Abstract
Postmenopausal osteoporosis poses a significant clinical challenge, as conventional therapies are often ineffective or poorly tolerated owing to adverse effects or underlying health conditions, underscoring the need for alternative treatments. This study investigated the anti-osteoporotic effects of a novel probiotic mixture combining Limosilactobacillus reuteri MGE 3301 (LR) and Weissella cibaria MGE 3110 (WC), which were selected for their anti-inflammatory properties and ability to modulate bone metabolism, in an ovariectomized rat model. Thirty-five female Wistar rats were randomly assigned to five groups: Sham, Ovariectomy (OVX), OVX with LR supplementation (OVX/LR), OVX with WC (OVX/WC), and OVX with a combination of LR and WC (OVX/LR/WC), under ARRIVE guidelines and ethical approval. Each probiotic group received 1 × 10⁹ CFU/mL/day for 16 weeks starting at 5 weeks post-OVX. Micro-computed tomography and histopathological analyses revealed that the OVX/LR/WC group had superior trabecular bone preservation compared with that in the OVX control group, with significant improvements in bone mineral density (+ 54.2%), bone volume fraction (+ 24.8%), trabecular thickness (+ 13.6%), and trabecular number (+ 20%), along with decreased trabecular separation (− 8.1%; p < 0.05). RT-qPCR analysis of bone marrow demonstrated that LR/WC suppressed osteoclastogenic mediators (RANKL: −1.35-fold; TNF-α: −2.5-fold; IL-6: −1.9-fold) while elevating osteoprotective osteoprotegerin expression (+ 3.14-fold; p < 0.05). Serum analysis showed reduced CTX-I (− 38.9%) and elevated calcium (+ 30.8%) levels in OVX/LR/WC versus OVX rats (p < 0.05), indicating suppressed bone resorption and enhanced mineral homeostasis. These findings indicate that LR/WC probiotic supplementation attenuates OVX-induced bone loss by modulating bone turnover markers and inflammatory cytokines. To our knowledge, this is the first study to assess the combined effects of LR and WC in an osteoporosis animal model, highlighting its potential as an adjunctive therapeutic candidate for osteoporosis. However, few notable imitations include undefined human dosing and the unassessed long-term safety of probiotics. Future clinical trials must validate the efficacy, elucidate mechanisms (e.g., gut-bone axis interactions), and assess safety in postmenopausal women to advance therapeutic applicability.
Similar content being viewed by others
Introduction
Osteoporosis is a chronic metabolic bone disease characterized by low bone mass and microarchitectural deterioration, leading to an increased risk of fractures due to an imbalance between bone resorption and formation1,2. However, postmenopausal women experience osteoporosis due to estrogen deficiency, which accelerates bone resorption by stimulating osteoclast activity3,4. Osteoporotic fractures, especially those of the hip, spine, and wrist, are associated with increased mortality, morbidity, and economic burden. According to the World Health Organization (WHO), osteoporosis affects approximately 200 million people worldwide, with annual healthcare costs exceeding $17 billion in the United States alone5. In addition, 50% of postmenopausal women in the United States experience osteoporotic fractures during their lifetime6. Therefore, osteoporosis-related fractures are becoming a leading cause of fatalities and disabilities7,8,9.
Conventional therapies for osteoporosis primarily include pharmacological agents such as bisphosphonates, selective estrogen receptor modulators (SERMs), and monoclonal antibodies such as denosumab, along with hormone replacement therapy (HRT), which help restore bone density and protect the bone from fractures10,11,12. Among these, bisphosphonates are the most widely prescribed drugs that inhibit osteoclast-mediated bone resorption, thus improving bone health13. However, several reports claim that long-term bisphosphonate treatment is related to atypical stress fractures of the femur and osteonecrosis of the jaw due to oversuppression of bone turnover14. SERMs function as estrogen agonists in bone tissue, replicating the protective effects of estrogen on bone remodeling; however, they pose an elevated risk of venous thromboembolism and hot flashes15. Receptor activator of nuclear factor-kappa B ligand (RANKL) plays a key role in promoting osteoclast activity and contributes to osteoporosis when its signaling becomes excessive or unregulated16. To counteract this pathology, denosumab, a monoclonal antibody targeting RANKL, prevents osteoclast differentiation; however, discontinuation triggers rebound bone loss and elevated fracture risk17. HRT, although useful in treating postmenopausal osteoporosis, has been associated with increased risks of breast cancer and endometrial hyperplasia18,19. In addition, patients aged > 70 years experience an increase in cardiovascular problems and cerebral stroke while receiving HRT20. These adverse effects underscore the need for safer and more sustainable alternatives to mitigate systemic toxicity while maintaining bone health17,18.
