Abstract
Dengue fever is a vector-borne, acute, febrile, and self-limiting systemic viral infection that affects tropical and subtropical regions, including Pakistan. Karachi has a significant burden of Aedes aegypti and Aedes albopictus due to suitable breeding sites, weather, and rapid and unplanned urbanization of squatter areas. The country has limited surveillance studies on circulating serotypes of the dengue virus and the patient’s clinical features evolving over temporal changes. This study aimed to bridge the gap by screening 1500 patients using immunochromatographic detection and clinically following up on 486 of them. RNA extraction and cDNA synthesis of positive patients were performed, followed by PCR and sequencing. Data analysis and graphs were done on Prism 8.0. Males (53.87%) had a higher infection rate than females (46.13), with ages 18–60 years having the highest infection rate (69.14%). Results showed that 57.8% of patients were positive for NS1, followed by IgM (39.8%), and IgG (89.77%). DENV 1 and DENV 2 were found to be circulating, representing 20% and 80% respectively. Data on fever, shortness of breath, body aches, headache, nausea, vomiting, diarrhea, and epistaxis revealed significant differences. We conclude that continuous surveillance of dengue and other Flaviviruses and their infections is necessary to improve the prognosis and management of vector-borne diseases, thereby reducing the associated mortality rate of patients in Pakistan.
Similar content being viewed by others
Introduction
Dengue fever (DF) is an acute, febrile, and self-limiting systemic viral infection transmitted by the Aedes aegypti and Aedes albopictus mosquitos worldwide. The disease is endemic in tropical and subtropical regions such as Southeast Asia, the Eastern Mediterranean Region, Sub-Saharan Africa, and Latin America, and affects about 40% of the global population1. It has been a major health concern for decades, causing about 390 million illnesses annually and killing up to 40% of severe patients2,3. The first instance of dengue fever in Pakistan was recorded in November 1994, when a hospitalized woman presented with multiple dengue symptoms. Since then, Pakistan has seen multiple cyclical dengue epidemics in different regions. This can be attributed to the dense population, poor socioeconomic conditions, poor healthcare systems, inadequate water and sanitation facilities, and frequent natural calamities such as floods, earthquakes, and climate changes4,5.
Dengue fever can present as mild fever, dengue hemorrhagic fever (DHF), or dengue shock (DSS) syndrome. DHF and DSS have been recorded in several patients in Pakistan during dengue epidemics in recent decades. The initial symptoms of DF include fever, headache, muscle soreness, discomfort, a transient rash, petechiae, ecchymosis, and venipuncture bleeding. In the critical phase, plasma leakage causes shock, fluid accumulation, and severe bleeding. Some patients get acute liver failure, renal dysfunction, and encephalopathy with convulsions, as well as lower gastrointestinal bleeding6. There is no specific medication for dengue fever, however, mild fever can be treated with antipyretics, but severe infections necessitate hospitalization and enough fluids7. Since the initial outbreak, the disease’s clinical symptoms have evolved over temporal changes and are constantly developing. These various clinical signs are assumed to be caused by the prevalence of a certain serotype (DENV 1–4) at a given time8,9. Pakistan lacks a surveillance program for vector-borne diseases like dengue fever and therefore relies on a passive approach limited to select areas. Hence, the characterization of the dengue virus serotypes and surveillance of associated clinical features of patients in Karachi would serve as a scientific basis for dengue and other flaviviral infection control and prevention, as well as dengue immunizations in Pakistan. In this study, we performed serotyping of dengue virus and clinical surveillance of dengue patients in Karachi, Pakistan, from 2019 to 2023.
Materials and methods
Study design, ethical approval, sample collection and preparation
This cross-sectional study was conducted between January 2019 and March 2023 at the National Institute of Virology, Dr. Panjwani Center for Molecular Medicine and Drug Research, University of Karachi in collaboration with Indus Hospital, Abbasi Shaheed Hospital, and Essa Diagnostic Laboratory in Karachi. The study protocol was approved by the institutional independent ethics committees and collaborating hospitals (ICCBS/IEC-042/HB-2018/ProtocoV1.0) and all methods were performed under the relevant guidelines and regulations.
