Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. Toll-like receptor 4 (TLR4) is a pattern-recognition transmembrane receptor that induces neuroinflammatory processes in response to injury. Tlr4 is highly expressed in ocular tissues and is known to modulate inflammatory processes in both anterior and posterior segment tissues. TLR4 activation can lead to mitochondrial dysfunction and metabolic deficits in inflammatory disorders. Due to its effects on inflammation and metabolism, TLR4 is a candidate to participate in glaucoma pathogenesis. It has been suggested as a therapeutic target based on studies using acute models, such as experimentally raising IOP to ischemia-inducing levels. Nevertheless, its role in chronic glaucoma needs further evaluation. In the current study, we investigated the role of TLR4 in an inherited mouse model of chronic glaucoma, DBA/2J. To do this, we analyzed the effect of Tlr4 knockout (Tlr4−/−) on glaucoma in DBA/2J mice. Our studies found no significant differences in intraocular pressure, iris disease, or glaucomatous progression in Tlr4−/− compared to Tlr4+/+ DBA/2J mice. Our data do not support a role for TLR4 as a treatment target in chronic glaucoma.
Similar content being viewed by others
Introduction
Glaucoma refers to a complex group of multifactorial diseases characterized by the progressive degeneration of retinal ganglion cells (RGCs) and visual field deficits1,2. Elevated intraocular pressure (IOP), genetics, and advanced age are considered the most important risk factors for glaucoma2,3. Both neuroinflammation and perturbed metabolism are implicated in the disease. Nevertheless, the mechanisms that lead to neural demise and degeneration in glaucoma are yet to be fully elucidated.
Neuroinflammation is involved in glaucomatous progression1,2,4,5,6,7,8. We have previously investigated the processes implicated in the initiation of glaucomatous damage and identified monocyte cell entry into the optic nerve head as an important pathogenic event in DBA/2J glaucoma4,6,9,10. Subsequently, macrophages were found to infiltrate axon bundles in the optic nerves of glaucoma patients11. Additionally, we and others have shown that mitochondrial dysfunction and impaired metabolism contribute to the development and progression of glaucoma8,12,13,14,15,16,17,18,19,20,21,22,23. In a vicious cycle, pro-inflammatory mediators (released by e.g., infiltrating macrophages) can trigger mitochondrial damage and amplify the neurodegeneration driven by mitochondrial dysfunction17,24.
Toll-like receptors (TLR) are a family of pattern-recognition transmembrane receptors and a centerpiece of the innate immune response25. They can recognize external pathogen-associated molecular patterns (PAMPs) like bacterial lipopolysaccharide (LPS), as well as endogenous damage-associated molecular patterns (DAMPs)26. They play a significant role in inflammatory conditions and neurodegenerative diseases27. TLR4, in particular, is highly expressed in cells of the central nervous system and the retina, and its activation triggers multiple signaling cascades that lead to the release of inflammatory cytokines and immune modulators28,29. TLR4 has been implicated in the regulation of glucolipid metabolism30and in mitochondrial dysfunction after LPS-induced TLR4 activation31. Additionally, TLR4 activation can induce metabolic changes in macrophages via mitochondrial reprogramming32.
TLR4 has been studied as a therapeutic target in preclinical models of eye disorders33,34,35. Its activation has been linked to ocular inflammation, retinal damage following acute experimental elevation of IOP to an ischemic level, and retinal/ischemia-reperfusion injury36,37,38. In murine optic nerve crush, an acute model with relevance to glaucoma due to direct axon injury, blocking TLR4 suppressed inflammatory reactions and RGC loss34,39. In human tissues, increased expression of TLR4 was reported in glaucomatous human trabecular meshwork (TM), a tissue relevant to IOP elevation, and in glaucomatous human optic nerve head40,41,42. Finally, polymorphisms in TLR4 are suggested to be involved in normal tension glaucoma and primary open-angle glaucoma (POAG), but further data are needed43,44,45,46.
Given the important effects of TLR4 on both metabolism and neuroinflammation, we functionally tested the role of TLR4 in a chronic glaucoma by comparing Tlr4-deficient (Tlr4−/−) and wild-type (Tlr4+/+) littermate mice on a DBA/2J background. DBA/2J mice are a model of hereditary glaucoma and are widely used for glaucoma research. Mutations in two genes impacting melanosomes cause pigment-dispersing iris disease, IOP elevation, and glaucoma in DBA/2J mice47. Variants in PMEL, a gene with a role in melanosome biology, cause pigmentary glaucoma in humans48. Thus, the initiating melanosomal etiology of DBA/2J glaucoma is similar to at least a subset of human pigmentary glaucoma. Importantly, findings in DBA/2J have been extended to POAG, the most common form of glaucoma, with initial promising outcomes in clinical trials12,15,49,50. In our experiments, we studied whether TLR4 plays a role in the development of DBA/2J glaucoma. We observed no significant differences in IOP, nerve damage, and other glaucoma-associated phenotypes between Tlr4+/+ and Tlr4−/− DBA/2J mice. Our data indicate that TLR4 is not necessary for disease progression in this chronic glaucoma model.
