Abstract
The tubers of Curcuma kwangsiensis are regarded as an important medicinal material in China. In C. kwangsiensis cultivation, tuber expansion is key to yield and quality, but the regulatory mechanisms are not well understood. In this study, metabolomic and transcriptomic analyses were conducted to elucidate the mechanism underlying tuber expansion development. The results showed that auxin (IAA), jasmonic acid (JA), gibberellin (GA3), ethylene (ETH), and brassinolide (BR) levels increased during tuber expansion development. Metabolomic analysis showed that 197 differentially accumulated metabolites (DAMs) accumulated during tuber expansion development and these also play important roles in the accumulation of carbohydrates and secondary metabolites. 6962 differentially expressed genes (DEGs) were enriched in plant hormone signal transduction, starch and sucrose metabolism, linoleic acid metabolism, MAPK signaling pathway as well as sesquiterpenoid and triterpenoid biosynthesis. Comprehensive analysis revealed that DEGs and DAMs of plant hormone signal transduction, ABC transporters and biosynthesis of phenylpropanoids and terpenoids are critical pathways in regulating tuber expansion. In addition, some transcription factors (ARF, C2H2, C3H, NAC, bHLH, GRAS and WRKY) as well as hub genes (HDS, HMGR, ARF7, PP2CA, PAL and CCOMT) are also involved in this process. This study lays a theoretical basis for the molecular mechanism of tuber expansion in C. kwangsiensis.
Similar content being viewed by others
Introduction
Curcuma kwangsiensis S. G. Lee et C. F. Liang, belonging to genus Curcuma, Zingiberaceae family, is a traditional Chinese medicinal herb widely cultivated in southwestern China. The rhizomes and tubers of C. kwangsiensis are recognized as one of the sources of Ezhu and Yujin, respectively, as documented in the Chinese Pharmacopoeia (2020 edition)1, with distinct therapeutic properties. Compared to rhizomes, tubers exhibit a higher yield, thus making tuber size a critical factor in deterimining the planting efficiency of C. kwangsiensis. The active ingredients of C. kwangsiensis tubers are volatile oils composed of structurally diverse sesquiterpenoid compounds, such as germacrone, curzerenone, beta-elemen and curcumenol2,3, offering anticancer, anti-inflammatory, antioxidant, and hepatoprotective benefits4,5. During artificial cultivation, the cultivated tubers display significant size variation, leading to low yield and disparity qualities6. Therefore, the mechanisms underlying the development of C. kwangsiensis tubers and the biosynthesis pathways of their metabolites is crucial for enhancing both their quality and yield, and also will provide a fundamental basis for optimizing production strategies.
Tuber plants are characterized by their expansion of underground storage organs. Tuber development mainly includes initiation, expansion, and maturation stages, accompanied by the accumulation of metabolites (starch, storage proteins and other metabolites)7,8,9. Great efforts have been made to explore tuber development commonly caused by environmental and endogenous signals10.Potato and yam are among the most important tuber crops, and their tubers are expansion stems originating from belowground stolon and hypocotyls, respectively. The short-day treatment accelerated tuber formation in potato and promoted tuber initiation and expansion growth in yam, but the responses varied among species and cultivars11,12,13. Endogenous phytohormones are involved in tuber developmental processes. High gibberellins (GA) and abscisic acid (ABA) levels inhibit tuber initiation and retard expansion in potato9, and GA, ABA, trans-zeatin (ZR) and jasmonic acid (JA) are also involved in accelerating tuber expansion growth of yam7. The StGA2ox1 (GA2-oxidase) expression level was upregulated in the subapical zone of the stolon and growing tuber before tuber expansion, which the ABRE-binding factor genes StABF4 and StABF2 positively regulate tuber induction, suggesting these genes play a role in tuber development14,15. DELLA (DELLA protein), Aux/IAA (auxin influx carrier/auxin-responsive protein), ARF (auxin response factors) and SAUR (small auxin up RNA) genes were significantly abundant in the tuber expansion stage16. In medicinal plants, enlarged tubers act as crucial medicinal organs accumulating abundant bioactive ingredients. There are several genes involved in C. longa tuber development were significantly enriched in the carbohydrate metabolism, phytohormone signaling, phenylpropanoid and transporter pathway, include IAA6 (auxin-responsive protein 6), PYR1 (abscisic acid receptor 1), ARF17, ABI5 (abscisic acid insensitive 5), GID1, MYC2/4, SWEET (sugars will eventually be exported transporters), STP5 (sugar transport protein 5), PAL, C4H (cinnamate 4-hydroxylase), 4CL (4-coumarate-CoA ligase), CCOMT (coffee acyl coenzyme A-3-O-methyl transferase) genes17. The terpenoid biosynthesis, plant hormone signal transduction, phenylpropanoid biosynthesis, flavonoid biosynthesis, and other synthesis pathways are found in tubers of C. wenyujin18. Nonetheless, there are still significant gaps in our knowledge of tuber development, particularly its molecular mechanisms in medicinal plants.
The analysis of the tuber development process of C. kwangsiensis is beneficial for improving their yield and quality during cultivation6. C. kwangsiensis tubers have active sesquiterpenoid compounds. Numerous studies have shown that sesquiterpenoids comprise an important component of their pharmacological activity4,19, and sesquiterpenoid synthesis has been identified by studying terpenoid biosynthesis20,21. The mevalonic acid (MVA) and 2-C-methyl-D-erythritol 4-phosphate (MEP) pathways are two synthetic routes to the biosynthesis of terpenoids. 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), 1-deoxy-D-xylulose-5-phosphate synthase (DXS), 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR), isoprene pyrophosphate synthase and terpene synthase (TPS), as well as other enzymes, have been reported to be key enzymes in terpenoid biosynthesis20,22.In addition, the genes encoding transcription factor (TFs), including bHLH (basic helix-loop-helix), WRKY, NAC, MYB (myeloblastosis), GRAS and ARF, and ATP-binding cassette transporter (ABC transporter), regulated terpenoid biosynthesis during tissue development in C. wenyujin, Aconitum heterophyllum, Agriophyllum squarrosum and celery18,23,24,25. However, the specific roles of the terpenoid-related gene-regulating network are still lacking and impede our understanding of the tuber development process in C. kwangsiensis.
