Abstract
Cervical spondylotic myelopathy (CSM) is a neurological disorder characterized by degenerative changes in the spinal cord and compression of the spinal cord and its adjacent structures due to various reasons, such as intervertebral disc herniation. The Japan Orthopaedic Association score is a disease-specific outcome tool that provides quantitative measurements for CSM patients. At present, no scholars have developed a model that can directly predict the prognosis of CSM patients. This study used binary logistic regression analysis to identify clinical independent predictive factors, and then used R language to construct a nomogram. The results showed that Hb (0.71 [0.51, 0.99]), LYM (0.32 [0.22, 0.46]), and ALB (0.32 [0.23, 0.45]) were independent predictive factors. Good prediction performance with modest errors was shown by the nomogram in both the training and validation groups. Hb, LYM, ALB are independent predictors of the prognosis of ACDF in CSM patients. The constructed nomogram can help clinicians assess and treat patients early, benefiting more patients.
Similar content being viewed by others
Introduction
Cervical spondylotic myelopathy (CSM) is a neurological disorder characterized by spinal degenerative changes and compression of the spinal cord and adjacent structures due to various reasons, such as disc herniation1. CSM often develops insidiously2,3, with early clinical symptoms including changes in hand dexterity, difficulty walking, and even falls. Currently, there are two treatment options for CSM: non-surgical and surgical. However, CSM is often considered a surgical disease, and studies have shown that the rate of significant functional limitations with non-surgical treatment is 6% at 1 year, 21% at 2 years, and 56% at 10 years4. In recent years, researchers have studied the incidence of perioperative and delayed complications associated with surgical treatment of CSM5,6. However, there is limited research on predicting the prognosis of CSM. Blood tests, as common admission examinations, are easily obtained and have significant guiding value for clinical treatment. Blood indicators have the potential to serve as prognostic markers for patient assessment.
The Japanese Orthopaedic Association (JOA) score was developed in 1975 as a disease-specific outcome tool that provides quantitative measurements for patients with CSM7,8. It assesses upper and lower extremity motor function, sensory function, trunk sensory function, and bladder function, with a total score ranging from 0 to 179. The JOA score is widely used in CSM as a prognostic tool10,11. The JOA recovery rate, calculated based on the JOA score, provides a more intuitive reflection of the improvement in patients’ condition three months after surgery.
Therefore, our research question is to construct a nomogram prediction model for the postoperative evaluation of the efficacy of CSM patients based on the relevant blood test results obtained at admission. This model will use the results of whole blood analysis at admission to predict the JOA recovery rate. Our goal is to provide an early, simple, and convenient prediction tool to assist clinicians in making timely decisions and implementing subsequent rehabilitation interventions.
Materials and methods
Patient section
This is a retrospective cohort study conducted at two large medical centers in China. The study included patients who underwent Anterior Cervical Discectomy and Fusion (ACDF) for cervical spondylotic myelopathy (CSM) between January 2015 and September 2020. The inclusion criteria were as follows: (1) a confirmed diagnosis of CSM with typical clinical manifestations such as limb weakness and sensory disturbances; (2) evidence of spinal cord compression or significant changes in spinal cord signal on magnetic resonance imaging or computed tomography; (3) all patients underwent ACDF surgery; (4) age > 18 years. The exclusion criteria were as follows: (1) presence of other spinal disorders (such as spinal tuberculosis, infection, or tumor); (2) presence of major systemic diseases affecting the blood or immune system; (3) exclusion of limb dysfunction caused by other etiologies, such as cerebral infarction or thoracic outlet syndrome. Patients who met the above criteria at the First Affiliated Hospital of Guangxi Medical University and the First Affiliated Hospital of Guilin Medical University were included in the study and divided into a training set and a validation set in a 7:3 ratio for the construction and validation of the nomogram.
As this is a retrospective study, the ethics committee approved the study protocol based on national laws and institutional consent, and patient informed consent was not required. In this study, personal identifying information of the patients will be anonymized and de-identified to ensure confidentiality.