The social science statistics online tool estimated a sample size of 385, with a 95% confidence level, a margin of error of ± 4, and a 0.5 estimated proportion. We adopted the purposive sampling approach including patients who experienced symptoms during infection and had their immune-chromatographic (ICT) results confirmed positive. Overall, 1500 samples were included in this study. Clinical observations were also recorded, including white blood cell (WBC) and platelet counts, and clinical signs and symptoms of selected 486 patients. All samples were collected using commercially available ethylenediamine tetraacetic acid (EDTA) anticoagulant tubes. Informed consent was obtained from all subjects and their legal guardian(s) about the DENV epidemiological and clinical surveillance before they were included in the study. The plasma was separated by centrifugation at 3000 rpm for five minutes, then collected into a fresh tube and stored at −20°C until needed. The demographic and clinical information of the patient was obtained from guardians and on-duty medical officers, respectively.
Extraction of RNA and synthesis complementary DNA (cDNA)
This study considered genotyping samples that tested positive for the NS1 (nonstructural protein 1) antigen of the dengue virus. QIAamp viral RNA isolation kit (Qiagen, Heldin, Germany) was used to extract RNA from 140 µl plasma sample in 50 µl elution buffer as per the manufacturer’s protocol. The quality and quantity of the extracted RNA were determined using NanoDrop Multiscan Go Spectrophotometer µDrop™ Plate. RevertAid First strand cDNA synthesis kit (Thermo Scientific Inc.) was used to synthesize cDNA from the extracted RNA. Briefly, RNA 1 µg, was incubated with DNase I in a 10 µL reaction mixture for 30 min at 37 ℃ followed by 10 min of incubation with 50 mM EDTA at 65 ℃. This RNA was used to synthesize cDNA in 20 µL reaction mixture at a thermal profile: incubation at 25 °C for five minutes followed by 42°C for 60 min, and termination at 70 °C for 5 min in the T100 Thermal Cycler (Bio-Rad). The resulting cDNA was used to classify all four dengue virus serotypes using multiplex PCR.
Multiplex PCR
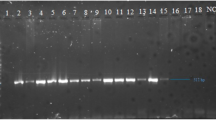
The first PCR reaction was carried out with dengue virus universal primers D1 and D210. The positive samples were further analyzed with specialized serotyping primers (TS1, TS2, TS3, and DEN4) based on their highest homology to four serotypes11. All primers were purchased from the macrogen’s commercially available services through a local supplier (Table 1). Briefly, a 25 µl reaction was prepared using absolute mastermix Molequle-On (Cat# AM-M-001–1250, New Zealand) and PCR was performed for 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 60 s, extension at 72 °C for 120 s12. Amplified PCR products (511 bp, 482 bp, and 119 bp gene fragments) were visualized in 2% TAE (1× Tris-acetate-EDTA) agarose gel. The respective regions of different serotypes in the gel, characterized by Thermo Scientific™ GeneRuler™ 100 bp DNA Ladder, were excised and purified using an MQ PCR Product Purification Kit (Cat# PPK-M-002–100, New Zealand).
Sanger sequencing
A total of 14 samples were subjected to Sanger sequencing using D1 and D2 primers. BigDye® Direct Sanger Sequencing Kit was used for sequencing PCR of these samples as per the manufacturer’s protocol. Purified PCR sequencing products were subjected to Sanger sequencing. Data were analyzed by the nucleotide basic local alignment search tool (BLAST).
Results
Demographic information of patients with DENV infection
This study analyzed 1500 dengue patients from Indus Hospital, Abbasi Shaheed Hospital, and Essa Diagnostic Laboratory in Karachi, from 2019 to 2023. The study included 53.87% (808/1500) males and 46.13% (692/1500) females. Patients were divided into three groups based on age differences, i.e., 1–17 years, 18–60 years, and > 60 years with contributions of 18.93% (284/1500), 69.14% (1037/1500), and 11.93% (179/1500) respectively. The distribution of samples among the three groups of the two genders was not significantly different (P = 0.357, χ2 = 2.059, 2).
Trends in seropositivity of patients with DENV infection
An analysis of seropositivity and co-presence of NS1 with IgM and IgG was made using results from ICT. The results showed that 57.8% (531/919) of patients were positive for NS1, 39.8% (488/1226) for IgM, 89.77% (1018/1135) for IgG, 39.2% (138/352) for NS1 + IgM, and 89.9% (96/108) for NS1 + IgG, and 85.7% (359/419) for IgM + IgG. The chi-square test revealed a significant difference (P = 0.001, χ2 = 54.83, 2) in positivity across immune-chromatographic detection (Fig. 2A; Table 2).