Recently, the genes and metabolites involved in the development of enlarged tissues in medicinal plants have been investigated through an intergrative approach combining metabolomics and transcriptomics, such as Dioscorea polystachya tubers26, Tetrastigma hemsleyanum tuberous root27, Panax notoginseng taproots28. C. kwangsiensis tuber possesses high medicinal value and economic benefits, with size influencing both the yield and quality6. The analysis of the tuber development process of C. kwangsiensis is beneficial for improving their yield and quality during cultivation. Understanding the metabolites and constructing the molecular mechanism of tuber expansion development can provide an important basis for its high-yield breeding and the synthesis of quality components in C. kwangsiensis. In this study, the initiation and expansion stages of tuber development were selected for metabolomic and transcriptomic analysis. This study aimed to uncover the key molecular network during tuber development in C. kwangsiensis.
Results
Morphological investigation of tuber expansion
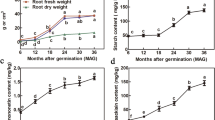
The tuber characteristics of C. kwangsiensis were significantly different at the initiation and expansion stages (ET and MT) (Fig. S1C). Hormonal analysis revealed distinct profiles at these two stages. ZR, ABA and salicylic acid (SA) were high at the initiation stage, while IAA, JA, GA, ethylene (ETH), SA and brassinolide (BR) were significantly accumulated at the expansion stage (Fig. S1D). The expansion of the tubers might be related to the hormonal level variations.
Metabolites identification and analysis
To explore the metabolite differences of the initiation and expansion stages (ET and MT) in C. kwangsiensis, the tubers were analyzed using a non-targeted UHPLC-MS/MS with positive and negative modes. A heat map cluster showed good correlations among replicates which indicated high statistical repeatability of the data (Fig. S2A). A total of 999 metabolites were identified (Table S1), of which 12 category classifications were annotated according to their chemical properties (Fig. 1A) and these included flavonoids (173), phenolic acids (162), lipids (118), amino acids and their derivatives (82), organic acids (79), alkaloids (61), nucleotides and their derivatives (48), lignans and coumarins (48), terpenoids (47), tannins (14), quinones (2) and others (165). PCA and OPLS-DA model analysis were used to decrease the data dimensions and improve their interpretability and effectiveness. The PCA score plot showed that the two samples were divided into two sections (Fig. 1B), and the OPLS-DA results showed a high degree of distinction between the sample groups (Fig. 1C), suggesting that the metabolites could be effectively separated with differences between the initiation and expansion stages being evident.
Metabolic analysis of sample relationship of metabolites. (A) Metabolites number. (B) Principal component analysis (PCA) at the initiation (ET) and expansion (MT) stages. (C) Orthogonal partial least-squares discriminant analysis (OPLS-DA) model at ET and MT stage. (D) A clustering heatmap of the differentially accumulated metabolites (DAMs). (E) KEGG enrichment pathway of DAMs.
According to the results of metabolomics analysis, 197 DAMs were found between the initiation and expansion stages, of which approximately two-thirds of DAMs were up-regulated (Fig. 1D). The metabolite differences significantly enriched metabolic pathways (Fig. 1E), including ABC transporters, galactose metabolism, starch and sucrose metabolism and biosynthesis of antibiotics. It is noteworthy that some important carbohydrate metabolites, secondary metabolites and amino acids in the initiation and expansion stages were significantly accumulated within pathway such as starch and sucrose metabolism, citrate cycle, galactose metabolism, phenylalanine metabolism, terpenoid compounds, ABC transporters, valine, leucine and isoleucine biosynthesis and degradation.
Transcripts identification and analysis
The transcriptome library was constructed from a pool of mixed RNA consisting of the initiation and expansion stages (named EZ). A total of 31,889 transcripts and 26,590 genes were obtained (Table S2). The Pearson correlation coefficient analysis provided evidence of the biological consistency (Fig. S2B). 26,274, 26,158, 19,038 and 23,559 genes were functionally annotated with 4 functional databases NR, KEGG, KOG and SwissProt, respectively, making a total of 26,348 genes (Table S3). 18,257 genes were commonly annotated in NR, KOG, KEGG and SwissProt databases (Fig. 2A). Based on the functional annotation results of the NR database, the proportions of different species in the notes of genes were calculated, and 14,634 genes were aligned to Musa acuminata (Fig. 2B). To investigate the gene expression differences with C. kwangsiensis tubers, we conducted analyses at the initiation and expansion stages using six RNA-Seq libraries. PCA of gene expression levels demonstrated a distinct separation between samples from the initiation and expansion stages (Fig. 2C). After significance analysis, 6962 DEGs were identified, of which approximately two-thirds were up-regulated in the expansion stage (Fig. 2D).
The enriched GO terms of DEGs in the paired groups ET-vs-MT, included carbohydrate catabolic process, NAD(P)H oxidase activity, cellulose synthase activity, cell wall polysaccharide catabolic process, and response to the hormone. In total, the unique gene families during tuber expansion were mainly related to energy functions, cell membrane composition, and cellular metabolic processes (Fig. 3A). The enriched pathways were hormone signal transduction, starch and sucrose metabolism, linoleic acid metabolism, MAPK signaling pathway and sesquiterpenoid and triterpenoid biosynthesis (Fig. 3B), suggesting several transcriptional regulations systems, as well as metabolite balance and environmental adaptation genes, were activated in C. kwangsiensis tuber expansion.