Data collection and definition
We gathered baseline laboratory data and clinical information for this study, such as coagulation function, blood biochemistry, and complete blood count. Gender, age, history of diabetes, history of hypertension, history of hepatitis, history of tuberculosis, history of alcohol use, and history of smoking were among the baseline clinical data.
In addition, this study collected the following data on patients’ laboratory test results upon admission: White blood cell count (WBC), Red blood cell count (RBC), Hemoglobin (Hb), Mean corpuscular volume (MCV), Neutrophils (NC), Lymphocyte (LYM), Monocyte (MONO), Eosinophils (Eos), Basophil (Baso), Albumin (ALB), Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Total bilirubin (TBIL), Creatinine (Cr), Blood urea nitrogen (BUN), Prothrombin time (PT), Activated partial thromboplastin time (APTT), Fibrinogen (FIB), and other relevant data.
Outcome
This study used the preoperative and postoperative JOA score calculation to determine the JOA improvement rate as the outcome event. A JOA recovery rate of ≤ 25% was defined as a positive result, while a JOA recovery rate of > 25% was defined as a negative result. The specific calculation formula is as follows: JOA recovery rate = [(post-treatment score − pre-treatment score)/(17 − pre-treatment score)] × 100%12. Generally, a JOA recovery rate of 100% is considered complete recovery, greater than 60% is considered significant improvement, 25-60% is considered effective, and less than or equal to 25% is considered ineffective13. In addition, according to previous reports, the minimum clinically important difference (MCID) in JOA score was 2.514. In this study, a JOA recovery rate of > 25% and JOA > 2.5 points at 3 months postoperatively was defined as the Improvement group, while the opposite was defined as the No Improvement group.
Statistical analysis
In this study, we first combined the data from two hospitals and used SPSS 21.0 (SPSS Inc., Chicago, IL) to compare the clinical data and test results between the Improvement group and the No Improvement group. For continuous data, we assessed their normality using the Shapiro-Wilk test. If the data followed a normal distribution, we presented them as mean ± standard deviation and compared the groups using independent one-way analysis of variance (ANOVA). If the data did not follow a normal distribution, we presented them as median (25th percentile, 75th percentile) and compared the groups using the Kruskal–Wallis test. Categorical data were described using frequencies (percentages), and group comparisons were performed using the chi-square test or Fisher’s exact test. We used Graphpad Prism 9.5.0 to create violin plots for Hb, LYM, and ALB in the Improvement group and the No Improvement group, as well as to plot ROC curves and calculate the area under the curve. We considered a two-sided p-value less than 0.05 to be statistically significant.
The collected independent variables in the data were classified into two based on the mean value. Variables below the mean were defined as group 1, while variables above the mean were defined as group 2. SPSS software was used to conduct single-factor logistic regression analysis on each factor. Subsequently, clinically significant factors identified in the single-factor analysis were included in the multiple-factor logistic regression analysis to calculate the independent predictive factors contributing to the occurrence of “No Improvement”.
Finally, we randomly divided the data into a training group and a validation group in a 7:3 ratio using SPSS. Based on the results of the multiple-factor logistic regression analysis, we used R Studio (version 4.2.2) to create a Nomogram for the independent predictive factors. We evaluated the predictive performance of the model using ROC curves and the area under the curve (AUC). We calculated the average error of the model using a calibration plot. Additionally, we analyzed the clinical benefits of the model using a Decision Curve Analysis (DCA) plot.
