Abstract
Helicobacter pylori (H. pylori) infection is an established cause of gastric cancer. Although H. pylori eradication is suggested to decrease gastric cancer risk, this has not been fully investigated in general populations. This analysis included 48,530 Japanese men and women aged 40–74 years from four cohort studies. At baseline, the participants provided a self-reported eradication history and serum anti-H. pylori IgG titers and the results of a pepsinogen (PG) test. We examined the association between eradication history and gastric cancer risk considering H. pylori positivity and PG testing using Cox proportional hazards regression models. From 2010 to 2018, 649 gastric cancer cases were diagnosed. Compared with those who were negative for both H. pylori and PG test as a reference, gastric cancer risk was 5.89 times higher (95%CI: 4.41–7.87) in those who were H. pylori-positive and/or PG test-positive and with no eradication at baseline. Gastric cancer risk among those who underwent eradication before baseline decreased after a temporal increase in risk following eradication (baseline to < 1y: HR 1.74, 95%CI 1.18–2.57; 1y to < 6y: HR 0.81, 95%CI 0.59–1.11; ≥ 6y: HR 0.44, 95%CI 0.28–0.68). In this large Japanese general population, H. pylori eradication was associated with a long-term reduction in gastric cancer incidence.
Similar content being viewed by others
Introduction
Gastric cancer affected 0.97 million people worldwide in 2022, making it the fifth-most common cancer and cause of cancer death globally that year1. In Japan, gastric cancer was the leading cause of death by cancer site until 1998 and ranked third in 20222.
Helicobacter pylori (H. pylori)infection is listed as a Group 1 Carcinogen by the International Agency for Research on Cancer (IARC). It is the most critical factor in the development of gastric cancer3,4. Gastric cancer develops in 5% of H. pylori-positive individuals over ten years, according to estimates of the Japanese population5. The effect of infection varies according to the infecting H. pyloristrain and is not uniform worldwide6,7. A meta-analysis that included randomized control trials confirmed that H. pylori eradication is associated with a reduced incidence of gastric cancer8. However, whether eradication prevents gastric cancer in the general population has not been determined9.
Here, we conducted a pooled analysis of ongoing population-based cohort studies in Japan to determine the risk of developing gastric cancer after H. pylori eradication in a general population setting.
Materials and methods
Study population
The study was conducted using prospective cohort study data of residents aged 40–74 years in four areas: Yamagata, Saku, Yokote, and Chikusei, Japan. The Yamagata area initiated the study in 2009 under the Yamagata Study protocol. Blood samples were collected with consent at the time of health examinations, and questionnaires were conducted at baseline and the 5-year follow-up survey. Medical information derived from health insurance records, cancer diagnoses by cancer registries, and deaths were captured during follow-up. The Saku, Yokote, and Chikusei areas initiated the study under the protocol of The Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT Study), which was initiated in 2011 and targeted residents in each area. Questionnaire surveys and blood sampling were conducted at baseline and at the time of the 5-year follow-up survey. Participants were followed for vital status, cause of death, and residential relocation by the residential registry or death certificates, with consent. Both the Yamagata Study and JPHC-NEXT Study were designed to identify lifestyle, environmental, and genetic factors in the development of lifestyle-related diseases. The two large cohort studies are described in detail elsewhere10,11.
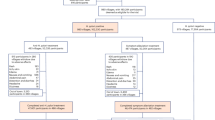
We conducted two main analyses. Analysis 1 included only the baseline survey results, while Analysis 2 included only those who responded to the baseline and 5-year follow-up surveys.
We initially included 49,140 residents aged 40–74 in the four areas who participated in the baseline survey and had blood sampling. After those with a history of gastric cancer as determined by self-report or the cancer registries before baseline were excluded (n = 610), 48,530 participants were regarded as eligible for analysis (Analysis 1). Among these, 32,196 participants answered the questionnaire of the 5-year follow-up survey. After excluding those who were treated for H. pylori eradication before the baseline survey (n = 3,658), were both H. pylori antibody- and PG test-negative (n = 13,240) or were treated for eradication at an unknown time (n = 2,214), participants who were H. pylori antibody-positive and/or PG test-positive (n = 13,084) were included in further analysis (Analysis 2).
The study flow is shown in Fig. 1.