Phylogenetic analysis and serotyping
Since only the serotype region (D1/D2 amplified region, Fig. 1A, B) was targeted for sequencing, mutational analysis was not in the scope rather confirmation of serotypes and serotyping primers were aimed. The set criteria were 10 samples of each serotype but no sample of serotypes 3 and 4 could be detected using serotyping primers. Moreover, many samples were rejected due to poor quality of DNA determined by Qubit Fluorometric quantification. A total of 14 samples were successfully sequenced and phylogenetic analysis confirmed four samples of DENV 1 and 10 samples of DENV 2. This result was in 100% agreement with PCR results representing 20% and 80% of the dengue virus circulating serotypes, 1 and 2 respectively (Fig. 2B).
Laboratory findings for patients with the DENV infection
The study investigated WBCs and platelet counts, and the clinical manifestations of 486 patients enrolled at Indus Hospital. Males and females contributed to 54.53% (265/486) and 45.47% (221/486) of the samples respectively. Similarly, the age groups 1–17, 18–60, and > 60 contributed 19.54% (95/486), 68.51% (333/486), and 11.93% (58/486) respectively. Chi-square test revealed no significant difference in WBCs (P = 0.734, χ2 = 0.618, 2, P = 0.267, χ2 = 2.641, 2, and P = 0.356, χ2 = 2.064, 2) as well as platelets count (P value 0.272, χ2 = 2.603, 2, P value 0.638, χ2 = 0.898, 2, and P value 0.158, χ2 = 3.686, 2) among the three age groups of two genders (Table 3).
Clinical surveillance of patients with DENV infection
Data on clinical presentations among the three groups showed that shortness of breath (SOB) (P value < 0.001, χ2 = 44.30, 2), fever (P value < 0.001, χ2 = 14.09, 2), body ache (P value < 0.002, χ2 = 12. 19, 2), headache (P value < 0.049, χ2 = 6.003, 2), nausea (P value < 0.003, χ2 = 11.13, 2), vomiting (P value < 0.001, χ2 = 13.03, 2), diarrhea (P value < 0.025, χ2 = 7.36, 2), and epistaxis (P value < 0.025, χ2 = 7.32, 2) revealed significant difference but weakness, chest pain, headache, Skin rashes, retro-orbital pain, abdominal pain, gum bleeding, epistaxis, ear canal bleeding, hematemesis, and melena were not significant (Table 4).
We also analyzed the effect of gender on significant clinical features. We found that SOB (P value < 0.918, χ2 = 0.010, 1), fever (P value < 0.565, χ2 = 330, 1), body ache (P value < 0.359, χ2 = 0.841, 1), head ache (P value < 0.057, χ2 = 3.611, 2), nausea (P value < 0.150, χ2 = 2.069, 1), diarrhea (P value < 0.202, χ2 = 1.621, 1), and epistaxis (P value < 0.410, χ2 = 0.677, 1) were not significant, however, vomiting (P value < 0.036, χ2 = 4.366, 1) was significantly more common in females than males (Table 5).
We also examined data on the duration of the significant clinical features. A significantly high number of patients had a fever (P value < 0.011, χ2 = 16.55, 3) for ≥ 1 – ≤ 7 days. However, the duration of SOB, body aches, nausea, vomiting, and diarrhea was not significant across the three age groups (Table 6).
Discussion
Pakistan, an Eastern Mediterranean country, has seen repeated dengue fever outbreaks in recent decades13,14,15. It lacks a surveillance program for vector-borne diseases like malaria and dengue, instead relying on a passive strategy restricted to specific areas. Karachi, one of the most populous cities of Pakistan, has a significant burden of Aedes aegypti and Aedes albopictus due to suitable breeding sites, weather, and rapid and unplanned urbanization of squatter areas1,16,17. This study examined the infectivity rate and clinical characteristics of dengue patients in Karachi from 2019 to 2023. We found that the seropositivity of the dengue patients was 57.8%, 39.8%, and 89.77% for NS1, IgM, and IgG respectively (Fig. 2A; Table 2), with 20 and 80% circulation of DENV 1 and DENV 2 serotypes (Fig. 2B). It showed that males are at a greater risk of dengue infections (53.87%) than females (46.13%), though not significantly. DENV infections varied with age, being highest in the 18–60 years (69.14%) and the lowest in the > 60 years (11.93%) age group. Clinical features such as SOB, fever, body pains, headache, nausea, vomiting, diarrhea, and epistaxis differed significantly amongst the three groups (Table 4), whereas vomiting differed significantly between the two genders only (Table 5). The duration of fever varied significantly between ≥ 1–≤ 7 days among the three age groups (Table 6).