Results
Relationship of clinical factors to predictive JOA recovery rate
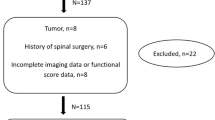
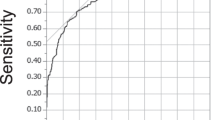
In this study, a total of 819 patients diagnosed with cervical sylotic myelopathy were collected from two large medical centers. After excluding 70 patients based on the aforementioned factors, a total of 749 patients were included in the retrospective study, as shown in Fig. 1. All the data were combined, and the patients were divided into two groups based on whether the postoperative JOA recovery rate was > 25%. The samples with a postoperative JOA recovery rate > 25% were defined as the Improvement group, with a total of 498 patients. The samples with a postoperative JOA recovery rate ≤ 25% were defined as the No Improvement group, with a total of 251 patients. The baseline data of the two groups were compared, as shown in Table 1. In the No Improvement group, there were 98 females (39.04%) and 153 males (60.96%). The number of patients with a history of hypertension, diabetes, heart disease, hepatitis, tuberculosis, smoking, and alcohol abuse were 57 (22.71%), 20 (7.97%), 10 (3.98%), 12 (4.78%), 5 (1.99%), 50 (19.92%), and 34 (13.55%), respectively. There were no statistically significant differences in baseline data between the groups (p > 0.05), indicating comparability. However, there were statistically significant differences in age, RBC, Hb, WBC, LYM, BAS, ALB, and ALT between the groups (p < 0.05). Subsequently, ROC curves were plotted for Hb, LYM, and ALB to predict postoperative No Improvement (Fig. 2A–C). The optimal cutoff values, sensitivity, and specificity were calculated. The results showed that the optimal cutoff value for Hb was 0.574, with a sensitivity of 0.586 and a specificity of 0.562. The optimal cutoff value for LYM was 0.628, with a sensitivity of 0.781 and a specificity of 0.476. The optimal cutoff value for ALB was 0.647, with a sensitivity of 0.677 and a specificity of 0.616. Violin plots were also created to visually demonstrate the distribution differences between the two groups for Hb, LYM, and ALB (Fig. 2D–F).
The clinical data were classified into two groups based on the mean value and in a single-factor binary logistic regression analysis. The results are shown in Table 2, where Age (1.58 [1.16–2.15]), RBC (0.71 [0.53, 0.97]), WBC (0.5 [0.34, 0.73]), LYM (0.31 [0.22, 0.44]), BAS (0.62 [0.43, 0.9]), and ALB (0.3 [0.22, 0.41]) were identified as independent predictive factors for cervical spondylotic myelopathy. The significant factors identified in the single-factor analysis were included in a multiple-factor binary logistic regression analysis using a stepwise method. The results showed that Hb (0.71 [0.51, 0.99]), LYM (0.32 [0.22, 0.46]), and ALB (0.32 [0.23, 0.45]) were independent predictive factors, as shown in Table 3.
Nomogram construction and validation
To further validate the predictive ability of various factors on tibial plateau fractures, a nomogram model was constructed using R Studio software based on the results of multifactor analysis (Hb, LYM, ALB). The collected data was randomly sampled in a 7:3 ratio and divided into a training group and a validation group. The training group data was used to construct a nomogram, as shown in Fig. 3, and the ROC curve was plotted to validate the predictive performance of the column chart (Fig. 4A). The C-index was 0.732, indicating good predictive performance. The calibration curve of the training group (Fig. 5A) showed an average error of 0.023. The decision curve analysis (DCA) of the training group (Fig. 6A) indicated good clinical benefits with a threshold between 0.09 and 0.55. The validation group data was used for validation, and the ROC curve of the validation group (Fig. 4B) yielded a C-index of 0.701. The calibration curve (Fig. 5B) showed an average error of 0.064. The DCA (Fig. 6B) indicated good clinical benefits with a threshold between 0.19 and 0.53. It is evident that the column chart exhibited good predictive performance and had small errors in both the training and validation groups, benefiting the majority of clinical patients.
Discussion
CSM typically occurs at the level of the intervertebral disc, its pathophysiology determines that surgical decompression of spinal cord compression is the most direct treatment for CSM15. After surgery, the JOA score is commonly used to assess postoperative improvement in patients. Currently, many studies focus on predicting poor prognosis in patients based on preoperative imaging. Wang et al. 16 analyzed the MRI results of 680 patients who visited a local hospital between January 2012 and June 2021. They quantified the results in conjunction with the JOA score and ultimately developed a clinical predictive nomogram for CSM patients with intramedullary signal intensity enhancement on T2-weighted images. Chen et al.17 collected and analyzed preoperative and postoperative clinical and imaging data from 369 patients, and developed a nomogram to predict postoperative kyphotic deformity. Although MRI imaging data can provide clinicians with rich information, MRI has the disadvantages of being expensive, difficult to complete MRI examinations in a timely manner, and poor patient compliance. Serological examinations can be completed very early, thereby achieving the effect of early prediction. Previous literature has shown that the HALP score system composed of hemoglobin, albumin, lymphocyte, and platelet is often used as a new prognostic biomarker to predict various clinical outcomes in various cancers18,19. Low HALP scores at admission are associated with cognitive impairment after ischemic stroke and are also associated with the prognosis of hemodialysis patients20,21. There is currently no literature indicating blood markers related to postoperative recovery in CSM patients.