Integrated analysis of the metabolites and genes
A Pearson correlation coefficients (PCC) > 0.8 was selected to analyze DAMs and DEGs by using Log2. The nine quadrant diagrams displayed a systematic view of variations in DAMs and DEGs in response to tuber expansion. As shown in quadrants 3 and 7, 1829 DEGs corresponding to 50 DAMs were positively correlated (Fig. 4A). They had similar consistent patterns, suggesting the change of metabolite accumulation may be regulated by genes in the tuber expansion stage. The enrichment pathways with DEGs and DAMs were displayed simultaneously in response to tuber expansion. It was found that DEGs and DAMs were simultaneously and significantly enriched in the pathways of terpenoid backbone, sesquiterpenoid, triterpenoid, monoterpenoid and phenylpropanoid biosynthesis as well as ABC transporters and plant hormone signal transduction (Fig. 4B).
Integrated analysis of the DEGs and DAMs. (A) Correlation quadrant diagram analysis.
Quadrant 5 shows unchanged DAMs and DEGs, quadrant 3 displays up-regulated DAMs and DEGs, and quadrant 7 exhibits down-regulated DAMs and DEGs. In quadrants 3 and 7, DAMs and DEGs are positively correlated with similar patterns. Quadrant 1 represents up-regulated DAMs and down-regulated DEGs, while quadrant 9 represents down-regulated DAMs and up-regulated DEGs. The DAMs and DEGs in quadrants 1 and 9 are negatively correlated with the opposite patterns. (B) KEGG enrichment analysis of DEGs and DAMs.
DEGs and DAMs related to plant hormone signal transduction
In this study, the plant hormone signal transduction pathway was enriched in the tuber expansion stage. Some DEGs were annotated to signal transduction pathways mediated by IAA, ZR, GA, ABA, ETH, BR, JA and SA, and with the majority exhibiting up-regulation in the tuber expansion stage (Fig. 5). Additionally, genes such as ARF, EIN3 (ethylene-insensitive 3), EBF1/2 (ein3-binding F-box 1 and 2), BIN2 (brassinosteroid insensitive 2), JAZ (jasmonate ZIM-___domain) and NPR1 (non-expressor of pathogenesis-related genes 1), which are involved in the IAA, ETH, BR, JA and SA pathways were highly expressed. This suggests they potential critical role in facilitating the completion of tuber expansion. Meanwhile, hormone-related metabolites are also involved in these pathways, including JA, ABA and SA.
DEGs and DAMs related to phenylpropanoid biosynthesis
In this study, the phenylpropanoid biosynthesis pathway was reconstructed with DEGs and DAMs of the tuber expansion stage (Fig. 6). Here, a total of 51 DEGs were assigned to this pathway. These DEGs included phenylalanine ammonia-lyase (PAL), 4-coumarate: CoA ligase (4CL), cinnamoyl CoA reductase (CCR), cinnamyl alcohol dehydrogenase (CAD), cinnamate 4-hydroxylase (C4H), coffee acyl coenzyme A-3-O-methyl transferase (CCOAOMT), shikimate O-hydroxycinnamoyl transferase (HCT), 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase (C3H). In addition, most of DAMs involved in the phenylpropanoid biosynthesis pathway were up-accumulated at tuber expansion stage, including L-phenylalanine, p-coumaric acid, p-coumaroyl shikimate, p-coumaroylquinic acid, caffeoylquinic acid, caffeic aldehyde, coniferaldehyde, sinapinaldehyde and sinapic acid.
DEGs and DAMs related to terpenoid biosynthesis
25 DEGs were found to be associated with the pathway of terpenoid backbone, sesquiterpenoid and triterpenoid biosynthesis in the tuber expansion stage (Fig. 7). Among them, one phospho-mevalonate kinase (PMK) gene, one DXS gene, one DXR gene, three (E)-4-hydroxy-3-methyl-but-2-enylpyrophosphate synthase (HDS) genes, one (E)-4-hydroxy-3-methylbut-2-enyl-pyrophosphate reductase (HDR) gene and three TPS genes were up-regulated, and two HMGR genes, one PMK gene, one 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase (MDC) gene, one DXS gene, one DXR gene, two HDS genes, two HDR genes and four TPS genes were down-regulated. There were also 16 terpenoid metabolites categorized as monoterpenoids, sesquiterpenoids and triterpene. Among the monoterpenoids and triterpene metabolites, compounds such as syringopocrogenin E, syringopocrogenin D, 2-hydroxyoleanolic acid and phytolaccagenin exhibited reduced levels during the tuber expansion stage. In contrast, sesquiterpenoid metabolites such as germacrone, curzerenone and beta-elemen showed increased levels. These findings suggest that DEGs and DAMs associated with the terpenoid biosynthesis pathway play a role in tuber expansion.
DEGs and DAMs related to ABC transporters
Among 18 ABC genes, ABCB and ABCC genes were related to 10 metabolic pathways according to the co-expressed network analysis (Fig. S3). ABCB and ABCC were linked to amino acids and nucleotides as well as their derivatives for the transport of metabolites. Also, certain secondary metabolites such as flavonoids, lignans and coumarins, tannins, alkaloid, terpenoids, organic acids and lipids showed connections in this network module.
Transcription factors co-expression network analysis
To further explore the regulatory mechanism underlying tuber expansion development in C. kwangsiensis, an analysis of TF interactions with key pathway genes association analysis was conducted to identify the core regulating TFs. Based on the metabolites and transcripts analysis data of DEGs in the three pathways, 24 differentially expressed TF families were screened and the co-expression network of TFs and key genes was constructed (Fig. 8A). In the molecular network diagram, the critical DEGs (HDS, HMGR, ARF7, PP2CA, PAL and COMT) were closely linked to the core TFs (ARF, C2H2, C3H, NAC, bHLH, GRAS and WRKY) and they were found to be embedded in the center of the network by hub genes screening.
Network analysis of metabolomic and transcriptomic data. (A) Network analysis of TFs and screened DEGs. The dashed red lines represent positive correlations, and the solid blue lines represent negative correlations. (B) A putative model of the molecular network associated with tuber expansion. The rectangle represents the genes, and the ellipse represents the metabolites. The solid red lines represent up-regulated, the green solid lines represent down-regulated, and the dashed red-green lines represent up-regulated or down-regulated.
