Abstract
Acute kidney injury (AKI) is common in critically ill patients, optimal timing for initiation of renal replacement therapy (RRT) for AKI but without life-threatening indications is unclear. A retrospective study was performed using the medical information mart for intensive care (MIMIC-IV), including AKI patients identified based on kidney disease improving global outcomes (KDIGO) definition. The time to initiate CRRT was defined as the interval from first diagnosis of AKI to the initiation of CRRT, analyzed as a continuous variable. The primary outcome was 28-day mortality, restricted cubic splines (RCS) to assess the relationship between CRRT initiation timing intervals and clinical outcomes. The study included 673 patients, with a 28-day mortality rate of 38.78%. RCS analysis revealed no significant association between variations in CRRT timing intervals and 28-day mortality (P > 0.05). In the subgroup of patients with non-renal SOFA scores < 8, observed an increase in 28 day mortality (OR 1.011 [95% CI 1.001–1.021], P < 0.05), along with a greater likelihood of reduced 28-day CRRT, mechanical ventilation (MV), and ICU-free days for each 1-h delay in CRRT initiation (OR − 0.037 [95% CI − 0.064 to − 0.010], P < 0.05; OR − 0.051 [95% CI − 0.078 to − 0.024], P < 0.05; OR − 0.056 [95% CI − 0.082 to − 0.003], P < 0.05). The findings indicate that while no significant relationship exists between mortality and the timing of CRRT initiation, patients with lower non-renal SOFA scores who initiate RRT promptly may experience improved clinical outcomes. Further exploration and validation require the adoption of novel research methodologies and more pertinent clinical studies.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is common among critically ill patients and at risk for increased morbidity and mortality1. Continuous renal replacement therapy (CRRT) is indicated when medical management is insufficient to maintain fluid balance or when complications of acute kidney injury (AKI) occur2,3,4,5. Determining the optimal timing of CRRT initiation remains a top research priority in the most recent Kidney Disease Improving Global Outcomes (KDIGO) Conference on Controversies in AKI6,7. In the past decade, considerable attention has been directed toward identifying the optimal timing for the initiation of CRRT. Several randomized controlled trials involving adult populations have examined the influence of initiation timing on CRRT outcomes, yet the findings remain inconclusive2,3,8,9,10,11.
This cohort study presents a quantitative analysis focused on the timing of CRRT initiation and its endpoints, aiming to assess the effect of CRRT initiation timing on 28-day mortality in patients with acute kidney injury (AKI) utilizing data from the MIMIC-IV database.
Methods
Study design and setting
We performed a retrospective observational cohort study at a single institution, utilizing data from the Medical Information Mart for Intensive Care IV version 2.2 (MIMIC-IV v2.2)12, a comprehensive publicly accessible database developed and maintained by the Computational Physiology Lab at the Massachusetts Institute of Technology (MIT). The database, sourced from Beth Israel Deaconess Medical Center in Boston, Massachusetts, USA, encompasses extensive, anonymized clinical data from over 380,000 patients admitted between 2008 and 2019. All data were obtained from PhysioNet (https://physionet.org/) following the completion of data permission applications and the signing of the necessary agreements. The institutional review board waived the necessity for written informed consent and ethical review, as the study utilized de-identified patient data and was conducted retrospectively. After finishing the online training for the Collaborative Institutional Training Initiative program (record ID: 55632299), one author of this study (MN) received access to the database. This study was conducted in compliance with the 2014 revision of the Declaration of Helsinki13,14. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline15.
Participants
The study population included 17,385 adult patients diagnosed with AKI as defined by the KDIGO criteria, identified within the MIMIC-IV version 2.2 database. For patients with multiple admissions, only the first hospitalization was taken into account. This analysis excluded individuals who did not receive CRRT, as well as those with a history of end-stage kidney disease (ESKD), peritoneal dialysis, or emergency CRRT necessitated by hyperkalemia (serum potassium level > 6.5 mmol/L), metabolic acidosis (pH < 7.15), or fluid overload unresponsive to diuretics with pulmonary edema prior to the diagnosis of AKI. Furthermore, patients who were pregnant, had malignancies, or had undergone kidney transplantation were also excluded, along with those with missing data exceeding 30% or lacking follow-up information. Ultimately, the final study cohort comprised 673 patients, who were categorized into two groups based on the primary outcome. (Fig. 1).