Studies conducted around the world revealed a variety of demographic trends in dengue infections. In North America either an equal or higher prevalence of dengue was seen in females than males. In contrast, in the Asian population, men are more affected than females. We also found a higher infection rate in males than females, but the difference was not significant. The difference between these observations can be related to prevailing social gender norms in different regions. For example, in Pakistan males have traditionally been responsible for outdoor activities, whereas women mostly work as housewives and are dressed to conceal their entire bodies, influencing their risk of contracting vector-borne disease18,19,20,21. Age group 18–60 has a high incidence of dengue infection followed by 1–17 similar to previously reported studies19,22,23,24.
Different studies use a variety of approaches to detect dengue virus infection. NS1, a glycoprotein of DENV, is implicated in host defense evasion, pathogenesis, and early stages of viral replication25. Sera from infected patients can carry it during the acute phase of the disease, and some studies have found its presence for up to 9–10 days after dengue symptoms manifest26,27,28. On the other hand, the host defense can be harnessed by detecting IgM and IgG antibodies for the presence of a primary or secondary infection. The sensitivity of each diagnostic method depends on whether the infection is primary or secondary, as well as the dengue serotype29.
We found that the seroprevalence of NS1 was 57.8%, IgM 39.8%, and IgG 89.77% (Fig. 2A; Table 2). In one study in the Khyber Pakhtunkhwa province, IgM was 32% and IgG was 20%30, whilst another reported that NS1 was 25.26, IgM 41.05%, and IgG 8.42%31. A study conducted in Multan showed IgM and IgG at 54.17% and 27.6%32. A study conducted in Karachi showed NS1 at 73.5% while a few at 2.6%, and 5.3% for IgM and IgG respectively, however, the sample size was small4. The disparities between our study and other reported studies could be attributed to changes in sample size, study duration, and temporal variation. The higher IgG values observed in our study could be attributed to unnoticed mild dengue infections that occurred over time in Karachi.
Typically, dengue manifests itself as fever, pain, or rash however bleeding and gastrointestinal complications might occur at varying degrees33. Studies showed fever in all patients of dengue with the most common associated features being nausea and/or vomiting, severe headache, anorexia, and retro-orbital pain34,35,36 as we found in the study Similarly, consistent with the previous studies37,38, the current study also shows a decreasing proportion of mucosal bleeding (epistaxis, gum bleeding, and otorrhagia), and GI bleeding (hematemesis and/or melena). Furthermore, unlike other studies that identified skin rash as a critical feature, we did not observe it significantly (Table 4). The decreased prevalence of skin rash could be attributed to prevalent secondary infection rather than primary infection24,39, as evidenced by 89.7% IgG and DENV 1 and DENV 2 serotype circulation40.
Vomiting and diarrhea were the most common digestive symptoms in this study, however abdominal pain and weakness were not frequent (Table 4). Studies also showed a significant increase in diarrhea and abdominal pain over the past decade41,42,43. Khan et al. and Guzman and Kouri 2003 observed that abdominal pain was more likely to occur in DEN-3 serotypes than in DEN-1 and DEN-240,44. We found DENV 1 and 2 serotypes were more prevalent in 2019–2023 in Karachi, which could explain the decrease in abdominal pain45, nevertheless, further evidence from substantial serotype-specific studies is needed.
Limitations
Diagnosis of dengue infections based on NS1 antigen, IgM, and IgG antibody assay may produce false positives from cross-reactivity with other flaviviral infections46. We performed RT-PCR confirmation on a small number of samples intended to be sequenced. The number sequenced was also small, due to cost constraints, which are some of the drawbacks of our study. The strength of this study is the inclusion of serologically confirmed positive cases, a large number of female samples, patients of all ages, a long period, and a detailed clinical profile of the enrolled cases, which can assist local health authorities in allocating resources for capacity development.
Data availability
Accession numbers and links for sequences analyzed in this work are available in the Gene Bank: PP990413: https://ncbi.nlm.nih.gov/nuccore/PP990413 PP990414: https://ncbi.nlm.nih.gov/nuccore/PP990414.