Discussion
The tubers of C. kwangsiensis are widely employed in traditional Chinese medicine to treat various ailments. The active ingredients of the tubers consist of a volatile oil composed of structurally diverse terpenoid compounds2, especially sesquiterpenoid, and the tuber expansion process is an important factor affecting the yield and quality formation of C. kwangsiensis6. Previous studies have focused on the sesquiterpenoid compound and pharmacological analysis of C. kwangsiensis2,29,30. A comparative transcriptome analysis of the rhizome tissues has previously been performed to investigate floral formation C. kwangsiensis under drought stress conditions31. Additionally, transcriptome profiling in C.longa, another species within the same genus, elucidated the involvement of phytohormone signaling and carbohydrate metabolism in the initiation of tuber formation17. However, little is known regarding the tuber development and metabolite accumulation in C. kwangsiensis. In this study, a systematically investigation into the molecular mechanisms underlying tuber expansion and metabolism accumulation was performed by selecting two different developmental stages of tuber formation (namely the initiation and expansion stages) for metabolomic and transcriptomic analysis. Over 999 metabolites and 26,590 genes were obtained from the samples and 197 DAMs and 6962 DEGs were identified. According to KEGG enrichment analysis, the metabolomic pathways related to carbohydrate metabolism, like starch and sucrose metabolism, citrate cycle, and galactose metabolism, were significantly enriched. These findings are similar to those observed in tuber formation of C.longa17, suggesting a potential relationship between tuber expansion and carbohydrate metabolism. Subsequently, an integrated analysis of metabolites and genes revealed a positive correlation between 50 DAMs and 1829 DEGs. According to the KEGG enrichment analysis of the DAMs and DEGs, pathways related to plant hormone signal transduction, phenylpropanoid biosynthesis, terpenoid backbone biosynthesis, sesquiterpenoid and triterpenoid biosynthesis and ABC transporters were involved. Furthermore, notable disparities were found in these DEGs and DAMs between the initiation and expansion stages. Based on our metabolomic and transcriptomic analysis, this study summarized a putative model of the molecular network associated with tuber expansion (Fig. 8B). Consequently, these pathways within the model were used to scrutinize the regulatory network governing tuber expansion.
Hormonal signaling regulation
Hormones play a coordinating role in tuber and root growth and the development of crops and herbs. High levels of ABA can stimulate potato tuberization, while high levels of GA can regulate tuber elongation9. ZR, GAs, IAA and JA have been found to regulate the tuber enlargement of the yam7. High IAA, ZR and GA3 levels have been found at the rapid expansion stage of tuberous roots, suggesting that these compounds might promote tuber enlargement32. The concentration of ZR increased significantly during the adventitious root initiation stage33. The current study showed that a high level of ZR, ABA and SA appeared at the initiation stage of tuber development, while the levels of IAA, JA, GA3, ETH, and BR content increased during the tuber expansion stage (Fig. S1D). Thus, ZR, ABA and SA are beneficial for the tuber initiation, while IAA, JA, GA3, ETH and BR are beneficial for tuber expansion. Moreover, a total of 82 genes were detected in the plant hormone signal transduction pathways, including IAA, ZR, GA, ABA, ETH, BR, JA and SA pathways (Fig. 5). The majority of the ARF genes, along with two GH3 genes, exhibited upregulation at the expansion stage, indicating they involvement in the regulation of the tuber expansion. This is consistent with the observed trend of the root swelling in T. hemsleyanum27. DELLA and PIF3 gene levels were also identified as correlating with the hypocotyl length of Arabidopsis34. DELLAs were also regulated in the GA-mediated rhizome development process35, and high GA levels could reduce the transcription abundance of DELLA genes, suggesting that there was some feedback regulation36. Similar to these studies, GA3 level increased during tuber expansion development, while one DELLA gene and two PIF genes were down-regulated. In yam, the JAR (JA-amino acid synthetase), JAZ and MYC2 family genes were highly expressed at the tuber rapid growth and expansion periods26, and most of the JAR and JAZ genes, along with two MYC2 genes were upregulated at the expansion stage, indicating that these genes are involved in regulating the tuber expansion development. High ABF expression levels were reported to inhibit potato tuber formation and yam tuber development15,26. The expression level of the ABF gene was upregulated in the expansion stage, which also indicated that the ABF gene played a negative role in tuber expansion. The type-B ARABIDOPSIS RESPONSE REGULATORS (B-ARR) gene can target with WUSCHEL (a key gene required for apical meristem maintenance) gene to impact shoot development37. One B-ARR gene was detected in the ZR pathway, which was highly expressed in the initiation stage, suggesting this gene can regulate the initiation stage of tuber development. In summary, ZR, ABA and SA are beneficial for tuber initiation, while IAA, JA, GA3, ETH and BR are beneficial for tuber expansion. ARF, GH3, DELLA, PIF, JAR, JAZ, MYC2, ABF and B-ARR genes may regulate the changes of hormone signaling pathways during tuber expansion of C. kwangsiensis.
Phenylalanine biosynthesis regulation
Lignin, as the primary constituent of the cell wall, plays a vital role in providing essential structural support during plant growth, development, and defense processes. Lignin is produced via the phenylalanine biosynthesis pathway in plant cells, and PAL, C4H, 4CL, HCT, COMT and CAD genes have been reported to be key enzymes for lignin biosynthesis38,39,40,41. In this study, a total of 51 genes were detected in the phenylpropanoid biosynthesis pathway, including the ones listed above (Fig. 6). Previous studies have shown that the expression of the CAD gene decreased in Arabidopsis resulting in a reduction of lignin content40, and silencing of the COMT gene in alfalfa resulted in reduced content of G-lignin42. Similarly, the expression levels of COMT and CAD genes were decreased during tuber expansion development in C. kwangsiensis, resulting in a decrease in lignin during this process. Similar to these studies, the expression of lignin-related genes was reduced at the root swelling stage in sweet potato and T. hemsleyanum27,43. We speculate that lignin is needed to construct fibrous roots in the initiation stage and that the lignin decreases as the carbohydrate and secondary metabolites increase during the expansion stage.