Results
Tlr4 knockout has no effect on pigment-dispersing iris disease or IOP
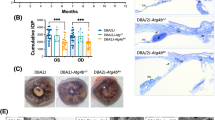
DBA/2J mice develop a pigment-dispersing iris disease that results in pigment accumulation in the ocular drainage tissues and subsequent IOP elevation. There were no differences in the onset or progression of the iris disease between mice of each Tlr4 genotype (Fig. 1A). At all ages (analyzed between 4 and 12 months old), both groups followed the usual anterior segment disease progression that we and others have previously characterized for DBA/2J mice51,52,53,54,55. DBA/2J mice typically have clear disease by 5 months of age, consisting of transillumination defects that involve progressive depigmentation and iris atrophy51,52,53. At 8 months of age, both Tlr4+/+ and Tlr4−/−mice presented peripupillary accumulation of pigment, dispersed pigment, and transillumination defects. The depigmenting iris disease worsened by 12 months of age, with severe transillumination defects and iris atrophy in both groups. In the DBA/2 J model, the IOP is increased in many mice by 8–9 months of age and starts declining after 12–13 months due to atrophy of the ciliary body52,53. We did not detect any differences in IOP between Tlr4+/+ and Tlr4−/− mice (p-value = 0.702, two-way ANOVA. Figure 1B).
Tlr4 deficiency does not influence the iris disease or the IOP phenotype of DBA/2J mice. (A) Representative slit-lamp images of Tlr4+/+ (wild-type DBA/2J) and Tlr4−/− eyes at 4, 8, and 12 months. The top row shows broad beam illumination, and the bottom row shows transillumination. The glaucoma-related changes in Tlr4−/− mice developed at the same time as in Tlr4+/+ mice. At 4 months of age, iris disease is not yet evident in either of the groups. Eight-month-old Tlr4+/+ and Tlr4−/− eyes present dispersed pigment and transillumination defects, which become more severe at 12 months of age. N > 20 mice per genotype at each age. (B) IOP distributions at key ages. There was no significant effect of the genotype on IOP levels at any age (two-way ANOVA; P = 0.702).
Tlr4 knockout has no effect on RGC numbers or axonal degeneration
To study the role of TLR4 in glaucomatous degeneration, we assessed the effects of Tlr4 deficiency on optic nerve damage using PPD-stained nerve cross-sections. We evaluated nerves at 10.5 and 12 months of age, two key glaucoma points frequently used for assessing glaucomatous neurodegeneration in this model4,56 (Fig. 2A-B). There were no significant differences in nerve damage between genotypes at the two ages analyzed. Because the Tlr4 mutant mice initially had a slightly higher incidence of severe damage at each age, we analyzed an even larger number of 12-month-old eyes. Despite the larger number of nerves, the difference remained statistically insignificant (Fisher’s test comparing Tlr4+/+ and Tlr4−/− mice at 10.5 months of age, P = 0.749; P = 0.169 at 12 months of age). At 10.5 months of age, 27% of the Tlr4+/+ nerves had severe nerve damage (> 50% axonal loss), and this proportion increased to 39% at 12 months of age. For the Tlr4−/− mice, 33.9% of the nerves had severe damage at 10.5 months of age, which increased to 48.8% by 12 months.
To evaluate potential axonal-somal uncoupling, where RGC somal survival is observed despite axon loss57,58,59, we analyzed RGC layer cell numbers in Nissl-stained flat-mounted retinas. To assess uncoupling, we counted and compared retinas of Tlr4+/+ and Tlr4−/− eyes that had severe or no glaucomatous damage (Fig. 2C-D). As expected, retinal cell counts in Tlr4+/+ and Tlr4−/− mice with severe nerve damage were considerably reduced with no effect of the genotype on this loss (P = 0.384 when comparing Tlr4+/+ and Tlr4−/− retinas with severe damage, one-way ANOVA and post hoc Tukey’s HSD test). Thus, knocking out Tlr4 does not prevent RGC death or glaucomatous nerve damage.
Lack of Tlr4 does not affect glaucomatous neurodegeneration. (A) Representative images of nerves with no glaucoma (NOE, no/early, see Methods), moderate (MOD), and severe (SEV) damage. Scale bar = 10 μm. (B) Frequency distribution of optic nerve damage at 10.5 and 12 months of age. No significant differences were observed between the two genotypes of mice at any age (Fisher’s test at 10.5 months, P = 0.749; P = 0.169 at 12 months of age). (C) RGC layer cell counts for eyes with no glaucoma (NOE, both genotypes combined) or severe (SEV, split by genotype) glaucoma based on optic nerve damage. No significant differences in RGC layer cell numbers were observed between eyes of either genotype with severe glaucoma, indicating that somal and axonal damage are not uncoupled by the Tlr4 mutation (one-way ANOVA and post hoc Tukey’s HSD test, Tlr4+/+ SEV vs. Tlr4−/− SEV; P = 0.384). (D) Representative images of the retinas of Tlr4+/+ and Tlr4−/− mice with no or severe nerve damage at 40X. Scale bar = 50 μm.
Discussion
TLR4 targeting strategies have been studied in multiple models of diseases characterized by an inflammatory microenvironment29,60. TLR4 is expressed by a variety of ocular tissues and plays an important role in inflammatory eye diseases61,62,63. A study in mice showed that TLR4 contributes to retinal ischemia/reperfusion injury via NF-kB signaling, increasing the expression of proinflammatory genes37. Knockout of Tlr4 led to a suppressed inflammatory response in the retina. Another study analyzed TLR4 downstream mechanisms and showed that TLR4 signaling mediated by caspase-8 controlled the production of the inflammatory cytokine interleukin 1β (IL-1β)36. Inhibition of either TLR4 or caspase-8 blocked the production of IL-1β and attenuated retinal ischemic damage after acute experimental IOP elevation. Tlr4−/−mice have also been shown to be more resistant to optic nerve crush injury than wild-type mice34.