Data collection
Data collection was conducted utilizing Structured Query Language (SQL) with PostgreSQL (version 16.1) to extract baseline characteristics, clinical interventions, and outcomes of patients from the MIMIC-IV version 2.2 database. The dataset included demographic information (age, gender, body mass index [BMI]), timing of admission, initiation of CRRT time, CRRT treatment dose, CRRT down time, diagnosis of AKI, source of intensive care unit (ICU) admission, comorbidities, Sequential Organ Failure Assessment (SOFA) score, non-renal SOFA score, severity of AKI, organ support interventions (vasoactive support, mechanical ventilation [MV], CRRT), and clinical outcomes (mortality, length of hospital stay, length of ICU stay). The CRRT initiation time defined as duration of AKI to initiate CRRT; AKI diagnoses and stage for AKI are defined according to the KDIGO standards (refer to supplementary material eFig. 3), The minimum serum creatinine (SCr) value recorded prior to ICU admission was utilized as the baseline SCr, while the peak SCr value within 24 h of AKI diagnosis was recorded as the SCr at the time of diagnosis. The calculation for 28 day MV, CRRT, and ICU-free days involves subtracting the duration of MV or CRRT utilization, as well as the length of ICU stay, from a total of 28 days. Patients who die within the 28 day period are assigned a value of 0 for this outcome measure, reflecting the competitive nature of this endpoint. Comorbidities were identified using codes from the International Classification of Diseases, 10th Revision (ICD-10) and ICD-9. The follow-up period commenced upon admission and concluded upon reaching the predefined endpoints of interest.
Main exposure
The variable of interest was the time to CRRT initiation, anchored to the first diagnosis of AKI. This was defined as the duration (in hours) from the initial diagnosis of AKI to the commencement of CRRT. The diagnosis of AKI was established in accordance with the KDIGO criteria, which encompass any of the following: an increase in SCr of ≥ 0.3 mg/dL (26.5 µmol/L) within 48 h; an increase in SCr to ≥ 1.5 times the baseline value within the preceding 7 days; or a urine output of < 0.5 mL/kg/hour for a duration of 6 h16.
Primary and secondary outcomes
The primary outcome of the study was 28 day mortality. Secondary outcomes included 90-day mortality, 1 year mortality, the number of 28 day MV-free days, 28 day CRRT-free days, and 28 day ICU-free days. The 28 day mortality was defined as death occurring within 28 days following the diagnosis of AKI. Definitions for 90 day mortality and 1 year mortality were derived from the 28 day mortality. Data on patient deaths in the MIMIC-IV database were obtained from death records during hospitalization or from the Massachusetts Department of Public Health database for deaths recorded post-discharge12. The calculation for 28 day MV, CRRT, and ICU-free days involves subtracting the duration of MV or CRRT utilization, as well as the length of ICU stay, from a total of 28 days. Patients who die within the 28 day period are assigned a value of 0 for this outcome measure, reflecting the competitive nature of this endpoint.
Subgroup analysis
Subgroup analyses were performed using the same statistical modeling methods applied to the primary outcome, stratified by gender, age, non-renal SOFA score, and history of pre-existing chronic kidney disease (CKD). The subgroup analyses included: (1) male versus female patients; (2) patients aged > 65 years compared to those aged ≤ 65 years; (3) patients with non-renal SOFA scores of < 8 versus ≥ 8; and (4) patients with and without a history of CKD prior to admission. The age subgroup was established based on classifications used in previous studies2,4,8,10,17. For the non-renal SOFA subgroup, participants were stratified according to the median (IQR) of the population. In constructing the baseline table for the non-renal SOFA subgroup, early and late initiation times of CRRT were treated as categorical variables for statistical analysis, with the median CRRT initiation time serving as the categorical cutoff.
Statistical analysis
Continuous variables were summarized as mean (standard deviation) or median (interquartile range), and group comparisons were performed using the Mann–Whitney U test, depending on the nature of the data. Categorical variables were presented as frequencies and percentages, and comparisons between groups were made using Fisher’s exact test or the Pearson chi-square test. Logistic regression and linear regression analyses were conducted to evaluate the odds ratios (OR) for primary and secondary outcomes, along with the corresponding 95% confidence intervals (CI). This study performed a restricted cubic spline (RCS) analysis to investigate the relationship between CRRT initiation timing intervals and the odds ratio for 28 day mortality. In this analysis, CRRT initiation timing intervals were treated as the independent variable, while the primary outcome was designated as the dependent variable. This approach elucidates the trends in the OR and the 95% CI for 28 day mortality in relation to variations in CRRT initiation timing intervals.