Assessment of H. pylori infection status
This study used serum samples obtained during health examinations or study-specific blood sampling opportunities. Serum anti-H. pylori IgG antibodies were measured using enzyme-linked immunosorbent assay (ELISA; E-Plate Eiken or E-Plate II Eiken; Eiken Chemical Co., Ltd., Japan). We defined values of 10 U/mL or higher as positive, the standard cut-off point when the study was initiated. As a marker of atrophic gastritis, serum levels of pepsinogen (PG) I and II were measured by latex agglutination (LZ test “Eiken” Pepsinogen I, II; Eiken Kagaku). The PG test was determined to be negative at PG I > 70 ng/mL or PG I/II > 3.0, or positive at PG I ≤ 70 ng/mL and PG I/II ratio ≤ 3.012. Participants were then categorized into five groups by H. pylori infection status according to the results for serum anti-H. pylori IgG antibodies and PG test, namely H. pylori-negative and PG test-negative; H. pylori-positive and PG test-negative; H. pylori-positive and PG test-positive; H. pylori-negative and PG test-positive; and self-reported history of H. pylori eradication before baseline survey.
Assessment of H. pylori eradication history
In the Yamagata Study, we inquired about participants’ H. pylori eradication status using the following questions: (1) “Have you ever been tested for H. pylori?” (Yes/No) (2) “Have you received H. pylori eradication?” (Yes/No), (3) “At what age did you receive H. pylori eradication?” In the JPHC-NEXT, we asked, “Have you ever received H. pylori eradication?” (No history/Received). Participants who reported previous eradication were additionally asked about the length of time between eradication and baseline (< 1 year, 1 year to < 6 years, ≥ 6 years). We matched the eradication age in the Yamagata Study participants to the JPHC-NEXT response categories. Those who underwent eradication after gastric cancer diagnosis were reclassified as no-eradication. The validity of this study’s self-reported H. pylori eradication history has been confirmed elsewhere13,14.
Follow-up and identification of gastric cancer
Participants were followed from the date of study entry at baseline until gastric cancer diagnosis, movement out of the study area, death, or the end of follow-up (December 31, 2018), whichever occurred first. Data on survival, movement out of the study area, and death were identified from residence records. Information on the study outcome – gastric cancer (Code C16.0–16.9) diagnosed during the study period – was obtained from major local hospitals as well as regional (until 2015) and national cancer registries (from 2016) by the Cancer Registry Promotion Act and prepared for analysis in this study and processed independently. Cases were coded using the International Classification of Diseases for Oncology (ICD-O), Third Edition15.
Statistical analysis
Hazard ratios (HRs) and 95% confidence intervals (CIs) for developing gastric cancer by category of years since eradication were determined using Cox proportional hazards models, controlling for the following potential confounders: sex, age at baseline (5-year categories), body mass index (< 20 kg/m2, 20 to < 25 kg/m2, 25 to < 30 kg/m2, or ≥ 30 kg/m2), smoking status (never, current, former), and alcohol consumption (pure ethanol per week; never or rarely, < 150 g/week, 150 to 300 g/week, 300 g/week or more). These variables were selected based on associations identified in a previous study16. Missing values were also incorporated as missing indicator variables in the analysis. Heterogeneity across the study areas was accounted for by including the study area as a strata variable in the Cox model. We also estimated HRs stratified by H. pylori infection status. Some participants who completed the 5-year follow-up survey underwent eradication between baseline and the 5-year follow-up survey. Additional sensitivity analyses were conducted based on complete-case analysis, excluding cases with missing values and participants with inconsistent responses between the baseline and 5-year follow-up survey. Testing of the proportional hazards assumption using Schoenfeld and scaled Schoenfeld residuals found no violation of proportionality. The p-values for differences in the Tables were calculated using ANOVA (normal distribution) or Pearson’s chi-squared test and reported as two-sided, with p < 0.05 set as the statistical significance level. All statistical analyses were performed using Stata 17 (Stata Corp).
Results
Analysis 1 included 48,530 participants for analysis (20,762 men and 27,768 women; average age at baseline, 60.3 years). During 283,379 person-years of follow-up (average follow-up period: 5.8 years), a total of 649 participants (463 men and 186 women) were newly diagnosed with gastric cancer. In Analysis 2, there were 323 diagnoses of gastric cancer during a mean follow-up period of 6.1 years. (Fig. 1). The baseline characteristics of the study participants according to their self-reported history of H. pylori eradication are shown in Table 1. A total of 11.7% of participants had an H. pylori eradication history at the baseline survey, with the largest proportion of participants from the Chikusei area (14.6%). Men had a higher proportion of eradication treatment history than women (14.4% vs 9.6%). With regard to drinking history, there were no significant differences in the distribution of whether and how much alcohol was consumed between the eradicated and non-eradicated groups for both men and women. Concerning smoking history, the proportion of current smokers was not significantly different between the eradicated and non-eradicated groups in men and women. Selected characteristics of the study participants according to H. pylori infection status and serum PG1 and PG2 assays are shown in Table S1.