Growing evidence suggests that mitochondrial and metabolic disturbances with bioenergetic insufficiency contribute to the demise of RGCs in glaucoma15,64,65,66,67, while an extensive literature implicates neuroinflammatory processes1,2,4,5. TLR4 plays a role in multiple metabolic processes, affects mitochondrial function in inflammatory diseases, and has been proposed to have a role in acute glaucoma based on an induced, acute model with very high IOP elevation (ischemia-inducing)36, but a role in chronic glaucoma is not assessed. Thus, we determined its role in the chronic DBA/2J glaucoma model. Our experiments did not detect any differences in the glaucoma-related phenotype between Tlr4−/− and Tlr4+/+ DBA/2J mice. Both genotypes developed comparable levels of IOP elevation, RGC loss, and optic nerve damage. Our data do not provide evidence for a role of TLR4 in this chronic glaucoma.
The difference between our results and those in more acute models is not surprising as different types and degrees of insult can induce differing damaging mechanisms. For example, in acute glaucoma, there is an overwhelming inflammatory response compared to chronic glaucoma, with slower, more cumulative damage mediated by different pathways (e.g., metabolic failure, oxidative stress, mitochondrial dysfunction, inflammation)12,15,36,64. The above-mentioned study in the acute model used an IOP of 110 mmHg36, and such a degree of IOP elevation does not occur in chronic glaucoma or even in most human patients with acute glaucoma. Additionally, optic nerve crush is meant to model direct axon injury in glaucoma, but there are differences to IOP-induced glaucoma, including transcriptomic differences68,69. Another study reported that Tlr4 mutation protects against TGFβ2- or fibronectin-EDA induced IOP elevation70. This finding differs from ours, as TLR4 does not affect IOP in DBA/2J mice. This may reflect a difference between mechanism of IOP elevation following pigmentary insults versus and other forms of IOP elevation.
Our studies indicate that the lack of Tlr4 does not influence glaucoma progression in DBA/2J glaucoma. Multiple factors can influence disease progression, such as genetic background and environment71,72,73, and various factors may influence the effects of TLR4 on glaucoma. TLR4 mediates inflammation in response to infection and endogenous molecules such as saturated fatty acids74,75. Thus, the presence of specific infectious or commensal microbes or the nature of diet, including saturated fat content, could possibly impact its role in glaucoma. The immune system often has redundant pathways to ensure robustness. Other TLRs (e.g., TLR2) may compensate for the lack of TLR476,77, sustaining the inflammatory response and glaucomatous progression. Additionally, non-TLR pathways, such as the tumor necrosis factor (TNF) signaling pathway, may still drive neuroinflammation and RGC damage independently of TLR478,79. The specific mutation may also play a role73, with some mutations in the human population possibly being activating. Nevertheless, our studies in a widely used model of chronic glaucoma do not support targeting of TLR4 as a treatment for chronic glaucoma.
Although pathologic neuroinflammation and TLR receptor signaling are implicated in a wide variety of neural diseases and inflammaging80, our results are not completely surprising. We and others have implicated dysregulated metabolism in glaucoma12,15,18,20,66,67, while there is growing evidence for reciprocal immuno-metabolic signaling interactions81,82. In addition to regulating inflammatory cascades, TLR4 is reported to influence the autonomic control of body temperature, heart rate, and metabolism83. Stimulation of TLR4 receptors leads to key phenotypic shifts in astrocytes and microglia, while it reprograms metabolism in both immune cells and glia. This reprogramming results in a shift towards glycolysis84,85. Inhibiting this shift has been suggested as a neuroprotective strategy86. On the other hand, TLR4 activation also induces AKT, a protein kinase with important roles in modulating both protein synthesis and promoting cell survival87,88. This protection involves the kinase TBK1 (in at least dendritic cells), a gene associated with glaucoma89. Importantly, TLR9 also has a role in energy metabolism and cell protection as demonstrated in neurons90, raising the possibility that TLR4 or other related receptors may also promote neuronal protection in specific stress or disease contexts. As further evidence of TLR-mediated protection: (1) deficiency of the TRF1 (important adaptor protein for TLR receptor function) exacerbates amyotrophic lateral sclerosis in mice (proposed astrocyte-related mechanism91; (2) while deficiency of MYD88 (another critical adaptor protein) exacerbates excitotoxic cortical neuron injury by kainic acid92. The lack of such protective effects in mice completely lacking TLR4 may counteract any beneficial effects of the mutation in lessening neuroinflammation and inflammaging in this glaucoma model. Similarly, lung integrity is compromised in Tlr4-mutant mice on at least some strain backgrounds93, possibly impacting oxygen supply and counterbalancing any beneficial effects. Clearly, further experiments in various models of glaucoma are needed to understand both the damaging and protective roles of TLR receptors/adaptors and reciprocal immunometabolic regulation. It will be important to determine their complex roles in different tissues and cell types at different stages of glaucoma as well as in chronic and acute forms of this disease.
Materials and methods
Ethical approval
All mice were treated in accordance with the Association for Research in Vision and Ophthalmology’s statement on the use of animals in ophthalmic research. All animal procedures were performed according to the protocols approved by the Jackson Laboratory and Columbia University’s Institutional Animal Care and Use Committee.