Terpenoid metabolites regulation
In the current study, monoterpenoid, sesquiterpenoid, and triterpenoid metabolites and 25 genes were detected in the terpenoid biosynthesis pathway during the tuber expansion development. The biosynthesis of terpenoids occurs via the MVA and MEP pathways. In this study, two HMGR genes, one DXS gene and one DXR gene were downregulated during the tuber expansion development, while other DXS and DXR genes were upregulated (Fig. 7). These genes have been reported to be key enzymes and function in the biosynthetic of terpenoids20. In addition, TPSs can catalyze the conversion of GPP, FPP and GGPP into monoterpenes, sesquiterpenes and diterpenes, respectively, which can be categorized as monoterpene, sesquiterpene and diterpene synthases44. Moreover, PhTPS1 can increase the content of sesquiterpenes and regulate seed formation45. In this study, the expression of TPS genes was also correlated with terpenoid content, indicating that they play a key role in terpenoid biosynthesis. Many studies have proposed that four sesquiterpenoids (germacrone, curzerenone, beta-elemen and curcumenol) were active components in the volatile oils of C. kwangsiensis tuber2,3. Sesquiterpene volatiles can affect reproductive organ development and seed yield in Petunia hybrida45. Germacrone, curzerenone, beta-elemen and curcumenol metabolites were abundantly accumulated during the tuber expansion development, suggesting these sesquiterpene metabolites can affect the tuber expansion of C. kwangsiensis.
ABC transporters regulation
The ABC transporters participate in various biological processes and play essential roles in the transmembrane transport of secondary metabolites46. In this study, 18 ABC genes were shown to be involved in 10 metabolic pathways (Fig. S3), and these genes were abundant in expression during tuber expansion development. ABC transporter genes have been reported to be involved in terpenoid metabolites. Some ABC families in Salvia miltiorrhiza, such as ABCB1, ABCB9, ABCC1, ABCC10, ABCD3 and ABCG2 genes were identified as regulating the transport and accumulation of tanshinone47. Seven ABC transporter-encoding transcripts were abundantly expressed in different tissues, and involved in long distance and transport of picrosides in Picrorhiza kurroa organs48. In this study, four ABCB (Isoform0000383, Isoform0000823, Isoform0001225 and Isoform0000924) and seven ABCC (Isoform0001855, Isoform0002772, Isoform0000254, Isoform0000318, Isoform0005480, Isoform0007637 and Isoform0009011) genes were highly co-expressed with four terpenoids (maslinic acid, mws1610, alphitolic acid, Lmzn106284, curcumenol, MWSmce591 and germacrone, MWSmce564). Furthermore, the AtABCG14 was involved in cytokinin biosynthesis and transported cytokinin from the roots to the shoots49. In the present study, one ABCB gene (Isoform0001239) and two ABCC genes (Isoform0000125, Isoform000062) were highly co-expressed with JA (pme1654). These results show that ABCB and ABCC genes are involved in terpenoids (maslinic acid, maslinic acid, curcumenol, germacrone) and JA hormone transport during the tuber expansion development of C. kwangsiensis.
Transcription factors regulation
In the current study, the hormone signal transduction, phenylpropanoid and terpenoid biosynthesis pathways of DEGs and DAMs were used to accurately determine the critical point during the transition from expansion in tubers, such that, performing multi-omics integrative analysis, these pathway hub genes identified the TFs from the constructed co-expression network. These hub genes, including HMGR, HDS, PP2CA, ARF7, PAL and COMT genes, were found to be associated with several TF families (ARF, C3H, C2H2, NAC, AP2, bHLH, GRAS, WRKY, MYB and MYB_related) in the co-expression network (Fig. 8A). HMGR5, HDS1 and FPPS3 genes were regulated by bHLH20, bHLH9, ERF21, NAC4, bHLH5, WRKY7 and WRKY8, which were previously identified in Agriophyllum squarrosum23. The NAC, HB, MYB, and WRKY, are lignin synthesis-associated TFs, and these possibly combine with C3H2, CCoAOMT, COMT and other genes involved in phenylpropanoid and lignin biosynthetic processes50,51. The 14 TF-encoding DEGs, such as WRKY, bHLH, GRAS, NAC and ARF, responded to hormone signal regulation and were found to regulate the accumulation of terpenoids in celery24. ARF, C3H, C2H2, NAC, AP2, bHLH, GRAS, WRKY, MYB and MYB_related family genes were suggested to respond to the hub genes of hormone signal transduction, phenylpropanoid and terpenoid biosynthesis pathways based on the co-expression network. Therefore, it can be inferred that these DEGs are involved in tuber expansion development in C. kwangsiensis.
Conclusion
The mechanism of tuber expansion development was studied by metabolomic and transcriptomic analysis of C. kwangsiensis. The hormone levels played coordinating roles in the tuber expansion development. Related candidate genes were detected, including ARF, GH3, DELLA, PIF, BRI1 and PP2CA. The phenylalanine biosynthesis pathway is an important way to produce lignin, and PAL and CCOM genes were significantly expressed. The HMGR and HDS genes, and sesquiterpene metabolites (gerermacrone, curzerenone, beta-elemen and curcumenol) accumulated during the tuber expansion development. Some ABC transporter genes and TFs were predominantly involved in tuber expansion development. These findings will provide a foundation-based mechanism for tuber expansion in C. kwangsiensis and will aid the cultivation of this type of medicinal plant.
Methods
Plant material
The tubers of C. kwangsiensis plants were planted in an experimental field (Guangxi Botanical Garden of Medicinal Plants) in Nanning City, Guangxi Province China (Fig. S1A, B). The experimental materials were gathered at 60 (tuber initiation stage) and 210 days (tuber expansion stage) after planting. The experimental material of the ten plants was mixed as a biological replicate and immediately quickly frozen in liquid nitrogen.