An emerging report highlights the importance of the gut-bone axis in skeletal health, suggesting probiotics as a possible therapeutic pathway for osteoporosis prevention and treatment. Probiotics, defined as live microbes that offer health benefits when adequately administered, affect bone metabolism via multifaceted mechanisms21. Probiotics have been reported to enhance calcium absorption by modulating the gut microbiome, primarily by increasing the production of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate. Consequently, SCFAs lower intestinal pH, solubilizing dietary calcium for improved passive absorption, while butyrate directly suppresses osteoclast differentiation and reduces bone resorption by downregulating pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interleukin-1 beta22,23,24. Additionally, probiotics contribute to the maintenance of gut barrier integrity by preventing the translocation of bacterial endotoxins, which can exacerbate inflammation and accelerate bone loss25.
Probiotics of various strains have been explored to ameliorate osteoporosis and lower the incidence of osteoporosis-related fractures26,27,28,29,30. Among many bacterial strains, Limosilactobacillus reuteri (L. reuteri) has demonstrated robust osteoprotective properties in clinical and preclinical models31,32,33,34,35,36,37. For instance, L. reuteri supplementation in early postmenopausal women effectively maintained bone health over an observation period of more than 2 years31. In preclinical studies, L. reuteri supplementation in ovariectomized mice attenuated trabecular bone loss by 50%, modulated the gut microbiome, and suppressed osteoclastogenesis markers (e.g., RANKL and TRAP5), ultimately reducing bone resorption36,37.
Weissella cibaria (W. cibaria), a probiotic strain recognized for its anti-inflammatory properties, has shown potential in treating periodontal disease by suppressing pro-inflammatory cytokines such as TNF-α and IL-638,39. Emerging evidence suggests that W. cibaria supplementation reduces bone loss in preclinical models. For instance, it preserved alveolar bone density in mice with experimental periodontitis by modulating osteoclast activity40. Mechanistically, W. cibaria inhibits osteoclastogenesis by interfering with the binding of RANKL to its receptor activator of nuclear factor kappa-B (RANK), a critical pathway driving osteoclast differentiation and bone resorption38,39,40. Given its dual anti-inflammatory and anti-osteoclastogenic effects, W. cibaria is a promising therapeutic candidate for addressing bone loss in conditions such as postmenopausal osteoporosis, including ovariectomy (OVX)-induced animal models.
Despite these advances, the combined effects of L. reuteri and W. cibaria on osteoporosis remain unclear. We hypothesized that the synergistic anti-inflammatory and osteoprotective properties of these strains would enhance their efficacy in preventing bone loss. This study aimed to evaluate the anti-osteoporotic potential of a probiotic mixture containing L. reuteri MGE 3301 (LR) and W. cibaria MGE 3110 (WC) in OVX-induced osteoporotic rats, a well-established model for postmenopausal osteoporosis. By investigating the effects of this novel combination on bone microstructure, gene expression, and serum biomarkers, we sought to provide new insights into the therapeutic potential of probiotics in osteoporosis management.
Materials and methods
Probiotic strains
The probiotic formulations were prepared in our laboratory facilities. LR was isolated from feces of healthy infants and WC was isolated from kimchi in our lab. The probiotic strains were cultured in MRS (de Man, Rogosa and Sharpe) broth (BD, Sparks, MD, USA) overnight and centrifuged at 5000 × g for 10 min at 4℃. The cell pellets were washed with 0.1% peptone water (BD) and resuspended in 10% skim milk (BD). The cell suspensions were freeze-dried and viable cell counts were determined. For test sample preparation, the viable cell counts were adjusted to approximately 1.0 × 109 CFU/0.1 g in each microtube. The viability of probiotic strains was evaluated before and after freeze-drying using the plate count method on MRS agar. Following lyophilization, formulations were stored at 4 °C to maintain cell viability. Periodic viability assessments conducted over the study duration confirmed that bacterial counts remained stable, indicating retention of probiotic potency under the tested storage conditions.
Ethical statement
All animal experiments were conducted in strict accordance with institutional and national guidelines for the care and use of laboratory animals. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Soonchunhyang University Bucheon Hospital, Bucheon, South Korea (Approval No. SCHBCA2022-03; Approval Date: 2022-03-24). Efforts were made to minimize animal suffering, and all procedures were performed in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines to ensure transparency, reproducibility, and ethical rigor in our research. The ARRIVE checklist was used to design the study, conduct the experiments, and report the results, including details on the study design, sample size determination, randomization, blinding, and statistical analysis.