Ordinal regression models were used to explore the trend relationships between 28 day CRRT-free days, MV-free days, ICU-free days, and CRRT initiation time18. The outcomes for 28 day CRRT-free days, MV-free days, and ICU-free days were estimated using the ordinal regression model, with a common OR and 95% CI calculated. Subgroup analyses were performed to assess correlations between each subgroup and the primary outcome, with results presented in the form of a forest plot. Kaplan–Meier curves were utilized to illustrate the relationship between the primary outcome and CRRT initiation time across subgroups. The Wald χ2 test was applied to evaluate the significance of the interaction term. A two-sided P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using R software, version 4.4.0, IBM SPSS 25.0, Microsoft Excel, and Stata MP18 (64-bit).
Results
Patient characteristics
A total of 17,385 adults with acute kidney injury (AKI), as defined by KDIGO criteria, were reviewed from the MIMIC-IV database. Ultimately, 673 patients were identified in the final cohort who met the specified inclusion and exclusion criteria (Fig. 1). Among the reviewed patients, 412 (61.22%) were classified into the 28 day survival group, while 261 (38.78%) were categorized as the 28 day non-survival group. The cohort comprised 401 males (59.60%), with a median age of 61.0 years (interquartile range [IQR] 50.0–70.0). The baseline characteristics of the cohort are summarized in Table 1.
Timing of CRRT initiation
The median time to initiation of CRRT was 39.93 h (IQR 14.23–80.23 h), CRRT treatment dose 27.58 h (IQR 25.46–35.87 h), CRRT down time 96.00 h (IQR 48.00–216.00 h). The median time to diagnosis of AKI was 6.87 h (IQR 1.51–10.69 h). Upon comparison of CRRT initiation timing with primary and secondary outcomes, no significant associations were identified (Fig. 2). Regression analysis examining the correlation between outcomes and CRRT initiation timing indicated that, although mortality rates and the number of 28 day free days did not demonstrate statistically significant differences, each 1 h delay in CRRT initiation was associated with an 8.0% increase in the likelihood of prolonged hospital stay (hospital stay: OR 0.08 [95% CI 0.04–0.118]; P < 0.05) (refer to supplementary material eTable 2, eFig. 1).
Primary and secondary outcomes
The primary outcome of the study was 28 day mortality, with 261 patients (38.78%) dying during this period. Secondary outcomes are summarised in Table 1. Among the 673 patients who received CRRT, restricted cubic spline (RCS) analysis indicated that 28 day mortality was not significantly associated with the timing of CRRT initiation (P > 0.05, Fig. 3A). Furthermore, linear regression analyses examining the relationship between CRRT initiation timing and both primary and secondary outcomes revealed no statistically significant differences in 28 day mortality, 90-day mortality, 1 year mortality, 28 day CRRT-free days, 28 day MV-free days, or 28 day ICU-free days (P > 0.05, Fig. 3B, C) (refer to supplementary material eTable. 2).
Subgroup analysis
The timing of CRRT initiation was not statistically associated with primary or secondary outcomes. Consequently, subgroup analyses were conducted to explore the relationships between primary and secondary outcomes and CRRT initiation timing within specific populations. These analyses were performed based on gender, age, non-renal SOFA score, and history of pre-existing CKD (Fig. 4).
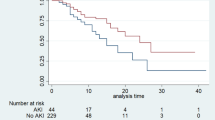
In the subgroup with non-renal SOFA scores < 8, each 1 h delay in CRRT initiation was associated with a 1.1% increase in the odds of 28 day mortality (OR 1.011 [95% CI 1.001–1.021], P < 0.05, Fig. 4). Kaplan–Meier curves for 28 day mortality in relation to CRRT initiation timing demonstrated that the survival rate in the non-renal SOFA < 8 group was significantly higher compared to the non-renal SOFA ≥ 8 subgroup (P < 0.05, Fig. 5, refer to supplementary material eTable. 3). Notably, the slope of the Kaplan–Meier curve for the non-renal SOFA < 8 group was relatively shallow at the initiation of CRRT, becoming steeper with further delays in initiation (Fig. 5). This observation indicates that the slope of the Kaplan–Meier curve reflects the rate of increase in 28 day mortality as the time to CRRT initiation lengthens. Furthermore, the RCS analysis comparing the non-renal SOFA < 8 group and time to CRRT initiation revealed that the odds ratio for 28-day mortality during the immediate CRRT initiation phase increased with delays in initiation, with statistically significant findings (non-renal SOFA < 8: P < 0.05 vs non-renal SOFA ≥ 8: P > 0.05, Fig. 5, eFig. 2).