In our research, the JOA recovery rate was used to assess the prognosis of patients. The results showed that Hb, ALB, and LYM were independent predictive factors for the improvement of limb function after CSM anterior cervical discectomy and fusion surgery. The decrease in Hb levels has been associated with poor prognosis in various diseases22,23,24. The decrease in Hb levels can lead to a decrease in tissue oxygen transport capacity25, thereby affecting the normal tissue repair process. ALB, as a common marker of nutritional status, can activate various cytokines26, stimulating the progress of tissue repair. LYM is commonly used to evaluate the degree of active immune response in patients, and it can increase to a certain extent during the body’s active resistance to disease progression27. Reviewing previous literature studies, the prognosis of postoperative patients can also be related to diabetes28, postoperative infection, age, BMI, surgery duration, preoperative hematocrit, and WBC29, among other factors. The nomogram model is a model that is easy for clinicians to use. It calculates the probability of a patient’s poor prognosis by adding up the scores of each indicator30. Our research results show that poor prognosis of CSM may be related to poor nutritional status and inflammatory response of patients. In the process of nursing and diagnosis and treatment, we can calculate the patient’s score when the patient is admitted to the hospital, so as to prevent the occurrence of adverse events at an early stage.
Improving postoperative function and reducing the occurrence of complications in patients with CSM is a common pursuit among orthopedic surgeons. Hematological indicators collected upon admission are important reference markers for evaluating the patient’s condition. In this study, a nomogram was constructed based on preoperative measurements of Hb, LYM, and ALB. The aim is to assist clinicians in early intervention for postoperative rehabilitation treatment and to achieve favorable treatment outcomes for patients after surgery.
This study has several strengths: (1) It is a retrospective study conducted at two centers in China, with a large amount of data, a reliable model, and simple and convenient application. (2) The hematological indicators involved in the study are routine tests conducted upon admission, and the results are easily obtainable, allowing for early prognosis prediction. (3) The training and validation models obtained in this study both demonstrate good predictive performance. However, there are also some limitations to this study: (1) Follow-up of postoperative and long-term occurrence rates in patients was not conducted. (2) The amount of data could be further expanded. (3) This is a retrospective study with certain selection bias and missing data. (4)The number of centers need to be further expanded. (5)This study did not pay attention to the dynamic changes of serology and did not integrate imaging data.
Conclusion
Hb, LYM and ALB are independent predictive factors for prognosis assessment in patients with CSM. The nomogram can assist clinicians in achieving early prevention and treatment goals, thereby benefiting more patients.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- CSM:
-
Cervical spondylotic myelopathy
- JOA:
-
Japanese orthopaedic association
- ACDF:
-
Anterior cervical discectomy and fusion
- WBC:
-
White blood cell count
- RBC:
-
Red blood cell count
- Hb:
-
Hemoglobin
- MCV:
-
Mean corpuscular volume
- NC:
-
Neutrophils
- LYM:
-
Lymphocyte
- MONO:
-
Monocyte
- Eos:
-
Eosinophils
- Baso:
-
Basophil
- ALB:
-
Albumin
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TBIL:
-
Total bilirubin
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thrombin time
- FIB:
-
Fibrinogen
- Cr:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- AUC:
-
The area under the curve
- DCA:
-
Decision curve analysis
- ROC:
-
Receiver operating characteristic
References
Fehlings, M. G., Tetreault, L. A., Riew, K. D., Middleton, J. W. & Wang, J. C. A Clinical Practice Guideline for the Management of Degenerative Cervical Myelopathy: Introduction, Rationale, and Scope. Global Spine J. 7, 21s–27s. https://doi.org/10.1177/2192568217703088 (2017).