Animals and experimental design
Thirty-five Wistar rats (11 weeks of age and 230–250 g of body weight) were purchased and used in this study. The animals were acclimatized for at least 1 week to the new environment and housed with two or three rats per cage. The rats were kept in a room of controlled temperature (around 25 °C) and relative humidity (around 55%) along with 12-hour light/dark cycles. The animals were randomly assigned to five experimental groups using a random number generator to minimize selection bias as follows: (I) Sham group, (II) Untreated OVX group, (III) LR-treated OVX group (OVX/LR), (IV) WC-treated OVX group (OVX/WC), and (V) LR and WC mixture-treated OVX group (OVX/LR/WC). All experimental groups were provided ad libitum water access along with a pelleted diet (Purina Rodent Chow, Hana Biotech, Gyeonggi-do, South Korea) containing 20.0% crude protein, 4.5% crude fat, 6.0% crude fiber, 6.0% ash, and 63.1% carbohydrates, with vitamins and minerals formulated to AIN-93G specifications, including 1.2% calcium, 0.8% phosphorus, and 4.0 IU/kg vitamin D₃. The cylindrical pellets (19.33 × 14.29 mm, brown coloration, hardness: 15.48 kg/cm²) were free of prebiotics, antibiotics, or probiotics to eliminate confounding interactions. Full nutritional details are available on the manufacturer’s website (https://www.hanabiotech.com). Figure 1 presents a graphical representation of the experimental design used in this study.
Preparation of OVX-induced osteoporotic animal models
The rats were ovariectomized, except for the rats in the sham group, to induce osteoporosis and prepare an osteoporosis model similar to that of postmenopausal estrogen deficiency-induced osteoporosis. OVX was performed according to the method described in our previous study30,41. The experimental animals were anesthetized with 4% isoflurane in an induction chamber and maintained at 2% isoflurane using a vaporizer mask throughout the surgical procedure. The surgical duration ranged from 20 to 30 min.
Briefly, a 4 cm bilateral dorsal skin incision was made, and dissection was performed in the retroperitoneal region to expose the ovary surrounded by whitish fat tissue. Next, the blood vessel was carefully ligated, followed by cutting off the fallopian tube and uterine horn connection. Sham rats underwent an incision, but the ovaries were kept intact. A sham group was included to confirm that the observed effects in ovariectomized rats resulted from ovarian loss and subsequent estrogen deficiency rather than from surgical procedures or anesthesia. The incision was then sutured layer by layer including muscle with absorbable suture [Coated VICRYL™ (polyglactin 910); Ethicon, Bridgewater, NJ, USA] and skin with non-absorbable suture (Black Silk Braided 24 PKT; Ailee Co., Ltd., Seoul, South Korea). The rats received intramuscular analgesic (ketoprofen 10 mg/kg, Unibiotech Co., Ltd., Chungnam, South Korea) and antibiotic injections (enrofloxacin 5 mg/kg, Baytril; Korea Elanco Animal Pharmaceuticals Co., Ltd., Seoul, South Korea) for 3 consecutive days to manage pain and prevent postoperative infection. The incision site was treated with 10% povidone-iodine for a few days after surgery. Postoperative recovery was monitored daily for any signs of pain or distress, including mobility level, suture site condition, behavioral changes, or altered food and water intake. The animals were allowed for 4 weeks after OVX to recover from injury and develop osteoporosis before starting probiotic supplementation as described in our previous studies30,41. Body weights of the animals were recorded at regular intervals until euthanasia day.
Probiotics supplementation
From the first day of the fifth week after OVX, probiotic supplements were administered via oral gavage to the OVX/LR, OVX/WC, and OVX/LR/WC groups. The probiotic supplements were prepared by adding 1 mL normal saline to the probiotic-containing tube and vortexing to dissolve and mix homogenously.
To ensure stability in saline solution, preliminary tests confirmed that the probiotic strains retained viability for at least two hours at room temperature, covering the preparation-to-administration window. Probiotics were administered via oral gavage at a dose of 1 × 10 CFU/mL/day to rats in the supplementation groups (OVX/LR, OVX/WC, and OVX/LR/WC) for 16 weeks. Probiotic dosing was selected based on the literature, where probiotic mixtures were tested in OVX-induced osteoporosis rat models28,33. On the other hand, the rats from sham and untreated OVX groups received 1 mL normal saline daily throughout the experimental period. Normal saline (0.9% NaCl) was selected as the vehicle control because of its inert properties and physiological compatibility, ensuring that the treatment effects could be attributed solely to probiotic activity. As an isotonic solution mimicking the body fluid composition, it avoids interference with bone metabolism pathways, inflammatory responses, and gut microbiota modulation. The administered volume was matched to the probiotic suspension dose to standardize fluid intake across groups.