In the non-renal SOFA < 8 group, secondary outcomes such as 90 day mortality and 1 year mortality also increased with longer time to CRRT initiation (P < 0.05, Fig. 6). Conversely, the number of 28 day CRRT-free days, MV-free days, and ICU-free days decreased with increasing time to CRRT initiation (P < 0.05, Fig. 6). In the non-renal SOFA ≥ 8 group, the correlations between secondary outcomes and time to CRRT initiation were not statistically significant (P > 0.05, Fig. 6 refer to supplementary material eTable. 3). Each 1 h delay in CRRT initiation was associated with a decrease in 28 day CRRT-free days, MV-free days, and ICU-free days, with the following odds ratios: 28 day CRRT free days: OR − 0.037 [95% CI − 0.064 to − 0.010], P < 0.05; 28-day MV free days: OR − 0.051 [95% CI − 0.078 to − 0.024], P < 0.05; and 28 day ICU free days: OR − 0.056 [95% CI − 0.082 to − 0.003], P < 0.05 (refer to supplementary material eTable. 2).
Discussion
This cohort study examined the timing of CRRT initiation in patients with acute kidney injury using data from the MIMIC-IV database12. Unlike previous studies that imposed strict definitions for early and delayed initiation, this study did not specify exact hour thresholds for CRRT initiation4,10,11,19,20.
Recent randomized controlled trials10,11,19,20 have yielded conflicting results. Observational studies and meta-analyses suggested potential benefits of early RRT21,22. Gaudry et al.23, found no significant difference in 60 day mortality when comparing early versus delayed RRT strategies. Another referenced study24 no survival benefit was observed for the delayed group.
The study used RCS curves to link CRRT timing with outcomes, a novel approach in CRRT research but seen in other fields. A recent study identified an optimal body mass index (BMI) interval using RCS curves25. Consistent with previous research2,11,23,26, this study found no correlation between 28 day mortality and the timing of CRRT initiation (Fig. 2).
Previous studies noted a lower 90 day mortality associated with early RRT initiation2, however, there were no significant differences concerning non-MV days or overall hospital days. Notably, while no statistical differences were found in primary or secondary outcomes, an increase in the length of hospital stay was observed with prolonged time to CRRT initiation, likely due to the study’s exclusive focus on patients receiving CRRT.
Subgroup analyses for the primary outcome were conducted according to gender, age, non-renal SOFA score, and history of CKD. In the subgroup with non-renal SOFA < 8, earlier CRRT initiation yielded a lower OR for mortality, with 28 day mortality increasing with delays in CRRT initiation, particularly sustained up to the 50 h mark (Fig. 4). Similarly, this subgroup exhibited statistically significant reductions in 90 day and 1 year mortality, as well as increases in 28 day CRRT-free days, MV-free days, and ICU-free days (P < 0.05, Fig. 5). While some studies have suggested that early CRRT initiation might extend ICU and MV days23, the findings of this study contradict this, possibly because all participants in this study underwent CRRT. A comparable trend was observed in patients with a history of comorbid CKD, although subgroup comparisons were limited due to the small number of patients without prior CKD.