Abbreviations
- BLAST:
-
Basic local alignment search tool
- Bp:
-
Base pair
- ℃:
-
Degrees celsius
- cDNA:
-
Complementary DNA
- DENV:
-
Dengue virus
- DF:
-
Dengue fever
- DHF:
-
Hemorrhagic fever
- DSS:
-
Dengue shock
- EDTA:
-
Ethylenediamine tetraacetic acid
- ICT:
-
Immune-chromatographic
- IgM:
-
Immunoglobulin M
- IgG:
-
Immunoglobulin G
- NS1:
-
Nonstructural protein 1
- PCR:
-
Polymerase chain reaction
- SOB:
-
Shortness of breath
- WBC:
-
White blood cell
References
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496 (7446), 504–507. https://doi.org/10.1038/nature12060 (2013).
WHO. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (2024).
Riaz, M. et al. Outbreak of dengue fever in Karachi 2006: a clinical perspective. J. Pak. Med. Assoc. 59 (6), 339 (2009). https://ecommons.aku.edu/pakistan_fhs_mc_med_intern_med/16
Mushtaq, S., Abro, T., M., Hussain & U., H. Dengue cases presenting to the emergency department of a tertiary care hospital in late 2021: A cross-sectional study in Karachi. Int. J. Public. Health. https://doi.org/10.3389/ijph.2024.1606753 (2024).
Khattak, A. et al. Burden and distribution of dengue infection in Pakistan (2000-19): a review. Braz. J. Biol.. 84, e267982. https://doi.org/10.1590/1519-6984.267982 (2023).
Estofolete, C. F. et al. Unusual clinical manifestations of dengue disease - Real or imagined? Acta Trop. 199, 105134. (2019). https://doi.org/10.1016/j.actatropica.2019.105134
Obi, J. O., Gutierrez-Barbosa, H., Chua, J. V. & Deredge, D. J. Current trends and limitations in dengue antiviral research. Trop. Med. Infect. Dis. 6 (4), 180. https://doi.org/10.3390/TROPICALMED6040180 (2021).
Khan, J., Ghaffar, A. & Khan, S. A. The changing epidemiological pattern of Dengue in Swat, Khyber Pakhtunkhwa. PLoS One. 13 (4), e0195706. https://doi.org/10.1371/journal.pone.0195706 (2018).
Haider, Z. et al. Dengue fever in Pakistan: A paradigm shift; Changing epidemiology and clinical patterns. Perspect. Public. Health. 135 (6), 294–298. https://doi.org/10.1177/1757913915599019 (2015).
Lanciotti, R. S., Calisher, C. H., Gubler, D. J., Chang, G. J. & Vorndam, A. V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30 (3), 545–551. https://doi.org/10.1128/jcm.30.3.545-551.1992 (1992).
Organji, S. R., Abulreesh, H. H. & Osman, G. E. H. Circulation of dengue virus serotypes in the city of Makkah, Saudi Arabia, as determined by reverse transcription polymerase chain reaction. Can. J. Infect. Dis. Med. Microbiol. https://doi.org/10.1155/2017/1646701 (2017).
Khawsak, P., Phantana, S. & Chansiri, K. Determination of dengue virus serotypes in Thailand using PCR based method. Southeast. Asian J. Trop. Med. Public. Health. 34 (4), 781–785 (2003).
Sherin, A. Dengue fever: A major public health concern in Pakistan. Khyber Med. Univ. J. 1, 1–3 (2011). https://www.kmuj.kmu.edu.pk/article/view/9230
Ahmad, S. et al. Epidemiology of dengue in Pakistan, present prevalence and guidelines for future control. Int. J. Mosq. Res. 4 (6), 25–32 (2017).
Iqtadar, S., Akbar, N., Mehmood, M. & Abaidullah, S. Clinical audit of dengue related deaths in 2011 at Mayo Hospital Lahore Pakistan. Pak. J. Med. Sci. 33(5), 1070–1073. https://doi.org/10.12669/pjms.335 (2017).
Mahmood, K. et al. Molecular characterization of human adenoviruses associated with pediatric respiratory infections in Karachi, Pakistan. BMC Infect. Dis. 24(1), 538. https://doi.org/10.1186/s12879-024-09415-9 (2024).