Animal euthanasia and experimental samples collection
The animals were euthanized after 20 weeks of the experimental period. Before euthanasia, the animals were anesthetized with 4% isoflurane in an induction chamber and complete sedation took approximately 3 min. Anesthesia was maintained with 2% isoflurane, and blood was collected via heart puncture using a 10 mL syringe. The animals were euthanized using a controlled inflow of CO₂ from a cylinder into an induction chamber, ensuring a displacement rate of 30–70% of the chamber volume per minute. The bone marrow was harvested from the femur using the phosphate-buffered saline (PBS) flush method. Briefly, the femur was carefully extracted, and the surrounding muscles and soft tissues were removed using sterile forceps and scissors. The bone marrow cavity was then exposed by cutting the ends of the femur at both epiphyses. A 3 mL syringe fitted with a 23G needle was used to flush the marrow cavity with sterile PBS. This flushing procedure was repeated three times to ensure complete collection of bone marrow. The harvested bone marrow was immediately centrifuged at 2000 revolutions per minute (RPM) for 5 min at 4 °C to obtain cell pellets, which were then stored at − 80 °C for further analysis. The tibias were extracted and fixed in 10% neutral formalin buffer at room temperature for at least 3 days. The fixed tibias were stored at 4 °C for future analyses, including micro-computed tomography (µ-CT) and histopathological observations.
µ-CT evaluation of the tibias
Formalin-fixed tibias were scanned using a high-resolution micro-CT scanner (Skyscan-1172; Skyscan NV, Kontich, Belgium). The NRecon application was utilized to reconstruct the scanned data, whereas DataViewer was employed to process 2D captures and datasets. Using the CTAn program, the datasets were analyzed to assess the proportion of bone volume in tissue volume (BV/TV; %), bone mineral density (BMD; mg/cm3), trabecular thickness (Tb.Th; mm), trabecular number (Tb.N; 1/mm), and trabecular separation (Tb.Sp; mm).
Histological observation
Following nondestructive µ-CT scanning, the tibias were histologically examined using hematoxylin and eosin (H&E) and Masson’s trichrome staining. In summary, the tibias were immersed in a 5% nitric acid solution for decalcification and checked regularly for flexibility, transparency, and bone penetrability using a sharp pin to assess the degree of calcification. The decalcified tissue samples were then dehydrated with an ethanol series (70–100%), followed by a dip in a xylene series to remove any leftover ethanol. The tissues were fixed in paraffin wax and sectioned at 3 μm using a microtome (RM2255 model; Leica Microsystems, Wetzlar, Germany). To evaluate the trabecular bone of the tibia, the processed sections were stained with H&E and Masson’s trichrome. The stained sections were mounted and micrographs were obtained using an optical microscope (Shandon Synthetic Mountant; Thermo Fisher Scientific, MA, USA).
RNA isolation and quantitative real-time polymerase chain reaction (RT-qPCR) analyses
RNA was extracted from the bone marrow cell pellets using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA yield was determined based on the absorbance at 260 nm using a Thermo Scientific NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The absorbance ratios at 260/280 and 260/230 nm were used to assess RNA quality. The resulting RNA was analyzed only when the absorbance ratios at A260/230 and A260/280 were between 1.8 and 2.0, with specific bands on gel electrophoresis26. The RNA was reverse transcribed into cDNA using the SuperScript™ III First-Strand Synthesis System kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RT-qPCR was carried out in a reaction mixture with 2 µL of cDNA in a total of 20 µL per reaction, using the 2x QuantiSpeedmSYBR Hi-Rox mix (PhileKorea, Seoul, Korea). Amplifications were run in a Thermal Cycler (StepOnePlus™ Real-Time PCR System, Thermo Fisher Scientific, Waltham, MA, USA) with the initial denaturation step at 95 °C for 2 min, followed by 40 cycles at 95 °C for 5 s, 58 °C for 10 s, and 72 °C for 20 s. The threshold cycle (CT value) was analyzed using the 2−ΔΔCT method, and the data were presented as fold changes. The forward and reverse primers sequences were purchased from Macrogen Co. (Seoul, South Korea) and the primers were diluted to a final concentration of 300 µL with nuclease-free water. The primer sequences are presented in Table 1. The relative expressions of genes including osteocalcin (OCN), RANKL, osteoprotegerin (OPG), TNF-α, and IL-6 were measured and all of the expression values of mRNA were normalized against the housekeeping Gapdh gene.