The non-renal SOFA < 8 patients baseline characteristics are detailed in the supplement (refer to supplementary material eTable 3). A recent post hoc analysis of the AKIKI trial27, stratified the original AKIKI cohort into septic and ARDS phenotypes, concluding that early initiation of RRT in this vulnerable subset of patients with concurrent ARDS or sepsis was associated with an increased 60 day mortality rate. Importantly, this mean SOFA score is higher than that observed in our study. Alternatively, employing a scoring methodology similar to the non-renal SOFA scoring utilized in this study could prove advantageous. Furthermore, a subset of patients in the late-stage cohort of the AKIKI trial did not receive CRRT, with all non-CRRT patients classified within this late-stage group. This allocation might artificially lower the mortality rates in the late-stage cohort, potentially leading to an overestimation of mortality rates in the early-stage group. Consequently, this study specifically targets the subgroup of patients who received CRRT, aiming to assess whether they derive any benefits from the earlier initiation of this therapeutic intervention. Previous observational studies17 have indicated that early initiation of CRRT can reduce mortality. Potential explanations include improved extracellular fluid volume, acid–base balance and electrolyte abnormalities with earlier RRT initiation. Although patients in this study with lower non-renal SOFA scores had a more fragile renal function due to their advanced age and multiple comorbidities, early initiation of CRRT was more likely to preserve renal function and mitigate other factors contributing to poor outcomes. In contrast, patients with higher non-renal SOFA scores faced multiple confounding risk factors associated with multi-organ dysfunction, which overall elevated mortality rates, suggesting that early CRRT initiation may not significantly alter final outcomes in this group.
Although a definitive optimal CRRT initiation time could not be established in this study, the effects of variations in outcomes with changes in CRRT initiation time were detectable in subgroup analyses by the RCS curve, providing a foundation for future exploration of optimal CRRT initiation timing in more specific patient populations.
Limitation
This study has several limitations. First, we included only patients who received continuous renal replacement therapy among the eligible cohort. During the screening process within the MIMIC-IV database, it proved challenging to identify patients who did not undergo CRRT following the diagnosis of acute kidney injury after excluding those with indications for urgent CRRT initiation. To mitigate bias, we deliberately excluded all patients who met the inclusion criteria but did not receive CRRT, and we aim to explore improved database screening methodologies in future research. The second limitation is inherent to the MIMIC database, which is a single-center, retrospective observational study; thus, the potential for selection bias is unavoidable. To address this concern, we implemented rigorous screening criteria based on nadir values and conducted subgroup analyses to compare differences between the unused subgroups and primary outcomes. Lastly, the inability to extract the latest biomarkers associated with acute renal insufficiency, as well as certain specialized laboratory indicators, poses a limitation due to the constraints of the database. Consequently, we could not assess prognosis in conjunction with these contemporary biomarkers. Future research will seek to supplement our findings by exploring additional databases to enhance the robustness of our results.
Conclusions
In conclusion, this study showed no significant relation of mortality between the variation of initiation time of RRT in patients with severe acute kidney injury but with no immediate, life-threatening complications linked to acute kidney injury, but patients with low non-renal SOFA, those who initiated RRT as early as possible benefited in clinical outcomes. Further exploration and validation require the adoption of novel research methodologies and more pertinent clinical studies.
Data availability
The MIMIC-IV database is available from https://mimic.physionet.org. The raw data were extracted using structure query language (SQL) and PostgreSQL, as well as using Excel 2019, R software, version 4.4.0, IBM SPSS 25.0 and Stata MP18 (64-bit) for data entry and analysis, respectively.
References
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Primers 7, 52 (2021).
Zarbock, A. et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically Ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA J. Am. Med. Assoc. 315, 2190–2199 (2016).
Wald, R. et al. Fluid balance and renal replacement therapy initiation strategy: a secondary analysis of the STARRT-AKI trial. Crit. Care 26, 360 (2022).
Wald, R. et al. Initiation of continuous renal replacement therapy versus intermittent hemodialysis in critically ill patients with severe acute kidney injury: a secondary analysis of STARRT-AKI trial. Intensive Care Med. 49, 1305–1316 (2023).
Wald, R. & Bagshaw, S. M. Integration of Equipoise into eligibility criteria in the STARRT-AKI Trial. Am. J. Respir. Crit. Care Med. 204, 234–237 (2021).
Ostermann, M. et al. Controversies in acute kidney injury: conclusions from a kidney disease: Improving global outcomes (KDIGO) conference. Kidney Int. 98, 294–309 (2020).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron 120, c179–c184 (2012).
Pan, H. C. et al. Accelerated versus standard initiation of renal replacement therapy for critically ill patients with acute kidney injury: a systematic review and meta-analysis of RCT studies. Crit. Care 25, 5 (2021).
Zampieri, F. G. et al. A Bayesian reanalysis of the standard versus accelerated initiation of renal-replacement therapy in acute kidney injury (STARRT-AKI) trial. Crit. Care 26, 255 (2022).
