R. & Nose, W. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: a prospective randomized study. Ophthalmology 114, 2050–2054 (2007).
Benard, Y., Lopez-Gil, N. & Legras, R. Optimizing the subjective depth-of-focus with combinations of fourth- and sixth-order spherical aberration. Vis. Res. 51, 2471–2477 (2011).
Nanavaty, M. A., Vasavada, A. R., Patel, A. S., Raj, S. M. & Desai, T. H. Analysis of patients with good uncorrected distance and near vision after monofocal intraocular lens implantation. J. Cataract. Refract. Surg. 32, 1091–1097 (2006).
Trindade, F., Oliveira, A. & Frasson, M. Benefit of against-the-rule astigmatism to uncorrected near acuity. J. Cataract. Refract. Surg. 23, 82–85 (1997).
Hayashi, K., Hayashi, H., Nakao, F. & Hayashi, F. Influence of astigmatism on multifocal and monofocal intraocular lenses. Am. J. Ophthalmol. 130, 477–482 (2000).
Cochener, B. Tecnis Symfony intraocular lens with a sweet spot for tolerance to postoperative residual refractive errors. Open. J. Ophthalmol. 7, 14–20 (2017).
Carones, F. Residual astigmatism threshold and patient satisfaction with bifocal, trifocal and extended range of vision intraocular lenses (IOLs). Open. J. Ophthalmol. 7, 1–7 (2017).
Macsai, M. S. & Fontes, B. M. Refractive enhancement following presbyopia-correcting intraocular lens implantation. Curr. Opin. Ophthalmol. 19, 18–21 (2008).
Atchison, D. A., Charman, W. N. & Woods, R. L. Subjective depth-of-focus of the eye. Optom. Vis. Sci. 74, 511–520 (1997).
Canovas, C. et al. Preclinical evaluation of tolerance to refractive errors with different intraocular lenses. Invest. Ophthalmol. Vis. Sci. 63, 3074–F0546 (2022).
Zheng, H. et al. The tolerance of refractive errors of extended depth of focus intraocular lens in patients with previous corneal refractive surgery. Int. Ophthalmol. 43, 3989–3997 (2023).
Villegas, E. A., Alcón, E. & Artal, P. Minimum amount of astigmatism that should be corrected. J. Cataract Refract. Surg. 40, 13–19 (2014).
Guirao, A., Tejedor, J. & Artal, P. Corneal aberrations before and after small-incision cataract surgery. Invest. Ophthalmol. Vis. Sci. 45, 4312–4319 (2004).
Fernández, J. et al. Standard for collecting and reporting outcomes of IOL-based refractive surgery: update for enhanced monofocal, EDOF, and multifocal IOLs. J. Cataract. Refract. Surg. 48, 1235–1241 (2022).
ISO 11979-7:2018 Ophthalmic implants-intraocular lenses-part 7: Clinical investigations of intraocular lenses for the correction of aphakia. https://www.iso.org/standard/69038.html (2018).
Wolffsohn, J. S. et al. Exploring the optimum step size for defocus curves. J. Cataract Refract. Surg. 39, 873–880 (2013).
Author information
Authors and Affiliations
Contributions
N.S., R.S., Rudy.N. and A.S.R.: conception, analyses and writing of the manuscript; P.A.: conception and critical review of the manuscript; Raghav.N. and R.R.: analyses, drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shetty, N., Shetty, R., Artal, P. et al. Inducing cylindrical and spherical defocus after implantation with new generation intraocular lenses improves intermediate and near visual acuity. Sci Rep 14, 31934 (2024). https://doi.org/10.1038/s41598-024-83387-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83387-3