Serum biochemical assay
Blood samples were collected via cardiac puncture on the day of euthanasia. The blood was placed into conical tubes and the serum was obtained by centrifugation at 3000 RPM for 10 min at 4℃. OCN, type I collagen C-telopeptide (CTX-I), and calcium (Ca) levels were analyzed using a Rat Osteocalcin ELISA Kit (LS Bio, USA), Rat CTX-1 ELISA Kit (Novus Biologicals, USA), and Rat Calcium ELISA Kit (USA), respectively. All serum analyses were performed according to the manufacturer’s instructions. The serum parameters were evaluated using a microplate absorbance reader (Infinite M200 PRO; TECAN, Switzerland).
Statistical analyses
Prior to statistical testing, data distribution was assessed using the Shapiro-Wilk test. As the normality tests indicated non-normal distribution (p < 0.05) for key outcome variables, non-parametric tests were chosen for analysis. Continuous variables are shown as the median (25% percentile, 75% percentile). The Kruskal–Wallis test was used to compare continuous variables between groups, and Dunn’s procedure was applied for multiple comparisons. Adjustment of p-values for multiple comparisons was performed using Bonferroni’s method. A retrospective power analysis was performed using BMD as the primary outcome variable, confirming that the sample size (n = 7 per group) provided sufficient statistical power (> 80%) to detect significant differences at an α level of 0.05. Statistical analyses were performed by Rex (Version 3.0.3, RexSoft Inc., Seoul, South Korea). A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Body weight variation
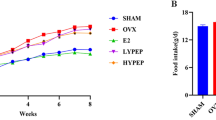
The mean body weight of the experimental animals on OVX day (Week 0), the starting day of probiotic supplementation (first day of week 5), and extraction day (Week 20) is displayed in Fig. 2, and it was observed that the probiotic treatment groups increased in body weight, but not significantly more than the sham group, during the testing period (p ˃ 0.05). However, the untreated OVX group showed substantially higher body weight than the sham group at the later phases of the experimental period including at the 20th week.
µ-CT data analysis
Figure 3 presents the µ-CT reconstructed imaging analysis of the tibias, demonstrating that OVX-induced bone loss was accelerated in the OVX-operated groups compared with that in the sham group, validating the successful execution of the OVX procedure. However, µ-CT revealed that the untreated OVX group experienced greater trabecular bone loss than the probiotic-supplemented OVX groups, particularly the probiotic mixture-treated group (OVX/LR/WC), suggesting that probiotic treatment exerts a beneficial effect on trabecular bone health. This finding is further supported by Fig. 4, which presents the trabecular bone parameters analyzed using µ-CT. The BMD, BV/TV, Tb.Th, and Tb.N showed improvement in the single-strain probiotic treatment groups (OVX/LR, OVX/WC); however, these differences were not statistically significant compared with those in the untreated OVX group (p > 0.05), except for BMD in the OVX/LR group. In contrast, the probiotic mixture group (OVX/LR/WC) exhibited significantly higher trabecular bone parameters than the untreated OVX group, with a 54.2% increase in BMD, 24.8% increase in BV/TV, 13.6% increase in Tb.Th, and 20% increase in Tb.N (p < 0.05), suggesting that probiotic mixture supplementation effectively counteracts osteoporotic bone loss. However, no statistically significant changes were observed in Tb.Sp among the OVX-operated groups, including those receiving probiotic supplementation (p > 0.05).
Trabecular bone parameters analyses. Trabecular bone elements of the tibias were assessed using µ-CT, and the results indicated that the OVX/LR/WC group had higher Tb.Th, Tb.N, BMD, and BV/TV than the bare OVX group. However, no significant variation of Tb.Sp was observed between them. *P < 0.05, **P < 0.01, and ns: no significant.
Histological observations
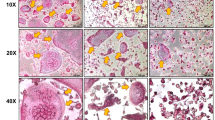
Consistent with the µ-CT reconstruction findings, histological analysis of tibial sections stained with H&E and Masson’s trichrome further demonstrated that the OVX and probiotic-supplemented OVX groups showed decreased bony trabeculae compared with the sham group. However, the probiotic mixture group (OVX/LR/WC) exhibited a relatively preserved trabecular structure compared with the bare OVX, OVX/LR, and OVX/WC groups, as shown in Fig. 5. Masson’s trichrome staining revealed a more intact collagen matrix in the probiotic mixture group (OVX/LR/WC), indicating improved bone integrity. Furthermore, H&E staining revealed a higher degree of adipose tissue infiltration in the bone marrow of the untreated OVX group, suggesting increased marrow adiposity associated with bone loss. These findings reinforce the protective effects of probiotic mixture supplementation in attenuating OVX-induced trabecular deterioration.