Determination of endogenous hormone
The hormone of tubers was extracted with 75% methanol containing 5% formic acid. The homogenates were then centrifuged for 10 min at 6000 rpm at 4℃ and the supernatants were collected. After concentrating at 35℃, the resulting dry residue was re-dissolved in 0.1 mL of 80% methanol. The levels of indole-3-acetic acid (IAA), jasmonate (JA), gibberellin (GA3), ethylene (ETH), salicylic acid (SA), brassinosteroid (BR), zeatin riboside (ZR) and abscisic acid (ABA) in tubers were determined with an ultra-performance liquid chromatography-tandem mass apectrometry (UHPLC-MS) system as previously described7.
Metabolomic profiling
Metabolomic analysis was conducted on tuber samples collected at two distinct developmental stages, and with each stage the determinations were replicated three times for robustness. Samples underwent initial preparation by immersion in 1.0 mL of 70% aqueous methanol at 4℃ overnight. Subsequently, extracts were processed through solid-phase extraction cartridges and filtered using a 0.22 μm pore size microporous membrane before LC-MS analysis. Utilizing a UPLC-MS system, chromatographic separation was achieved by employing a Waters C18 column with mobile phases comprising 0.04% acetic acid in water (Phase A) and 0.05% acetic acid in acetonitrile (Phase B) at 40 °C. The solvent gradient transitioned linearly over 15 min, ranging from 95:5 Phase A/Phase B to 5:95 Phase A/Phase B and returning to the initial conditions. The flow rate was maintained at 0.4 mL/min, with a 2 µL injection volume. High-resolution mass spectra were acquired in the positive ion mode using electrospray ionization. Data processing encompassed filtering, peak detection, alignment and calculations facilitated by Analyst 1.6.1 software. Metabolite identification involved accessing internal and public databases such as MassBank, KNApSAcK, HMDB, MoTo DB, and METLIN. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were employed to discern the metabolites with significantly distinct levels (p-value < 0.05). The differentially altered metabolites (DAMs) were identified based on a log2 fold change (FC) ≥ 2 or p-value ≤ 0.5 and variable importance in projection (VIP) scores > 1. Finally, metabolites were subjected to pathway analysis utilizing KEGG52 and MetaboAnalyst 4.0 software for comprehensive elucidation.
RNA extraction, isoform sequencing and Illuminea sequencing
The total RNA from two different developmental stages of tuber formation was extracted using the Trizol™ reagent kit (Invitrogen, Carlsbad, CA, USA) by following the manufacturer’s instructions. The purity, concentration and integrity of the RNA samples were assessed by using a NanoDrop micro-spectrophotometer (Thermo Scientific) and Agilent 2100 Bioanalyzer. To obtain an accurate reference for the genes in the C. kwangsiensis plant, full-length transcriptome sequencing was performed. Total RNA from two tissue samples was uniformly combined to create an Iso-Seq library by following the Clontech SMARTer PCR cDNA Synthesis Kit protocol and then sequenced on the PacBio SequelII platform (Gene Denovo Biotechnology Co. Ltd., Guangzhou, China). Primary Iso-Seq data were processed using SMRTlink v5.0.1 software to generate read sequences, and circular consensus sequences were derived following error correction. Sequences were then classified into non-full-length and full-length sequences based on 5’primers, 3’primers and polyA structures. The clustering of full-length sequences facilitated the extraction of cluster consensus sequences, which were refined to obtain full-length consensus sequences for subsequent analysis.
The sequence annotation of gene function was performed with NR (NCBI non-redundant protein database, https://www.ncbi.nlm.nih.gov/), Pfam (http://pfam.xfam.org/), NT (NCBI nonredundant nucleotide sequences), KO (Kyoto Encyclopedia for Genes and Genomes (KEGG) database, https://www.genome.jp/kegg)52, GO (Gene Ontology), Swiss-Prot (http://www.expasy.ch/sprot), and KOG/COG (http://www.ncbi.nlm.nih.gov/COG/) to obtain a refined gene reference sequence (subsequently named EZ).
High throughput transcriptomic analysis
Individual tuber stage total RNA samples were utilized to construct Illumina sequencing cDNA libraries by using the NEBNext® UltraTM RNA Library Prep Kit for Illumina, with subsequent RNA-seq conducted on the Novaseq 6000 platform in paired-end mode platform (Illumina, Gene Denovo Biotechnology Co. Ltd., Guangzhou, China). A total of six sets of transcriptome raw data were obtained. FASTP V0.18.0 was applied to the data for quality control. Bowtie2 V2.2.8 was used for read assembly, and the resulting clean reads were used to calculate gene abundance. The clean reads were mapped to the reference transcriptome sequence (named EZ) by TOPHAT V2.0.9. Gene expression levels were quantified using fragments per kilobase of transcript per million mapped reads (FPKM). The differentially expressed genes (DEGs) were identified with the NOISeq method, with criteria set at |log2 (fold change) | > 2 and a statistically significant p-value < 0.05. GO enrichment and KEGG pathway enrichment analysis were conducted, with significance determined at a corrected p-value threshold ≤ 0.05, considering the enrichment factor.
Correlation network analysis of the transcriptomic and metabolomic data
To elucidate the data relationships, normalization and statistical analyses were applied to both transcriptome and metabolome datasets. Functional analysis, metabolic pathway enrichment, and correlation analysis were subsequently employed to identify pivotal genes, metabolites, and pathways. Pearson correlation analysis between the DEGs and DAMs utilized the normalized data in the R language. Correlation and KEGG enrichment analyses were based on Pearson Correlation Coefficient (PCC) values with |PCC| ≥ 0.8 for both DEGs and DAMs. Additionally, a network diagram depicting the interplay between genes and metabolites was constructed using CYTOSCAPE V.3.7.2.
Quantitative real-time PCR (qRT-PCR) analysis
To validate the results of RNA-seq, 14 DEGs were selected for the qRT-PCR analysis, while the actin gene was used as an internal reference. The sequence primers were designed by Primer 5.0 (Table S4). The total RNA of the samples was extracted and reverse-transcribed into cDNA with three technical replicates for each biological replicate. qRT-PCR was performed using the LightCycler 96 instrument (Roche, Basel, Switzerland), with reaction conditions of 95 ℃ for 5 s, 60 ℃ for 10 s, and 72 ℃ for 15 s for 40 cycles. According to a previous study53, the 2–ΔΔCt method was employed to calculate the relative expression levels of the mRNAs. ACTIN was used as the reference gene (Table S4), the initiation stage was used as the control sample. When the transcriptomic and qRT-PCR data were combined, the candidate genes showed similar patterns of expression (Table S4).