Kim, M. W., Kang, C. N. & Choi, S. H. Update of the Natural History, Pathophysiology, and Treatment Strategies of Degenerative Cervical Myelopathy: A Narrative Review. Asian Spine J. 17, 213–221. https://doi.org/10.31616/asj.2022.0440 (2023).
Harrop, J. S., Hanna, A., Silva, M. T. & Sharan, A. Neurological manifestations of cervical spondylosis: an overview of signs, symptoms, and pathophysiology. Neurosurgery 60, S14–S20. https://doi.org/10.1227/01.Neu.0000215380.71097.Ec (2007).
Fehlings, M. G., Tetreault, L. A., Riew, K. D., Middleton, J. W. & Wang, J. C. A Clinical Practice Guideline for the Management of Degenerative Cervical Myelopathy: Introduction, Rationale, and Scope. Global Spine J. 7, 21S–27S (2017).
Fehlings, M. G. et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study. J. Neurosurg. Spine. 16, 425 (2012).
Ghogawala, Z. et al. Effect of Ventral vs Dorsal Spinal Surgery on Patient-Reported Physical Functioning in Patients With Cervical Spondylotic Myelopathy: A Randomized Clinical Trial. Jama 325, 942–951. https://doi.org/10.1001/jama.2021.1233 (2021).
Yonenobu, K., Abumi, K., Nagata, K., Taketomi, E. & Ueyama, K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa. 1976). 26, 1890–1894. https://doi.org/10.1097/00007632-200109010-00014 (2001). discussion 1895.
Hirabayashi, K., Miyakawa, J., Satomi, K., Maruyama, T. & Wakano, K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa. 1976). 6, 354–364. https://doi.org/10.1097/00007632-198107000-00005 (1981).
Oshima, Y. et al. Comparison Between the Japanese Orthopaedic Association (JOA) Score and Patient-Reported JOA (PRO-JOA) Score to Evaluate Surgical Outcomes of Degenerative Cervical Myelopathy. Global Spine J 12, 795–800. https://doi.org/10.1177/2192568220964167 (2022).
Kadaňka, Z. et al. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur. Spine J. 20, 1533–1538. https://doi.org/10.1007/s00586-011-1811-9 (2011).
Xu, C. et al. Intervertebral-spreader-assisted anterior cervical discectomy and fusion prevents postoperative axial pain by alleviating facet joint pressure. J. Orthop. Surg. Res. 17, 91. https://doi.org/10.1186/s13018-022-02983-z (2022).
Cheng, X. J., Jin, L., Wang, X., Zhang, W. & Shen, Y. Predictors of poor outcome in cervical spondylotic myelopathy patients underwent anterior hybrid approach: focusing on change of local kyphosis. J. Orthop. Surg. Res. 15, 369. https://doi.org/10.1186/s13018-020-01905-1 (2020).
Jiang, Z., Wang, A., Wang, C. & Kong, W. Analysis of compliance and efficacy of integrated management of whole process in the choice of percutaneous full-endoscopic surgery for patients with cervical disc herniation. J. Orthop. Surg. Res. 15, 381. https://doi.org/10.1186/s13018-020-01920-2 (2020).
Oshima, Y. et al. Comparison Between the Japanese Orthopaedic Association (JOA) Score and Patient-Reported JOA (PRO-JOA) Score to Evaluate Surgical Outcomes of Degenerative Cervical Myelopathy. Global Spine J. 12 (5), 795–800. https://doi.org/10.1177/2192568220964167 (2022).
Chen, Y. et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur. Spine J. 22, 1539–1546. https://doi.org/10.1007/s00586-013-2772-y (2013).
Wang, J., Li, H. & Yang, B. Predictive Nomogram for Clinical Prognosis in Cervical Spondylotic Myelopathy With Intramedullary T2-Weighted Increased Signal Intensity: A Novel Digital Tool for Patient Prognosis Education. Front. Public. Health. 10, 898242. https://doi.org/10.3389/fpubh.2022.898242 (2022).