ARRIVE guidelines
The study is reported in accordance with the ARRIVE guidelines. Study design: all phenotypes were compared between Tlr4−/− and Tlr4+/+ mice. The experimental unit is individual eyes. Sample size is shown in figure legends for each experiment. Given the variability of DBA/2J glaucoma, at least 40 nerves for each genotype were examined when looking at the effect of genotype on neurodegeneration. No data points were excluded. No randomization was used. Both genotypes were housed together and IOP measurements were taken at the same time of the day to minimize potential confounders. Blinding: the researchers were masked for genotypes when conducting experiments, evaluating nerve damage, and counting RGCs. Outcome measures: effect of Tlr4 genotype on IOP and neurodegeneration. Statistical methods are stated in methods and figure legends.
Mice
B6.B10ScN-Tlr4lps−del/JthJ mice (strain #007227) were obtained from The Jackson Laboratory94,95. These mice are Tlr4-deficient due to lack of exon 3 in the Tlr4 gene. The Tlr4 mutation was backcrossed to strain DBA/2J (strain #000671) for > 10 generations to produce congenic mice with the Tlr4-deficient allele on the DBA/2J background (all experimental mice were ≥ N10). For this study, congenic DBA/2J Tlr4 heterozygote mice were intercrossed to produce Tlr4 wild-type and homozygous mutant littermates. Both genotypes were housed together and analyzed simultaneously. All mice were housed in a 21 °C environment with a 14-h light and 10-h dark cycle, fed with a 6% fat diet, and acidified (pH 2.8–3.2) drinking water96. Both female and male mice were used for analysis.
Clinical slit-lamp examination
Mice underwent regular examinations using a slit lamp bio-microscope at 40X magnification throughout their lifespan, as described in previous studies47,52,97. The analysis started at 4 months of age, with subsequent examinations at 6, 8, 10, and 12 months. Phenotypic assessment of iris disease included the evaluation of iris atrophy, pigment dispersion, and transillumination. Mice were examined without anesthesia using standard handling, and all photographs were captured with identical camera settings.
IOP measurement
IOP was assessed using the microneedle method as previously outlined in detail98,99. In brief, mice were anesthetized with an intraperitoneal injection of a combination of ketamine (99 mg/kg; Ketlar, Parke-Davis, Paramus, NJ, USA) and xylazine (9 mg/kg; Rompun, Phoenix Pharmaceutical, St Joseph, MO, USA) immediately before the IOP measurement. All IOP measurements for both genotypes were taken at the same time of the day.
Nerve staining and evaluation of damage
The intracranial segments of the optic nerves were fixed in 0.8% paraformaldehyde, 1.22% glutaraldehyde, and 0.08 M Phosphate Buffer pH 7.4 at 4 °C for 12 h, followed by processing and embedding in plastic. The retro-orbital ends of the nerves were cut into 1 μm thick sections and stained with paraphenylenediamine (PPD) as previously described59,100,101. PPD stains the myelin sheath of all axons, while specifically darkly staining the axoplasm of damaged axons101. At least 3 sections were examined to determine the level of damage for each nerve. The optic nerves were determined to have 3 levels of damage as previously reported and validated against axon counting9,12: (1) No or early damage (NOE) - less than 5% of axons were damaged, no gliosis, indistinguishable from no glaucoma controls. These nerves have no damage by conventional criteria but are called no or early as some of them have early molecular changes that precede neurodegeneration. (2) Moderate damage (MOD) - had an average of 30% axonal loss and early gliosis. (3) Severe damage (SEV) - had more than 50% axonal loss and damage with prominent gliosis4,10,23,100. All nerves were evaluated by at least two masked investigators. In cases where the two investigators did not agree on the damage level a third investigator (also masked) analyzed the nerve, and the most assigned damage level was used100.
Retinal wholemounts Nissl staining and cell counting
Retinas from 12-month-old DBA/2J mice were Nissl-stained with cresyl violet as previously reported57. In short, eyes were fixed in 4% paraformaldehyde in 0.1 M Phosphate Buffer pH 7.4 overnight at 4 °C and then transferred into 0.4% paraformaldehyde in 0.1 M Phosphate Buffer pH 7.4. Whole retinas were dissected from the eye, processed with 0.3% Triton X-100 and 3% hydrogen peroxide, flat-mounted onto glass slides, and stained for 1 h in 1% cresyl violet in distilled water before being differentiated in 95% alcohol, 100% alcohol, and xylene. Eight 40x brightfield images were obtained (two per quadrant) of peripheral retina, equidistant from the peripheral edge. RGC layer cells were manually counted (endothelial cells excluded) and averaged across all eight images per retina.
Statistical analysis
IOP measurements were compared between Tlr4+/+ and Tlr4−/− mice at all ages with a two-way ANOVA. The level of nerve damage between Tlr4+/+ and Tlr4−/− mice at each age was compared using Fisher’s exact test. RGC numbers were compared with a one-way ANOVA with post hoc Tukey’s HSD test.
Data availability
The raw datasets produced during the current study are available from the corresponding author upon reasonable request.
References
Adornetto, A., Russo, R. & Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural Regen Res. 14, 391–394. https://doi.org/10.4103/1673-5374.245465 (2019).
Russo, R. et al. Retinal ganglion cell death in glaucoma: exploring the role of neuroinflammation. Eur. J. Pharmacol. 787, 134–142. https://doi.org/10.1016/j.ejphar.2016.03.064 (2016).