Table 2 shows the results of gastric cancer risk by length of time between eradication and baseline (Analysis 1). Among those with no history of eradication, participants who were H. pylori-positive and/or PG test-positive had a higher risk of gastric cancer (HR 5.89, 95%CI: 4.41–7.87) compared with those who were H. pylori-negative and PG test-negative. Further, the risk was increased in those who were H. pylori-positive and PG test-negative (HR 3.62, 95% CI: 2.60–5.03) and further increased in H. pylori-positive and PG test-positive (HR 7.67, 95% CI: 5.69–10.34) and in H. pylori-negative and PG test-positive (HR 8.79, 95%CI: 6.04–12.80). When analysis was restricted to participants with H. pylori infection (including those both with and without a history of eradication), compared with those with no history of eradication as reference, risk was increased for those who had undergone eradication less than one year before baseline (HR 1.74, 95%CI: 1.18–2.57) but decreased for those who had undergone eradication 1–6 years and 6 or more years before baseline (HR 0.81, 95%CI: 0.59–1.11; HR 0.44, 95%CI: 0.28–0.68, respectively). Although there was a statistically significant difference between the H. pylori-seropositive and PG test-negative group and the H. pylori-seropositive and PG test-positive group (p = 0.04), there was no significant difference between the H. pylori-seropositive and PG test-positive group and H. pylori-seronegative and PG test-positive group (p = 0.54). For those with gastric mucosal atrophy confirmed by a PG test, the difference in risk of developing gastric cancer was not observed by H. pylori infection status, either seropositive or seronegative.
Table 3 shows gastric cancer risk by period after H. pylori eradication based on the 5-year follow-up survey (Analysis 2). Among participants who followed from baseline to the 5-year follow-up survey, only those with a positive H. pylori infection status (H. pylori-positive and/or PG test-positive) and who self-reported an eradication history at the baseline and 5-year follow-up surveys were included. Compared to those with no eradication, gastric cancer risk declined after eradication between baseline and the 5-year follow-up survey, which was not statistically significant (HR 0.84, 95%CI: 0.56–1.26 for < 1 year at the 5-year follow-up survey; and HR 0.80, 95%CI: 0.63–1.03 for 1–6 years at the 5-year follow-up survey).
The complete case analysis was performed as a sensitivity analysis, and the analysis by age group showed no significant differences from the original analysis (data not shown).
Discussion
We examined the risk of developing gastric cancer after H. pylori eradication treatment by combining data from population-based cohort studies in four areas comprising a total of 48,530 Japanese participants. To our knowledge, this is the first report to examine the association between H. pylori eradication and gastric cancer risk in a large-scale prospective cohort study of a general Japanese population. We observed that the risk of gastric cancer among those who underwent eradication before baseline decreased with increasing time. This followed a temporal increase in risk immediately after eradication compared to those with H. pylori infection and no history of eradication. This finding suggests that gastric cancer risk is decreased in the long term by H. pylori eradication for those at high risk in a general population setting (Table 2). One possible reason for the temporary increase in risk is the inevitable rise in the number of endoscopies before and after eradication aimed at the possible diagnosis of early gastric cancer. Additionally, oversight of early gastric cancer during pre-eradication endoscopy complicates accurate risk evaluation after eradication. Risk showed a decreasing trend among participants who first underwent eradication between baseline and the 5-year follow-up survey (Table 3). These differences from the risk trends in participants eradicated before baseline may be partly due to the inclusion of participants with shorter observation periods. H. pylori eradication was shown to be more likely to reduce gastric cancer risk if performed in the early phase of infection both in an experiment17and an epidemiological study for individuals with few symptoms or mild gastric atrophy18.
One mechanism underlying the association between H. pylori infection and gastric cancer development may be deoxyribonucleic acid (DNA) methylation induced by chronic H. pylori infection-associated inflammation19,20. Cytotoxin-associated Gene A (CagA), a toxic agent of H. pylori, plays an important role in carcinogenesis6by promoting genetic mutations through interaction with breast cancer susceptibility gene 1 (BRCA1)21. H. pylori eradication decreases the effects of DNA methylation abnormalities and CagA, suggesting a preventive effect on carcinogenesis. However, if abnormalities occur in gastric epithelial cells and stem cells22, they may persist after eradication, which might explain why carcinogenic risk does not fully recover to healthy levels23.