Chen, G. et al. Nomogram for predicting kyphotic deformity after laminoplasty in cervical spondylotic myelopathy patients without preoperative kyphotic alignment. Clin. Neurol. Neurosurg. 199, 106284. https://doi.org/10.1016/j.clineuro.2020.106284 (2020).
Farag, C. M., Antar, R., Akosman, S., Ng, M. & Whalen, M. J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 14, 153–172. https://doi.org/10.18632/oncotarget.28367 (2023).
Xu, H., Zheng, X., Ai, J. & Yang, L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int. Immunopharmacol. 114, 109496. https://doi.org/10.1016/j.intimp.2022.109496 (2023).
Xu, M. et al. The HALP (hemoglobin, albumin, lymphocyte, and platelet) score is associated with early-onset post-stroke cognitive impairment. Neurol. Sci. 44 (1), 237–245. https://doi.org/10.1007/s10072-022-06414-z (2023).
Zhang, F. et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) is a potent indicator for the prognosis in hemodialysis patients. Med. (Baltim). 102 (19), e33650. https://doi.org/10.1097/MD.0000000000033650 (2023).
Liu, L. et al. Home enteral nutrition after esophagectomy for esophageal cancer: A systematic review and meta-analysis. Med. (Baltim). 99, e21988. https://doi.org/10.1097/md.0000000000021988 (2020).
Huang, Y. et al. The prognostic impact of decreased pretreatment haemoglobin level on the survival of patients with lung cancer: a systematic review and meta-analysis. BMC Cancer. 18, 1235. https://doi.org/10.1186/s12885-018-5136-5 (2018).
Hamai, Y. et al. Hemoglobin level influences tumor response and survival after neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma. World J. Surg. 38, 2046–2051. https://doi.org/10.1007/s00268-014-2486-2 (2014).
Yilmaz, G. & Shaikh, H. Normochromic Normocytic Anemia. In StatPearls, (StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.). (2023).
Chojkier, M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J. Clin. Gastroenterol. 39, 143–146. https://doi.org/10.1097/01.mcg.0000155514.17715.39 (2005).
Li, X. et al. Analysis of Results of Continuous Invasive Arterial Blood Pressure Monitoring in Postoperative Patients. Altern. Ther. Health Med. 29, 466–473 (2023).
Wang, P., Liu, B., Rong, T. & Wu, B. Is diabetes the risk factor for poor neurological recovery after cervical spine surgery? A review of the literature. Eur. J. Med. Res. 27, 263. https://doi.org/10.1186/s40001-022-00879-6 (2022).
Cabrera, A. et al. Use of random forest machine learning algorithm to predict short term outcomes following posterior cervical decompression with instrumented fusion. J. Clin. Neurosci. 107, 167–171. https://doi.org/10.1016/j.jocn.2022.10.029 (2023).
Li, X. D. & Li, M. M. A novel nomogram to predict mortality in patients with stroke: a survival analysis based on the MIMIC-III clinical database. BMC Med Inform Decis Mak. ;22(1):92. Published 2022 Apr 6. doi: (2022). https://doi.org/10.1186/s12911-022-01836-3
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Zhao Huang and Mingjian Lin designed this study. He Ling, Gaoyong Deng and Shaohuai Xia are responsible for writing articles, conducting statistical analysis, reviewing articles, and creating images.Wencai Li and Rongbin Lu responsible for collecting data and conducting statistical analysis.All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the the First Affiliated Hospital of Guangxi Medical University Ethics Review Committee (2023-E717-01). The requirement for informed consent was waived by the First Affiliated Hospital of Guangxi Medical University Ethics Review Committee because of the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ling, H., Deng, G., Xia, S. et al. Construction of a nomogram for predicting the prognosis of patients with cervical spondylotic myelopathy: a retrospective study. Sci Rep 14, 31919 (2024). https://doi.org/10.1038/s41598-024-83429-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83429-w