Shestopalov, V. I., Spurlock, M., Gramlich, O. W. & Kuehn, M. H. Immune responses in the glaucomatous retina: regulation and dynamics. Cells 10 https://doi.org/10.3390/cells10081973 (2021).
Williams, P. A. et al. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol. Neurodegener. 14, 6. https://doi.org/10.1186/s13024-018-0303-3 (2019).
Baudouin, C., Kolko, M., Melik-Parsadaniantz, S. & Messmer, E. M. Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog Retin Eye Res. 83, 100916. https://doi.org/10.1016/j.preteyeres.2020.100916 (2021).
Howell, G. R. et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Invest. 122, 1246–1261. https://doi.org/10.1172/JCI61135 (2012).
Harder, J. M. et al. Complement peptide C3a receptor 1 promotes optic nerve degeneration in DBA/2J mice. J. Neuroinflammation. 17, 336. https://doi.org/10.1186/s12974-020-02011-z (2020).
Harder, J. M. et al. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc. Natl. Acad. Sci. U S A. 114, E3839–E3848. https://doi.org/10.1073/pnas.1608769114 (2017).
Howell, G. R. et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 121, 1429–1444. https://doi.org/10.1172/JCI44646 (2011).
Williams, P. A., Braine, C. E., Foxworth, N. E., Cochran, K. E. & John, S. W. M. GlyCAM1 negatively regulates monocyte entry into the optic nerve head and contributes to radiation-based protection in glaucoma. J. Neuroinflammation. 14, 93. https://doi.org/10.1186/s12974-017-0868-8 (2017).
Margeta, M. A., Lad, E. M. & Proia, A. D. CD163 + macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 256, 2449–2456. https://doi.org/10.1007/s00417-018-4081-y (2018).
Williams, P. A. et al. Vitamin B(3) modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760. https://doi.org/10.1126/science.aal0092 (2017).
Williams, P. A., Harder, J. M. & John, S. W. M. Glaucoma as a metabolic optic neuropathy: making the case for nicotinamide treatment in Glaucoma. J. Glaucoma. 26, 1161–1168. https://doi.org/10.1097/IJG.0000000000000767 (2017).
Zhang, Z. Q., Xie, Z., Chen, S. Y. & Zhang, X. Mitochondrial dysfunction in glaucomatous degeneration. Int. J. Ophthalmol. 16, 811–823. https://doi.org/10.18240/ijo.2023.05.20 (2023).
Harder, J. M. et al. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or Rapamycin. Proc. Natl. Acad. Sci. U S A. 117, 33619–33627. https://doi.org/10.1073/pnas.2014213117 (2020).
Leruez, S. et al. A metabolomics profiling of Glaucoma points to mitochondrial dysfunction, senescence, and polyamines deficiency. Invest. Ophthalmol. Vis. Sci. 59, 4355–4361. https://doi.org/10.1167/iovs.18-24938 (2018).
Jassim, A. H., Inman, D. M. & Mitchell, C. H. Crosstalk between dysfunctional mitochondria and inflammation in glaucomatous neurodegeneration. Front. Pharmacol. 12, 699623. https://doi.org/10.3389/fphar.2021.699623 (2021).
Casson, R. J., Chidlow, G., Crowston, J. G., Williams, P. A. & Wood, J. P. M. Retinal energy metabolism in health and glaucoma. Prog. Retin. Eye Res. 81 https://doi.org/10.1016/j.preteyeres.2020.100881 (2021).
Chrysostomou, V., Rezania, F., Trounce, I. A. & Crowston, J. G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 13, 12–15. https://doi.org/10.1016/j.coph.2012.09.008 (2013).
Tribble, J. R. et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 43 https://doi.org/10.1016/j.redox.2021.101988 (2021).
Williams, P. A. & Casson, R. J. Glycolysis and glucose metabolism as a target for bioenergetic and neuronal protection in glaucoma. Neural Regen Res. 19, 1637–1638. https://doi.org/10.4103/1673-5374.389638 (2024).
Williams, P. A., Harder, J. M., Cardozo, B. H., Foxworth, N. E. & John, S. W. M. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun. Integr. Biol. 11, e1356956. https://doi.org/10.1080/19420889.2017.1356956 (2018).
Williams, P. A. et al. Nicotinamide and WLD(S) act together to prevent neurodegeneration in Glaucoma. Front. Neurosci. 11, 232. https://doi.org/10.3389/fnins.2017.00232 (2017).
van Horssen, J., van Schaik, P. & Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 710, 132931. https://doi.org/10.1016/j.neulet.2017.06.050 (2019).
Arancibia, S. A. et al. Toll-like receptors are key participants in innate immune responses. Biol. Res. 40, 97–112. https://doi.org/10.4067/s0716-97602007000200001 (2007).
Piccinini, A. M. & Midwood, K. S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 https://doi.org/10.1155/2010/672395 (2010).
Zheng, Z. et al. The toll-like receptor 4-mediated signaling pathway is activated following optic nerve injury in mice. Brain Res. 1489, 90–97. https://doi.org/10.1016/j.brainres.2012.10.014 (2012).
Farooq, M., Batool, M., Kim, M. S. & Choi, S. Toll-Like receptors as a therapeutic target in the era of immunotherapies. Front. Cell. Dev. Biol. 9, 756315. https://doi.org/10.3389/fcell.2021.756315 (2021).
Kuzmich, N. N. et al. TLR4 signaling pathway modulators as potential therapeutics in inflammation and Sepsis. Vaccines (Basel). 5. https://doi.org/10.3390/vaccines5040034 (2017).