Cao-Lormeau Van-Mai. Tropical Islands as New Hubs for Emerging Arboviruses. Infect. Dis. 22(5), 183–184. https://doi.org/10.1016/S0002-9394(14 (2016).
Ahmad, S. et al. Surveillance of intensity level and geographical spreading of dengue outbreak among males and females in Punjab, Pakistan: A case study of 2011. J. Infect. Public Health. 11 (4), 472–485. https://doi.org/10.1016/j.jiph.2017.10.002 (2018).
Zohra, T. et al. Demographic and clinical features of dengue fever infection in Pakistan: A cross-sectional epidemiological study. Trop. Dis. Travel Med. Vaccines. https://doi.org/10.1186/s40794-024-00221-4 (2024).
Mohan, K. et al. Clinical profile and atypical manifestation of dengue fever cases between 2011 and 2018 in Chennai, India. J. Fam. Med. Prim. Care 9(2), 1119. https://doi.org/10.4103/jfmpc.jfmpc_926_19. (2020).
Wenham, C. et al. Gender mainstreaming as a pathway for sustainable arbovirus control in Latin America. PLoS Negl. Trop. Dis. https://doi.org/10.1371/journal.pntd.0007954 (2020).
Khan, M. Y., Venkateshwarlu, C., Sandeep, N. & Krishna, A. H. A study of clinical and laboratory profile of dengue fever in a tertiary care hospital, Nizamabad, Telangana State, India. Headache 115, 76–77 (2016).
Patil, P. S. et al. A retrospective study of clinical and laboratory profile of dengue fever in tertiary care Hospital, Wardha, Maharashtra, India. J. Pure Appl. Microbiol. 14 (3), 1935–1939. https://doi.org/10.22207/JPAM.14.3.32 (2020).
Palomares-Reyes, C. et al. Dengue diagnosis in an endemic area of Peru: Clinical characteristics and positive frequencies by RT-PCR and serology for NS1, IgM, and IgG. Int. J. Infect. Dis. 81, 31–37. https://doi.org/10.1016/j.ijid.2019.01.022 (2019).
Scaturro, P., Cortese, M., Chatel-Chaix, L., Fischl, W. & Bartenschlager, R. Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1005277 (2015).
Krishnananthasivam, S. et al. Evaluation of a commercial rapid test kit for detection of acute dengue infection. Southeast. Asian J. Trop. Med. Public. Health. 46 (4), 602–610 (2015).
Suleman, M. et al. NS1 antigen: A new beam of light in the early diagnosis of dengue infection. Asian Pac. J. Trop. Med. 9 (12), 1212–1214. https://doi.org/10.1016/j.apjtm.2016.10.007 (2016).
Gaikwad, S., Sawant, S. S. & Shastri, J. S. Comparison of nonstructural protein-1 antigen detection by rapid and enzyme-linked immunosorbent assay test and its correlation with polymerase chain reaction for early diagnosis of dengue. J. Lab. Phys. 9(3), 177–181. https://doi.org/10.4103/0974-2727. (2017).
Da Costa, V. G., Marques-Silva, A. C. & Moreli, M. L. A meta-analysis of the diagnostic accuracy of two commercial NS1 antigen ELISA tests for early dengue virus detection. PLoS One 9(4), e94655. (2014).
Ali, A. et al. Seroepidemiology of dengue fever in Khyber Pakhtunkhawa, Pakistan. Int. J. Infect. Dis. https://doi.org/10.1016/j.ijid.2013.01.007 (2013).
Ahmad, S. et al. Epidemiological and clinical manifestation of dengue virus infection: A Recent Report of 2018 from District Battagram Khyber Pakhtunkhwa, Pakistan. Int. J. Mosq. Res. 7 (6), 5–8 (2020). https://www.researchgate.net/publication/366394167
Mukhtar, M. U., Mukhtar, M. & Iqbal, N. Dengue fever is an emerging public health concern in the city of Multan, Pakistan: Its seroprevalence and associated risk factors. Microbiol. Immunol. 62 (11), 729–731. https://doi.org/10.1111/1348-0421.12649 (2018).
Hasan, S., Jamdar, S. F., Alalowi, M., Al, A. A. & Beaiji, S. M. Dengue virus: A global human threat: Review of literature. J. Int. Soc. Prev. Commun. Dent.. 6 (1), 1–6. https://doi.org/10.4103/2231-0762.175416 (2016).