RT-qPCR analyses
Relative gene expression in the femoral bone marrow of rats after treatment with probiotic supplements is shown in Fig. 6. As expected, the mRNA expression levels of OCN, RANKL, TNF-α, and IL-6 were upregulated while the mRNA expression level of OPG was downregulated in the OVX-operated groups including probiotic-treated OVX groups compared with those in the sham group. Notably, treatment with the probiotic mixture significantly downregulated the expression of key osteoclastogenic and inflammatory genes compared to the untreated OVX group, with RANKL reduced by 1.35-fold, TNF-α by 2.5-fold, and IL-6 by 1.9-fold (p < 0.05). Moreover, the mRNA expression of OPG was significantly upregulated by 3.14-fold in the probiotic mixture group compared with that in the untreated OVX group (p < 0.05). These findings suggest that the probiotic mixture inhibited the osteoclastogenic and pro-inflammatory signaling pathways, thereby ameliorating OVX-induced osteoporosis in rats. The osteoblast formation marker OCN was significantly upregulated in bare OVX, OVX/LR, and OVX/WC group compared with that in the sham group ( < 0.05). In addition, no difference was noted among the OVX groups including the bare OVX and probiotic treatment groups. More interestingly, no significant difference was observed in the mRNA expression of these analyzed genes between the sham and probiotic mixture treatment groups (p > 0.05). Therefore, the relative mRNA expressions of the analyzed genes were comparable between the probiotic mixture and sham-operated groups, suggesting that the probiotic mixture treatment had bone protective effects in OVX-induced osteoporosis animal models.
Effect of the probiotic mixture (LR/WC) on mRNA expression levels of osteoclastogenesis and inflammation-related markers in femoral bone marrows of the ovariectomized rats. Relative expression of (a) osteocalcin (OCN), (b) receptor activator of nuclear factor kappa-Β ligand (RANKL), (c) osteoprotegerin (OPG), (d) tumor necrosis factor alpha (TNF-α), and (e) interleukin 6 (IL-6) in experimental groups. Results are expressed as mean ± SD. *P < 0.05, **P < 0.01, and #P < 0.0001.
Serum biochemical assay
Figure 7 graphically presents the levels of serum bone markers, including OCN, CTX-I, and Ca. The serum OCN level was significantly higher in the bare OVX group than in the sham group (p < 0.05). The probiotic-supplemented OVX groups, especially the probiotic mixture group (OVX/LR/WC), showed lower OCN levels than the OVX group, but the difference did not reach statistically significance in the investigation (p > 0.05). Treatment with the probiotic mixture significantly reduced the CTX-I level by 38.9%, a key marker of bone resorption, compared to the untreated OVX group (p < 0.05). This suggests that supplementation with the probiotic mixture effectively decreased bone resorption in OVX-induced osteoporotic rats. Moreover, calcium content analysis showed that the probiotic mixture-treated group had a significantly higher Ca level (30.8% increase) than the untreated OVX group (p < 0.05), indicating improved mineral homeostasis.
Serum biochemical analysis. Serum bone markers for osteoporosis revealed that the OVX/LR/WC group had significantly lower level of CTX-I than the bare OVX group, indicating recued bone resorption. The OVX/LR/WC group also showed lower level of osteocalcin (OC) than the bare OVX group, but the difference did not reach statistically significant in this study. Calcium (Ca) levels were significantly higher in the OVX/LR/WC group compared to the bare OVX group. *P < 0.05 and **P < 0.01.
Discussion
Osteoporosis is a prevalent skeletal disorder in postmenopausal women primarily caused by a significant decline in estrogen levels. This hormonal deficiency disrupts bone remodeling by stimulating osteoclast-mediated bone resorption and suppressing osteoblast-driven bone formation, leading to reduced BMD, microarchitectural deterioration, and an increased risk of fragility fractures42,43. Moreover, osteoporosis may occur during aging via a reduction in osteoblast progenitor cells in the bone marrow44. OVX rat models closely mimic the postmenopausal status, wherein estrogen levels are dramatically decreased, and as a result, bone fragility increases because of increased bone resorption45. Therefore, OVX rats are widely used in preclinical research on postmenopausal osteoporotic bone loss and treatment. In this study, we established an OVX-induced osteoporosis rat model and administered probiotic formulations for 16 weeks. We evaluated the potential effects of probiotics on bone health by assessing key parameters, including bone mineral density, bone microarchitecture, and biochemical markers of bone turnover. Our findings revealed that probiotic supplementation exerted a protective influence on bone metabolism by reducing bone resorption while supporting the balance of the bone remodeling process.