Zeng, F. et al. Physiological mechanisms of TLR4 in glucolipid metabolism regulation: potential use in metabolic syndrome prevention. Nutr. Metab. Cardiovasc. Dis. 33, 38–46. https://doi.org/10.1016/j.numecd.2022.10.011 (2023).
Katare, P. B., Bagul, P. K., Dinda, A. K. & Banerjee, S. K. Toll-Like receptor 4 Inhibition improves oxidative stress and mitochondrial health in Isoproterenol-Induced cardiac hypertrophy in rats. Front. Immunol. 8, 719. https://doi.org/10.3389/fimmu.2017.00719 (2017).
Balic, J. J. et al. STAT3 Serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1beta expression. Nat. Commun. 11, 3816. https://doi.org/10.1038/s41467-020-17669-5 (2020).
Coburn, P. S. et al. TLR4 modulates inflammatory gene targets in the retina during Bacillus cereus endophthalmitis. BMC Ophthalmol. 18, 96. https://doi.org/10.1186/s12886-018-0764-8 (2018).
Morzaev, D. et al. Toll-like receptor-4 knockout mice are more resistant to optic nerve crush damage than wild-type mice. Clin. Exp. Ophthalmol. 43, 655–665. https://doi.org/10.1111/ceo.12521 (2015).
Tsioti, I. et al. Endothelial Toll-like receptor 4 is required for microglia activation in the murine retina after systemic lipopolysaccharide exposure. J. Neuroinflammation. 20, 25. https://doi.org/10.1186/s12974-023-02712-1 (2023).
Chi, W. et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc. Natl. Acad. Sci. U S A. 111, 11181–11186. https://doi.org/10.1073/pnas.1402819111 (2014).
Dvoriantchikova, G., Barakat, D. J., Hernandez, E., Shestopalov, V. I. & Ivanov, D. Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Mol. Vis. 16, 1907–1912 (2010).
Allensworth, J. J., Planck, S. R., Rosenbaum, J. T. & Rosenzweig, H. L. Investigation of the differential potentials of TLR agonists to elicit uveitis in mice. J. Leukoc. Biol. 90, 1159–1166. https://doi.org/10.1189/jlb.0511249 (2011).
Nakano, Y. et al. Toll-like receptor 4 inhibitor protects against retinal ganglion cell damage induced by optic nerve crush in mice. J. Pharmacol. Sci. 133, 176–183. https://doi.org/10.1016/j.jphs.2017.02.012 (2017).
Mzyk, P., Hernandez, H., Le, T., Ramirez, J. R. & McDowell, C. M. Toll-Like receptor 4 signaling in the trabecular meshwork. Front. Cell. Dev. Biol. 10, 936115. https://doi.org/10.3389/fcell.2022.936115 (2022).
Sharma, T. P., Curry, S. & McDowell, C. M. Effects of Toll-Like receptor 4 Inhibition on transforming growth Factor-beta2 signaling in the human trabecular meshwork. J. Ocul Pharmacol. Ther. 36, 170–178. https://doi.org/10.1089/jop.2019.0076 (2020).
Geiduschek, E. K., Milne, P. D., Mzyk, P., Mavlyutov, T. A. & McDowell, C. M. TLR4 signaling modulates extracellular matrix production in the lamina cribrosa. Front. Ophthalmol. (Lausanne). 2. https://doi.org/10.3389/fopht.2022.968381 (2022).
Chaiwiang, N. & Poyomtip, T. The association of toll-like receptor 4 gene polymorphisms with primary open angle glaucoma susceptibility: a meta-analysis. Biosci. Rep. 39 https://doi.org/10.1042/BSR20190029 (2019).
Janssen, S. F., Gorgels, T. G., van der Spek, P. J., Jansonius, N. M. & Bergen, A. A. In Silico analysis of the molecular machinery underlying aqueous humor production: potential implications for glaucoma. J. Clin. Bioinforma. 3, 21. https://doi.org/10.1186/2043-9113-3-21 (2013).
Lin, Z., Huang, S., Sun, J., Xie, B. & Zhong, Y. Associations between TLR4 polymorphisms and open angle glaucoma: A Meta-Analysis. Biomed. Res. Int. 2019 (6707650). https://doi.org/10.1155/2019/6707650 (2019).
Shibuya, E. et al. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest. Ophthalmol. Vis. Sci. 49, 4453–4457. https://doi.org/10.1167/iovs.07-1575 (2008).
Anderson, M. G. et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 30, 81–85. https://doi.org/10.1038/ng794 (2002).
Lahola-Chomiak, A. A. et al. Non-Synonymous variants in premelanosome protein (PMEL) cause ocular pigment dispersion and pigmentary glaucoma. Hum. Mol. Genet. 28, 1298–1311. https://doi.org/10.1093/hmg/ddy429 (2019).
De Moraes, C. G. et al. Nicotinamide and pyruvate for neuroenhancement in Open-Angle glaucoma: A phase 2 randomized clinical trial. JAMA Ophthalmol. 140, 11–18. https://doi.org/10.1001/jamaophthalmol.2021.4576 (2022).
Hui, F. et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 48, 903–914. https://doi.org/10.1111/ceo.13818 (2020).
Howell, G. R. et al. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 8, 45. https://doi.org/10.1186/1471-2156-8-45 (2007).
John, S. W. et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 39, 951–962 (1998).