Alvarado-Castro, V. M. et al. Clinical profile of dengue and predictive severity variables among children at a secondary care hospital of Chilpancingo, Guerrero, Mexico: Case series. Boletín Méd. Del. Hosp. Infantil México (English Edition). 73 (4), 237–242. https://doi.org/10.1016/j.bmhime.2016.06.003 (2016).
Chen, C. H., Huang, Y. C., Kuo, K. C. & Li, C. C. Clinical features and dynamic ordinary laboratory tests differentiating dengue fever from other febrile illnesses in children. J. Microbiol. Immunol. Infect. 51 (5), 614–620. https://doi.org/10.1016/j.jmii.2016.08.018 (2018).
Deshwal, R., Qureshi, I. & Singh, R. Clinical and laboratory profile of dengue fever. J. Assoc. Physicians India. 63 (12), 30–32 (2015). http://emedicine.medscape.com/
Pervin, M. et al. Sera-epidemiology of dengue virus infection in clinically suspected patients attended in Dhaka Medical College Hospital during January to December 2016. J. Dhaka Med. Coll. 26 (2), 111–116. https://doi.org/10.3329/jdmc.v26i2.38825 (2018).
Sultana, N. et al. Frequency of dengue infection in febrile patients attended Dhaka Medical College Hospital during January to December 2018. J. Dhaka Med. Coll. 28 (1), 105–111. https://doi.org/10.3329/jdmc.v28i1.45765 (2020).
Cobra, C., Rigau-Perez, J. G., Kuno, G. & Vorndam, V. Symptoms of dengue fever in relation to host immunologic response and virus serotype. Puerto Rico Am. J. Epidemiol. 142 (11), 1204–1211 (1995).
Khan, E. et al. The Clinical features of co-circulating dengue viruses and the absence of dengue hemorrhagic fever in Pakistan. Front. Public. Health. https://doi.org/10.3389/fpubh.2020.00287 (2020).
Htun, T. P., Xiong, Z. & Pang, J. Clinical signs and symptoms associated with WHO severe dengue classification: A systematic review and meta-analysis. Emerg. Microbes infect. 10 (1), 1116–1128. https://doi.org/10.1080/22221751.2021.1935327 (2021).
Sajid, A., Ikram, A. & Ahmed, M. Clinical profile of children presenting at Madina Teaching Hospital Faisalabad. J. Univ. Med. Dent. Coll. 3 (1), 42–47 (2012). https://jumdc.com/index.php/jumdc/article/view/346
Khan Jehangir et al. Dengue outbreak. : Clinical profile of patients presenting at DHQ Burner and THQ Shangla, Khyber Pakhtunkhwa, Pakistan. Immun. Dis. 3, a11. (2013).
Guzman, M. G. & Kouri, G. Dengue and dengue hemorrhagic fever in the Americas: Lessons and challenge. J. Clin. Virol. 27 (1), 1–13. https://doi.org/10.1016/S1386-6532(03)00010-6 (2003).
Ahmad, M. H. et al. The sensitivity, specificity and accuracy of warning signs in predicting severe dengue, the severe dengue prevalence and its associated factors. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph15092018 (2018).
Zammarchi, L., Spinicci, M. & Bartoloni, A. Zika virus: A review from the virus basics to proposed management strategies. Mediterranean J. Hematol. Infect. Dis. https://doi.org/10.4084/MJHID.2016.056 (2016).
Funding
This study was supported by an institutional research grant from Dr. Panjwani Center for Molecular Medicine and Drug Research, ICCBS, University of Karachi.
Author information
Authors and Affiliations
Contributions
Khalid Mahmood: Conceptualization, Methodology, Data curation, Validation, Supervision, Writing –original draft, Writing – review & editing. Muhammad Rashid: Conceptualization, Methodology, Data curation, Validation, Supervision, Writing – original draft, Writing – review & editing. Sabeeta Kanwal Ansari: Methodology, Investigation, Data curation. Fatima Kanani: Methodology, Investigation, Data curation. Thomas Iftner: Conceptualization, Investigation, Supervision, review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahmood, K., Rashid, M., Ansari, S.K. et al. Clinical characteristics of dengue virus infections in Karachi from 2019 to 2023: a cross-sectional study. Sci Rep 14, 31910 (2024). https://doi.org/10.1038/s41598-024-83425-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83425-0