Chen, W. Y. et al. The timing of continuous renal replacement therapy initiation in sepsis-associated acute kidney injury in the intensive care unit: the CRTSAKI Study (continuous RRT timing in sepsis-associated AKI in ICU): study protocol for a multicentre, randomised controlled trial. BMJ Open 11, e40718 (2021).
Fayad, A. I., Buamscha, D. G. & Ciapponi, A. Timing of kidney replacement therapy initiation for acute kidney injury. Cochrane Database Syst. Rev. 11, D10612 (2022).
Johnson, A. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1 (2023).
Czarkowski, M. Helsinki declaration–next version. Pol. Merkur Lekarski 36, 295–297 (2014).
Snaedal, J. The Helsinki declaration. Laeknabladid 100, 135 (2014).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 4, e297 (2007).
Lameire, N. H. et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179 (2013).
Wald, R. & Bagshaw, S. M. The timing of renal replacement therapy initiation in acute kidney injury: is earlier truly better?*. Crit. Care Med. 42, 1933–1934 (2014).
Liu, Q., Shepherd, B. E., Li, C. & Harrell, F. J. Modeling continuous response variables using ordinal regression. Stat. Med. 36, 4316–4335 (2017).
Gist, K. M. et al. Time to continuous renal replacement therapy initiation and 90-day major adverse kidney events in children and young adults. JAMA Netw. Open 7, e2349871 (2024).
Jeong, R., Wald, R. & Bagshaw, S. M. Timing of renal-replacement therapy in intensive care unit-related acute kidney injury. Curr. Opin. Crit. Care 27, 573–581 (2021).
Schneider, A. G., Uchino, S. & Bellomo, R. Severe acute kidney injury not treated with renal replacement therapy: characteristics and outcome. Nephrol. Dialysis Transplant. 27, 947–952 (2012).
Gaudry, S. et al. Acute kidney injury in critical care: experience of a conservative strategy. J. Crit. Care 29, 1022–1027 (2014).
Gaudry, S. et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N. Engl. J. Med. 375, 122–133 (2016).
Bellomo, R. et al. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 361, 1627–1638 (2009).
Bhaskaran, K., Dos-Santos-Silva, I., Leon, D. A., Douglas, I. J. & Smeeth, L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 6, 944–953 (2018).
Barbar, S. D., Dargent, A. & Quenot, J. P. Timing of renal-replacement therapy in acute kidney injury and sepsis. N. Engl. J. Med. 380, 399 (2019).
Gaudry, S. et al. Timing of renal support and outcome of septic shock and acute respiratory distress syndrome a post hoc analysis of the AKIKI randomized clinical trial. Am. J. Respir. Crit. Care Med. 198, 58–66 (2018).
Funding
This work was supported by the Beijing Municipal Natural Science Foundation (No. 7224335).
Author information
Authors and Affiliations
Contributions
Mangsuer Nuermaimaiti and Ran Lou: created the study protocol, Mangsuer Nuermaimaiti and Meiping Wang: performed the statistical analyses, and wrote the first manuscript draft. Li Jiang and Meiping Wang assisted the analysis and explained statistical methods, Mangsuer Nuermaimaiti and Nan Wang collaborated on writing the code for data extraction from the database and data extraction. Tingting Wang and Quan Si assisted in screening and verifying the accuracy of the extracted data. Quan Si assisted in funding acquisition. Li Jiang assisted with manuscript revision and data confirmation. Li Jiang and Mangsuer Nuermaimaiti contributed to data interpretation and manuscript revision. All authors read and approved the final manuscript. All authors were aware of and approved the publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and consent statement
The Medical Information Mart for Intensive Care-IV database was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH) under award numbers R01-EB001659 (2003–2013) and R01-EB017205 (2014–2018). In these databases, the true identity information about the patient is hidden. Thus, obtaining the patient’s informed consent was not needed. The author of this study (MN) completed the relevant course training and obtained the certificate to access these databases (record ID: 55632299).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nuermaimaiti, M., Wang, M., Lou, R. et al. The impact of initiation timing of continuous renal replacement therapy on outcomes in critically ill patients with acute kidney injury a retrospective study from the MIMIC-IV database. Sci Rep 15, 10922 (2025). https://doi.org/10.1038/s41598-024-84435-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84435-8