Libby, R. T. et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 22, 637–648. https://doi.org/10.1017/S0952523805225130 (2005).
Hirt, J., Porter, K., Dixon, A., McKinnon, S. & Liton, P. B. Contribution of autophagy to ocular hypertension and neurodegeneration in the DBA/2J spontaneous glaucoma mouse model. Cell. Death Discov. 4, 14. https://doi.org/10.1038/s41420-018-0077-y (2018).
Yang, X. L. et al. Age-related changes in eye, brain and visuomotor behavior in the DBA/2J mouse model of chronic Glaucoma. Sci. Rep. 8, 4643. https://doi.org/10.1038/s41598-018-22850-4 (2018).
Donahue, R. J., Fehrman, R. L., Gustafson, J. R. & Nickells, R. W. BCLX(L) gene therapy moderates neuropathology in the DBA/2J mouse model of inherited glaucoma. Cell. Death Dis. 12, 781. https://doi.org/10.1038/s41419-021-04068-x (2021).
Libby, R. T. et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 1, 17–26. https://doi.org/10.1371/journal.pgen.0010004 (2005).
Syc-Mazurek, S. B., Fernandes, K. A. & Libby, R. T. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell. Death Dis. 8, e2945. https://doi.org/10.1038/cddis.2017.338 (2017).
Howell, G. R. et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell. Biol. 179, 1523–1537. https://doi.org/10.1083/jcb.200706181 (2007).
Ahmad, A. et al. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One. 8, e57208. https://doi.org/10.1371/journal.pone.0057208 (2013).
Lee, H. S. et al. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest. Ophthalmol. Vis. Sci. 53, 5632–5640. https://doi.org/10.1167/iovs.12-9547 (2012).
Chang, J. H., McCluskey, P. J. & Wakefield, D. Toll-like receptors in ocular immunity and the Immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 90, 103–108. https://doi.org/10.1136/bjo.2005.072686 (2006).
Titi-Lartey, O., Mohammed, I. & Amoaku, W. M. Toll-Like receptor signalling pathways and the pathogenesis of retinal diseases. Front. Ophthalmol. 2 https://doi.org/10.3389/fopht.2022.850394 (2022).
Duarte, J. N. Neuroinflammatory Mechanisms of Mitochondrial Dysfunction and Neurodegeneration in Glaucoma. J Ophthalmol 4581909 (2021). (2021). https://doi.org/10.1155/2021/4581909
Ju, W. K. et al. Glaucomatous optic neuropathy: mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog Retin Eye Res. 95, 101136. https://doi.org/10.1016/j.preteyeres.2022.101136 (2023).
Lee, S. et al. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp. Eye Res. 93, 204–212. https://doi.org/10.1016/j.exer.2010.07.015 (2011).
Tolman, N. et al. Single-cell profiling of trabecular meshwork identifies mitochondrial dysfunction in a glaucoma model that is protected by vitamin B3 treatment. bioRxiv (2024). https://doi.org/10.1101/2024.11.01.621152
Kalesnykas, G. et al. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 53, 3847–3857. https://doi.org/10.1167/iovs.12-9712 (2012).
Wang, J., Struebing, F. L. & Geisert, E. E. Commonalities of optic nerve injury and glaucoma-induced neurodegeneration: insights from transcriptome-wide studies. Exp. Eye Res. 207, 108571. https://doi.org/10.1016/j.exer.2021.108571 (2021).
Roberts, A. L. et al. Fibronectin extra ___domain A (FN-EDA) elevates intraocular pressure through Toll-like receptor 4 signaling. Sci. Rep. 10, 9815. https://doi.org/10.1038/s41598-020-66756-6 (2020).
Imai, Y. et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249. https://doi.org/10.1016/j.cell.2008.02.043 (2008).
Morales-Nebreda, L., Mutlu, G. M., Budinger, S., Radigan, K. A. & G. R. & Loss of TLR4 does not prevent influenza A-induced mortality. Am. J. Respir Crit. Care Med. 189, 1280–1281. https://doi.org/10.1164/rccm.201401-0193LE (2014).
Shirey, K. A., Blanco, J. C. G. & Vogel, S. N. Targeting TLR4 signaling to blunt Viral-Mediated acute lung injury. Front. Immunol. 12, 705080. https://doi.org/10.3389/fimmu.2021.705080 (2021).
Rocha, D. M., Caldas, A. P., Oliveira, L. L., Bressan, J. & Hermsdorff, H. H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 244, 211–215. https://doi.org/10.1016/j.atherosclerosis.2015.11.015 (2016).
Rogero, M. M. & Calder, P. C. Obesity, inflammation, Toll-Like receptor 4 and fatty acids. Nutrients 10 https://doi.org/10.3390/nu10040432 (2018).
Ji, Y. et al. Toll-like receptors TLR2 and TLR4 block the replication of pancreatic beta cells in diet-induced obesity. Nat. Immunol. 20, 677–686. https://doi.org/10.1038/s41590-019-0396-z (2019).
Verweij, S. P. et al. TLR2, TLR4 and TLR9 genotypes and haplotypes in the susceptibility to and clinical course of Chlamydia trachomatis infections in Dutch women. Pathog Dis. 74, ftv107. https://doi.org/10.1093/femspd/ftv107 (2016).
Soto, I. & Howell, G. R. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect. Med. 4 https://doi.org/10.1101/cshperspect.a017269 (2014).