Data availability
The datasets for this study can be found in the National Center of Biotechnology Information with the BioProject accession code PRJNA1089530 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1089530/). This published article and its supplementary information files include all data generated or analyzed in this work.
References
Pharmacopeia, C.o.N. Pharmacopoeia of People’s Republic of China (ed.Pharmacopeia, C.o.N.) 217 (China Medical Science and Technology, 2020).
Liao, H. B., Feng, W. Y., Wang, H. S. & Liang, D. Sesquiterpenoid compounds from Curcuma kwangsiensis. Chem. Biodivers. 16(5), e1900123 (2019).
Qin, B., Xie, J. X., Yang, H. L. & Xiong, P. Study on the content of curcumin by HPLC and the anti-tumor effect in vivo from different processed products of Curuma kwangsiensis. Zhongyaocai 33, 1379–1382 (2010).
Liu, J., Zhang, L., Zhang, J., Li, B. & Zhang, M. Curcuma kwangsiensis extracts produced antioxidant effects against injury induced by H2O2 on PC12 cells. Rec Nat. Prod. 13, 254–267 (2019).
Zeng, J. et al. Apoptosis-induced anti-tumor effect of Curcuma kwangsiensis polysaccharides against human nasopharyngeal carcinoma cells. Carbohydr. Polym. 89, 1067–1072 (2012).
Zhao, Y. et al. Traits, volatile oil content and composition of different strains of Curcuma kwangsiensis. Cent. South. Pharm. 17, 216–219 (2019).
Gong, M. et al. Phytohormone profiling during tuber development of Chinese yam by ultra-high performance liquid chromatography–triple quadrupole tandem mass spectrometry. J. Plant. Growth Regul. 36, 362–373 (2016).
Aksenova, N. P., Konstantinova, T. N., Golyanovskaya, S. A., Sergeeva, L. I. & Romanov, G. A. Hormonal regulation of tuber formation in potato plants. Russ J. Plant. Physiol. 59, 451–466 (2012).
Xu, X., van Lammeren, A. A., Vermeer, E. & Vreugdenhil, D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant. Physiol. 117, 575–584 (1998).
Zierer, W., Rüscher, D., Sonnewald, U. & Sonnewald, S. Tuber and tuberous root development. Annu. Rev. Plant. Biol. 72, 551–580 (2021).
Chen, S. C., Shiwachi, H. & Sanada, A. Theobroxide and day-length effects on the growth of yam (Dioscorea spp). J. Int. Soc. Southeast. Asian Agricultural Sci. 16, 22–30 (2010).
Shiwachi, H., Ayankanmi, T. & Robert, A. Effect of day length on the development of tubers in yams (Dioscorea Spp.). Trop Sci. 42, 162–170 (2002).
Rodríguez-Falcón, M., Bou, J. & Prat, S. Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu. Rev. Plant. Biol. 57, 151–180 (2006).
Kloosterman, B. et al. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant. J. 52, 362–373 (2007).
Muñiz García, M. N., Stritzler, M. & Capiati, D. A. Heterologous expression of Arabidopsis ABF4 gene in potato enhances tuberization through ABA-GA crosstalk regulation. Planta 239, 615–631 (2014).
Zhou, Y. et al. Integrated mRNA and miRNA transcriptome analysis reveals a regulatory network for tuber expansion in Chinese yam (Dioscorea opposita). BMC Genom. 21, 117 (2020).
Yin, Y. et al. A chromosome-scale genome assembly of turmeric provides insights into curcumin biosynthesis and tuber formation mechanism. Front. Plant. Sci. 13, 1003835 (2022).
Jiang, C. et al. Tissue-specific transcriptome and metabolome analyses reveal a gene module regulating the terpenoid biosynthesis in Curcuma wenyujin. Ind. Crops Prod. 170,113758 (2021).
Yuan, H. L. et al. Anti-inflammatory and antinociceptive effects of Curcuma kwangsiensis and its bioactive terpenoids in vivo and in vitro. J. Ethnopharmacol. 259,112935 (2020).
Huang, Y., Xie, F. J., Cao, X. & Li, M . Research progress in biosynthesis and regulation of plant terpenoids. Biotechnol. Biotechnol. Equip. 35, 1800–1809 (2021).
Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 148, 63–106 (2015).
Gong, D. Y., Chen, X. Y., Guo, S. X., Wang, B. C. & Li, B. Recent advances and new insights in biosynthesis of dendrobine and sesquiterpenes. Appl. Microbiol. Biotechnol. 105, 6597–6606 (2021).
Yin, X. et al. Comparative transcriptome analysis to identify genes involved in terpenoid biosynthesis in Agriophyllum squarrosum, a folk medicinal herb native to Asian temperature deserts. Plant. Biotechnol. Rep. 15, 369–387 (2021).
Yan, J. et al. Integrated metabolome and transcriptome analysis reveals candidate genes involved in metabolism of terpenoids and phthalides in celery seeds. Ind. Crops Prod. 172,114011 (2021).
Pal, T., Malhotra, N., Chanumolu, S. K. & Chauhan, R. S. Next-generation sequencing (NGS) transcriptomes reveal association of multiple genes and pathways contributing to secondary metabolites accumulation in tuberous roots of Aconitum heterophyllum Wall. Planta 242, 239–258 (2015).
Cao, T. et al. Transcriptome and metabolome analysis reveals the potential mechanism of tuber dynamic development in yam (Dioscorea polystachya Turcz). Lwt 181,114764 (2023).
Hang, S. et al. Integrative analysis of the transcriptome and metabolome reveals the developmental mechanisms and metabolite biosynthesis of the tuberous roots of Tetrastigma hemsleyanum. Molecules 28(6), 2603 (2023).