The main strengths of this investigation are its large, well-characterized Japanese cohort and collection of information before a diagnosis of gastric cancer, eliminating recall bias. Nevertheless, some methodological limitations also warrant mention. First, participants’ self-reported H. pylori eradication history was not confirmed using their treatment history. Eradication history may have been misclassified due to misidentification, with accidental eradication being a possible cause23. We assume that the results of medical records survey13and medical claims data14 have sufficient validity. Second, we did not consider the treatment assessment result or secondary eradication due to the high success rate of first-time eradication. This omission may cause the effect of eradication to be underestimated. In addition, some gastric cancers were diagnosed during the follow-up period among those classified as both H. pylori- and PG test-negative; these might be gastric cancers unrelated to H. pylori or attributable to misclassification by accidental eradication from other treatments or forgotten eradication reports. Third, we could not analyze gastric cancer risk by stratifying participants with a history of eradication, which should also be stratified by the presence or absence of mucosal atrophy at the time of eradication. The present study obtained information on gastric atrophy at the baseline survey only when the time had passed since eradication. As a result, we could not evaluate these data, which may have reflected gastric mucosal changes due to the eradication. Fourth, participants who had undergone eradication before baseline may have had advanced symptoms, such as peptic ulcers. This is because eradication was covered by health insurance for these individuals. Insurance coverage was subsequently extended to include “H. pylori-gastritis” after February 2013, which resulted in a marked nationwide increase in the number of patients undergoing eradication, from approximately 650,000 per year in 2001–2012 to 1.38 million per year in 201324. The effectiveness of eradication during the short follow-up period may have been influenced by the timing of eradication, specifically whether it was performed before or after the introduction of health insurance coverage. Additionally, the presence or absence of symptoms at the time of eradication may have also impacted the outcomes. In addition, a simulation study estimated that the 2013 coverage of eradication for H. pylori-gastritis in Japan will decrease the prevalence of H. pylori infection by 2050 to 5%, versus 22% if coverage had not been provided24. In the present study, initiating health insurance coverage for eradication of H. pylori-gastritis during the study period may have resulted in differences in participant backgrounds before and after the coverage began. However, we were unable to investigate these differences in detail. Given these changes in medical conditions, risk reduction by early eradication and early detection using appropriate long-term endoscopic examination should be considered essential components of any gastric cancer prevention strategy.
In conclusion, we found that H. pylori eradication was associated with a decrease in long-term gastric cancer risk in a large Japanese general population. However, a temporary risk increase was observed during the post-eradication observation period. These findings may aid risk stratification for strategic gastric cancer prevention and promote future prevention efforts.
Data availability
JPHC-NEXT and Yamagata studies are not publicly available because they are open only to researchers of Japanese nationality who meet the research requirements. They are available from the corresponding author upon reasonable request. Investigators planning to access both study data must receive approval from relevant committees. For JPHC-NEXT, approval is required from both the JPHC-NEXT Steering Committee (SC) and the Institutional Review Board (IRB) of the National Cancer Center (NCC). Researchers must submit a Project Protocol (including research question, objectives, background, design, and analysis plan) for review by the JPHC-NEXT SC. Similarly, the Yamagata Study requires approval from the Cohort Steering Committee and the Institute’s Steering Committee at the Yamagata University Well-Being Institute, with additional approval from the School of Medicine Ethics Review Committee for post-approval data. Requests can be made by contacting the JPHC SC directly ([email protected]) or contacting the Cohort Management Promotion Department ([email protected]) for information on applying for the Yamagata Study.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Ferlay, J EM., Lam, F., Laversanne, M., Colombet, M., Mery, L., Piñeros, M., Znaor, A., Soerjomataram, I., Bray, F. Global Cancer Observatory: Cancer Today (version 1.1). Lyon, France: International Agency for Research on Cancer. (2024) accessed 02 Aug 2024.
Cancer WIAfRo. IARC monographs on the identification of carcinogenic hazards to humans (2022).
Inoue, M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer 20, 3–7 (2017).
Charvat, H. et al. Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: The JPHC study cohort II. Int. J. Cancer 138, 320–331 (2016).
Sasazuki, S. et al. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: A nested case-control study. Cancer Epidemiol. Biomark. Prev 15, 1341–1347 (2006).
Suzuki, H. & Mori, H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J. Gastroenterol. 53, 354–361 (2018).
Lee, Y. C. et al. Association between helicobacter pylori eradication and Gastric Cancer Incidence: A systematic review and meta-analysis. Gastroenterology 150, 1113–1124.e5 (2016).
Lin, Y. et al. Effects of Helicobacter pylori eradication on gastric cancer incidence in the Japanese population: A systematic evidence review. Jpn. J. Clin. Oncol. 51, 1158–1170 (2021).