Tezel, G., Li, L. Y., Patil, R. V. & Wax, M. B. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 42, 1787–1794 (2001).
Kim, H. J., Kim, H., Lee, J. H. & Hwangbo, C. Toll-like receptor 4 (TLR4): new insight immune and aging. Immun. Ageing. 20, 67. https://doi.org/10.1186/s12979-023-00383-3 (2023).
Tezel, G. Molecular regulation of neuroinflammation in glaucoma: current knowledge and the ongoing search for new treatment targets. Prog Retin Eye Res. 87, 100998. https://doi.org/10.1016/j.preteyeres.2021.100998 (2022).
Coyle, S. et al. Targeting the NLRP3 inflammasome in Glaucoma. Biomolecules 11 https://doi.org/10.3390/biom11081239 (2021).
Okun, E. et al. Toll-like receptors 2 and 4 modulate autonomic control of heart rate and energy metabolism. Brain Behav. Immun. 36, 90–100. https://doi.org/10.1016/j.bbi.2013.10.013 (2014).
Tannahill, G. M. & O’Neill, L. A. The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett. 585, 1568–1572. https://doi.org/10.1016/j.febslet.2011.05.008 (2011).
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749. https://doi.org/10.1182/blood-2009-10-249540 (2010).
Vizuete, A. F. K. et al. Targeting Glycolysis for neuroprotection in early LPS-induced neuroinflammation. Brain Behav. Immun. Health. 42, 100901. https://doi.org/10.1016/j.bbih.2024.100901 (2024).
Bauerfeld, C. P. et al. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J. Immunol. 188, 2847–2857. https://doi.org/10.4049/jimmunol.1102157 (2012).
Everts, B. et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15, 323–332. https://doi.org/10.1038/ni.2833 (2014).
Ritch, R. et al. TBK1 gene duplication and normal-tension glaucoma. JAMA Ophthalmol. 132, 544–548. https://doi.org/10.1001/jamaophthalmol.2014.104 (2014).
Shintani, Y. et al. TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc. Natl. Acad. Sci. U S A. 110, 5109–5114. https://doi.org/10.1073/pnas.1219243110 (2013).
Komine, O. et al. Innate immune adaptor TRIF deficiency accelerates disease progression of ALS mice with accumulation of aberrantly activated astrocytes. Cell. Death Differ. 25, 2130–2146. https://doi.org/10.1038/s41418-018-0098-3 (2018).
Larochelle, A., Bellavance, M. A. & Rivest, S. Role of adaptor protein MyD88 in TLR-mediated preconditioning and neuroprotection after acute excitotoxicity. Brain Behav. Immun. 46, 221–231. https://doi.org/10.1016/j.bbi.2015.02.019 (2015).
Kim, S. J. et al. Endothelial toll-like receptor 4 maintains lung integrity via epigenetic suppression of p16(INK4a). Aging Cell. 18, e12914. https://doi.org/10.1111/acel.12914 (2019).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088. https://doi.org/10.1126/science.282.5396.2085 (1998).
Vogel, S. N., Hansen, C. T. & Rosenstreich, D. L. Characterization of a congenitally LPS-resistant, athymic mouse strain. J. Immunol. 122, 619–622 (1979).
Tolman, N. G. et al. Genetic background modifies vulnerability to glaucoma-related phenotypes in Lmx1b mutant mice. Dis. Model. Mech. 14 https://doi.org/10.1242/dmm.046953 (2021).
Chang, B. et al. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat. Genet. 21, 405–409. https://doi.org/10.1038/7741 (1999).
John, S. W., Hagaman, J. R., MacTaggart, T. E., Peng, L. & Smithes, O. Intraocular pressure in inbred mouse strains. Invest. Ophthalmol. Vis. Sci. 38, 249–253 (1997).
Savinova, O. V. et al. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2, 12. https://doi.org/10.1186/1471-2156-2-12 (2001).
Anderson, M. G., Libby, R. T., Gould, D. B., Smith, R. S. & John, S. W. High-dose radiation with bone marrow transfer prevents neurodegeneration in an inherited glaucoma. Proc. Natl. Acad. Sci. U S A. 102, 4566–4571. https://doi.org/10.1073/pnas.0407357102 (2005).
Smith, R. S., John, S. W. M., Nishina, P. M. & Sundberg, J. P. Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods (CRC, 2002).
Acknowledgements
The authors would like to thank Amy Bell for measuring intraocular pressure in mice, and Pete Finger for optic nerve sectioning and PPD staining.
Funding
This project was supported by a Vision Core grant P30EY019007 (Columbia University) and an unrestricted departmental award from Research to Prevent Blindness. Also partially supported by National Eye Institute grants (to S.W.M.J: EY11721, EY032507, EY032062, and EY018606 (MPI)), startup funds at Columbia University including the Precision Medicine Initiative, and the New York Fund for Innovation in Research and Scientific Talent (NYFIRST; EMPIRE CU19-2660). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
S.W.M.J. conceived the project; C.Z., J.M.H., and S.W.M.J. designed the research; C.Z., H.L., Q.W., M.S., J.M.H., and S.W.M.J. performed research and analyzed data; C.M. managed mouse colony; C.Z., M.S., and S.W.M.J. wrote the manuscript. All authors read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Simón, M., Harder, J.M. et al. TLR4 deficiency does not alter glaucomatous progression in a mouse model of chronic glaucoma. Sci Rep 15, 16852 (2025). https://doi.org/10.1038/s41598-025-00638-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00638-7