Li, X. J. et al. Comparative transcriptome and metabolome analyses provide new insights into the molecular mechanisms underlying taproot thickening in Panax notoginseng. BMC Plant. Biol. 19(1), 451 (2019).
Dai, W., Zhang, L., Liu, Y. & Zhang, M. A. New 4,5-secofurancadinene from the rhizome of Curcuma kwangsiensis. Rec Nat. Prod. 14, 297–300 (2020).
Xiang, F. F., He, J. W., Liu, Z. X., Peng, Q. Z. & Wei, H. Two new guaiane-type sesquiterpenes from Curcuma kwangsiensis and their inhibitory activity of nitric oxide production in lipopolysaccharide-stimulated macrophages. Nat. Prod. Res. 32, 2670–2675 (2018).
Feng, X., Zhou, L., Sheng, A., Lin, L. & Liu, H. Comparative transcriptome analysis on drought stress-induced floral formation of Curcuma kwangsiensis. Plant. Signal. Behav. 17(1), 2114642 (2022).
Yao, S. et al. Hormonal and transcriptional analyses provides new insights into the molecular mechanisms underlying root thickening and isoflavonoid biosynthesis in Callerya speciosa (Champ. Ex Benth.) Schot. Sci. Rep. 11, 9 (2021).
Wen, S. et al. Physiology and transcriptomic analysis of endogenous hormones regulating in vitro adventitious root formation in tree peony. Sci. Hortic. 318, 112122 (2023).
Li, K. et al. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 7, 11868 (2016).
Yang, M. et al. Transcriptomic analysis of the regulation of rhizome formation in temperate and tropical lotus (Nelumbo nucifera). Sci. Rep. 5, 13059 (2015).
Ito, T., Okada, K., Fukazawa, J. & Takahashi, Y. DELLA-dependent and -independent gibberellin signaling. Plant. Signal. Behav. 13, e1445933 (2018).
Xie, M. et al. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat. Commun. 9, 1604 (2018).
Liu, Q., Luo, L. & Zheng, L. Lignins: biosynthesis and biological functions in plants. Int. J. Mol. Sci. 19(2), 335 (2018).
Liu, H. et al. 4-Coumarate-CoA ligase-like gene OsAAE3 negatively mediates the rice blast resistance, floret development and lignin biosynthesis. Front. Plant. Sci. 7, 2041 (2016).
Thévenin, J. et al. The simultaneous repression of CCR and CAD, two enzymes of the lignin biosynthetic pathway, results in serility and dwarfism in Arabidopsis thaliana. Mol. Plant. 4, 70–82 (2011).
Schilmiller, A. L. et al. Mutations in the cinnamate 4-hydroxylase gene impact metabolism, growth and development in Arabidopsis. Plant. J. 60, 771–782 (2009).
Guo, D., Chen, F., Inoue, K., Blount, J. W. & Dixon, R. A. Downregulation of caffeic acid 3-O-methyltransferase and caffeoyl CoA 3-O-methyltransferase in transgenic alfalfa. impacts on lignin structure and implications for the biosynthesis of G and S lignin. Plant. Cell. 13, 73–88 (2001).
Lee, J. J. et al. A comparative study of proteomic differences between pencil and storage roots of sweetpotato (Ipomoea batatas (L.) Lam). Plant. Physiol. Biochem. 87, 92–101 (2015).
Zhou, F. & Pichersky, E. More is better: the diversity of terpene metabolism in plants. Curr. Opin. Plant. Biol. 55, 1–10 (2020).
Boachon, B. et al. Natural fumigation as a mechanism for volatile transport between flower organs. Nat. Chem. Biol. 15, 583–588 (2019).
Higgins, C. F. ABC transporters: physiology, structure and mechanism – an overview. Res. Microbiol. 152, 205–210 (2001).
Lin, C. et al. Spatiotemporal and transcriptional characterization on tanshinone initial synthesis in Salvia miltiorrhiza roots. Int. J. Mol. Sci. 23(21), 13607 (2022).
Pandey, R., Sharma, A., Sood, H. & Chauhan, R. S. ABC transporters mined through comparative transcriptomics associate with organ-specific accumulation of picrosides in a medicinal herb, Picrorhiza kurroa. Protoplasma 260, 453–466 (2023).
Poitout, A. A. O. et al. Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. Plant. Cell. 30, 1243–1257 (2018).
Guo, H. Y. et al. Metabolome and transcriptome analysis of eleutheroside B biosynthesis pathway in Eleutherococcus senticosus. Heliyon 8, e09665 (2022).
Ohtani, M. & Demura, T. The quest for transcriptional hubs of lignin biosynthesis: beyond the NAC-MYB-gene regulatory network model. Curr. Opin. Biotechnol. 56, 82–87 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2022).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25, 402–408 (2001).
Acknowledgements
We are grateful to Dr. Dev Sooranna, Imperial College London and YMUN, for English language editing of the manuscript.
Funding
This research was funded by the National Science Foundation of Guangxi (2022GXNSFBA035631, 2024GXNSFAA010196), the National Natural Science Foundation of China (32260071), the Scientific Research funding project of Guangxi Botanical Garden of Medicinal Plants (GYJ202104), the Guangxi Youth Qihuang scholars training program (GXQH202411), the Guangxi Appropriate Technology Development and Promotion project (GZSY-23-06) and the Key Subjects Construction project of Chinese Medicinal Resources Science (GZXK-Z-20-65).
Author information
Authors and Affiliations
Contributions
Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Y. Z. and C.P.; Data Curation, Methodology, Visualization, Investigation, L.Y.(Lixiang Yao); Methodology, Visualization, Investigation, Y.X.; Visualization, Investigation, B.H., Y.L. and X.H.; Supervision, Resource mobilization, Validation, L.Y.(Liying Yu); All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Yao, L., Xie, Y. et al. Metabolic and transcriptional analysis of tuber expansion in Curcuma kwangsiensis. Sci Rep 15, 1588 (2025). https://doi.org/10.1038/s41598-024-84763-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84763-9