Narimatsu, H. Constructing a contemporary gene-environmental cohort: Study design of the yamagata molecular epidemiological cohort study. J. Hum. Genet. 58, 54–56 (2013).
Sawada, N. et al. The Japan public health center-based prospective study for the next generation (JPHC-NEXT): Study design and participants. J. Epidemiol. 30, 46–54 (2020).
Dinis-Ribeiro, M. et al. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J. Med. Screen 11, 141–147 (2004).
Sasaki, Y. et al. Reliability of self-reported questionnaire for epidemiological investigation of Helicobacter pylori eradication in a population-based cohort study. Sci. Rep. 11, 15605 (2021).
Kihara, T. et al. Validity of self-reported Helicobacter pylori eradication treatment from questionnaire and interview surveys of the JPHC-NEXT study: Comparison with prescription history from insurance claims data. J. Epidemiol https://doi.org/10.2188/jea.JE20230168 (2024).
Fritz, A. et al. In International classification of diseases for oncology 3rd edn. (World Health Organization, 2000).
Sauvaget, C. et al. Vegetables and fruit intake and cancer mortality in the Hiroshima/Nagasaki life span study. Br. J. Cancer 88, 689–694 (2003).
Nozaki, K. et al. Effect of early eradication on Helicobacter pylori-related gastric carcinogenesis in Mongolian gerbils. Cancer Sci. 94, 235–239 (2003).
Take, S. et al. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J. Gastroenterol. 46, 318–324 (2011).
Maeda, M., Moro, H. & Ushijima, T. Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: Aberrant DNA methylation pathway. Gastric Cancer 20, 8–15 (2017).
Nakajima, T. et al. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol. Biomark. Prev. 15, 2317–2321 (2006).
Imai, S. et al. Helicobacter pylori CagA elicits BRCAness to induce genome instability that may underlie bacterial gastric carcinogenesis. Cell Host Microb. 29, 941–958.e10 (2021).
Tsugawa, H. et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microb. 12, 764–777 (2012).
Dinis-Ribeiro, M. et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European society of gastrointestinal endoscopy (ESGE), European helicobacter study group (EHSG), European society of pathology (ESP), and the Sociedade portuguesa de endoscopia digestiva (SPED). Endoscopy 44, 74–94 (2012).
Hiroi, S. et al. Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan: Retrospective observational study and simulation study based on real-world data. BMJ Open 7, e015855 (2017).
Acknowledgements
We appreciate all participants and staff members in each study area for their efforts in conducting the baseline and follow-up surveys.
Funding
This work was funded by the National Cancer Center Research and Development Fund [23-A-31(toku), 26-A-2, 29-A-4] (since 2011), Research and Development by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (2011–2013), and by Practical Research for Innovative Cancer Control, Japan Cancer Research Project, Japan Agency for Medical Research and Development (AMED) (17ck0106277h0001, 18ck0106277h0002, 19ck0106277h0003, 20ck0106561h0001, 20ck0106561h0001RR, 21ck0106561h0002, 21ck0106561h0002RR, 22ck0106561h0003, 22ck0106561 h0003RR, 23ck0106887h0001, 24ck0106887h0002, 25ck0106887h0003), and in part by the Japan Society for the Promotion of Science KAKENHI Grant Number JP20 K08350 for the Yamagata Study.
Author information
Authors and Affiliations
Contributions
MI was responsible for the concept and design of the study and supervised the project. AO, ST, NS, AG, ST, IM, KY, YS, YA, and TY contributed to data acquisition. AO, NS, and MI contributed to the analysis and interpretation of data. AO and MI drafted the manuscript. ST, NS, AG, ST, IM, KY, YS, YA, TK, YU, ES, TY, MI, and MI critically revised the manuscript for important intellectual content. All authors reviewed the manuscripts and contributed to the revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Ethics
The study was conducted conforming to the Declaration of Helsinki and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects and was initiated in each cohort by obtaining written informed consent from all participants at the time of study enrollment. The individual study protocols were approved by the Institutional Review Board of Yamagata University Faculty of Medicine (#2019–175) and the National Cancer Center Japan (2011–186), and the pooled analysis protocol was approved by Yamagata University Faculty of Medicine (#2019–175) and the National Cancer Center Japan (2017–243).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ono, A., Tanaka, S., Sawada, N. et al. Helicobacter pylori eradication and gastric cancer prevention in a pooled analysis of large-scale cohort studies in Japan. Sci Rep 15, 21307 (2025). https://doi.org/10.1038/s41598-025-00713-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00713-z