Several studies have demonstrated that probiotics have beneficial effects on bone health and are advantageous over conventional drugs or treatments because of their safety and absence of side effects. For example, multiple probiotic strains have been reported to inhibit bone loss in OVX-induced osteoporotic rats26,28,29,30,41,45. The mechanism by which probiotics improve bone health and inhibit bone loss is not yet clearly understood; however, several studies have hypothesized that multiple compounds secreted by probiotics affect various pathways in the host46. Probiotics have been reported to secrete multiple vitamins and enzymes, including vitamins D, vitamin K, and folate, which are essential for bone growth and matrix production47. In addition, probiotics may produce SCFAs that can reduce the intestinal tract pH and increase the absorption of minerals48. In brief, growth factors, vitamins, and enzymes secreted from probiotics interact with the intestinal epithelial barrier along with the lamina propria. The secreted factors then target antigen-presenting cells such as dendritic cells in the lamina propria, modulate their immune response, and reduce pro-inflammatory cytokines, leading to the uptake of minerals from the intestinal lumen. Secreted factors can then pass into the bloodstream and be transported to the bone, where they modulate bone and immune cells. This could reduce the expression of pro-inflammatory and pro-osteoclastogenic cytokines and oxidative stress while enhancing mineral apposition and Wnt10b expression. This modulation results in reduced osteoclast formation, subsequently leading to increased bone46. In the present study, we assessed the anti-osteoporotic effects of a probiotic mixture containing LR and WC strains via supplementation in OVX rats for 16 weeks. Based on serum biochemical analysis, gene expression by RT-qPCR, µ-CT, and histological findings, the probiotic mixture prevented bone loss by decreasing bone resorption and maintaining trabecular bone parameters better than in OVX control rats.
During the postmenopausal period, body weight and fat tissue typically increase owing to estrogen deficiency, which accelerates inflammation in the muscles45. Similarly, the body weight of OVX animals also increases as they consume more food than sham-operated or healthy animals. In this study, the mean body weight significantly increased in the OVX group compared with that in the sham-operated group at the later stages of the experimental period. However, despite having increased body weight, the probiotic-supplemented groups did not differ significantly from the sham group (Fig. 2).
Typically, substantial trabecular bone loss occurs in OVX-induced osteoporotic animals models, and µ-CT imaging effectively detects changes in trabecular microarchitecture30. µ-CT scanning of the tibias, as shown in Fig. 3, demonstrated that the untreated OVX group exhibited significant loss and weak trabecular bone structure, whereas the sham-operated group had normal bony trabecular structure. In our study, probiotic supplementation, particularly in the OVX/LR/WC group, significantly preserved trabecular bone, consistent with previous studies reporting the beneficial effects of various probiotic strains on bone health in ovariectomized rodent models20,29,30,41,49,50,51. However, our findings provided distinct insights compared towith those of earlier studies. For examplesexample, Yu et al.20 supplemented Lactobacillus brevis AR281 to OVX mice at a dose of 1 × 109 CFU/mL/day for 7 weeks. The authors demonstrated that probiotic supplementation reduced osteoclast differentiation by analyzing bone markers, gene expression, and trabecular microarchitecture, which is consistent with our dosing and findings. However, the aforementioned study investigated short-term effects, whereas our study evaluated the effects of 16 weeks of supplementation. Additionally, our study started probiotic supplementation 4 weeks postoperatively, which is crucial for analyzing the effects on the well-developed osteoporotic condition. Moreover, our study further strengthens this evidence by incorporating serum biochemical analysis of the bone formation marker OCN and the bone resorption marker CTX-I, along with comprehensive histopathological evaluation. These additional assessments enhance the novelty of our study by demonstrating the positive impact of the probiotic mixture on osteoporosis. Similarly, Lee et al.29 reported that Lactobacillus gasseri BNR17 improved postmenopausal symptoms in OVX rats after supplementation for 14 weeks at a dose of 1 × 1010 CFU twice per day, but they did not focus on detailed trabecular microstructural parameters. In contrast, even with a smaller dose and once a day, our study supports the beneficial role of the probiotic mixture in postmenopausal osteoporosis by demonstrating significant increases in BV/TV, BMD, Tb.Th, and Tb.N, and decreased Tb.Sp (Fig. 4), as previously observed with Lactiplantibacillus plantarum supplementation30,41. We focused on the trabecular bone parameters because of their functionality in the osteoporotic condition. BMD is a common biomarker for osteoporosis examination, and a relatively low BMD value leads to osteopenia and ultimately, osteoporosis52. In addition, the mechanical features of cancellous bone and its trabecular microstructure can be evaluated using another parameter, BV/TV53. All OVX groups had lower BMD and BV/TV than the sham group; however, the probiotic mix OVX/LR/WC group exhibited significantly greater BMD and BV/TV than the bare OVX group (Fig. 4a and b), conveying that probiotic mixture-treated rats had a stronger bone structure. When other trabecular bone indicators, including Tb.Th, Tb.N, and Tb.Sp, were analyzed, the OVX/LR/WC group outperformed the bare OVX group in terms of Tb.Th and Tb.N, but there was no difference in Tb.Sp among the OVX groups (Fig. 4). Taken together, \(\:{\upmu\:}\)-CT analysis suggested that the probiotic mixture maintained trabecular bone parameters and prevented OVX-induced osteoporotic bone loss.
Osteoclastogenesis and pro-inflammatory markers including RANKL, OPG, TNF-α, and IL-6 are closely linked to postmenopausal osteoporosis and bone loss. For example, RANKL is a key marker of osteoclastogenesis that binds to its receptor RANK, which is expressed on the surface of myeloid and dendritic cells. This interaction plays a crucial role in osteoclast activation, proliferation, and differentiation, ultimately enhancing the osteoclastic activity16,45. Moreover, RANKL may induce bone inflammation by increasing the inflammatory cytokine TNF-α, leading to osteoclastogenesis and subsequently bone resorption and bone loss. Thus, in patients with osteoporosis, increased inflammation triggers osteoclast activation in the bones, leading to bone resorption54. Therefore, downregulating the gene expression of inflammatory markers may be an effective method to ameliorate postmenopausal osteoporosis45. Another osteoclast activity regulator and bone metabolism marker is OPG, which exhibits anti-osteoclastic activity. OPG competes with RANK to bind to RANKL and thereby limit its activity55,56. Prior studies have shown that probiotic effects on bone loss are microbiota-dependent49 and and probiotics are able to alter osteoclastogenesis and pro-inflammatory cytokine-related markers, and our findings support this concept by showing that probiotic supplementation upregulated the anti-osteoclastogenic marker OPG, downregulated osteoclastogenesis-related genes (RANKL) and inflammatory cytokines (TNF-α and IL-6), and modulated bone turnover markers (Figs. 6 and 7). In addition, histological analyses (H&E and Masson’s trichrome staining) further confirmed that the trabecular bone was better preserved in OVX/LR/WC rats than in the OVX control group (Fig. 5). This suggests that LR/WC supplementation effectively attenuates osteoporosis-related bone loss, potentially through gut microbiota modulation and reduced inflammatory signaling, reinforcing the growing evidence supporting the use of probiotics as adjunctive therapies for osteoporosis.
Another bone biomarker, OCN, the most abundant non-collagenous protein, is present in the bone and is released by mature osteoblastic cells, indicating its bone-forming potential57. In this study, OCN gene expression did not vary significantly across the OVX-operated groups, but there was a significant difference between the sham and OVX-operated groups, except for the OVX/LR/WC group, indicating the protective effect of the probiotic mixture against bone loss. In the present study, RT-qPCR demonstrated that the tested probiotic mixture inhibited osteoclastic activity, indicating decreased bone resorption and loss in ovariectomized rats. Therefore, we believe that the probiotic mixture has a beneficial effect on OVX-induced osteoporotic rats.
Serum bone turnover markers, especially CTX and OCN, are crucial for characterizing bone resorption and formation features, respectively58. Studies of probiotic strains administered to OVX-induced osteoporotic animals have revealed that probiotic-fed animals show a significant variance in serum biochemical markers. Our previous studies demonstrated that Lactiplantibacillus plantarum probiotics decreased the levels of CTX-I and OCN in the blood compared with those in bare OVX rats30,41. Cegieła et al.43 studied the Lacticaseibacillus rhamnosus strain with or without azithromycin in OVX rats and revealed that serum markers, such as CTX-I and Ca levels, did not differ compared with those in the OVX control. However, in rats treated with probiotics or drugs, bone formation was enhanced by increasing serum OCN levels. In the present study, the OVX/LR/WC probiotic mixture group showed significantly lower levels of CTX-I than the bare OVX group, indicating that treatment with the probiotic mixture reduced bone resorption (Fig. 7b). However, no significant differences were observed between the single probiotic groups (OVX/LR and OVX/WC) and the bare OVX group in terms of OCN and CTX-I levels (Fig. 7a and b). In addition, the OVX/LR/WC group had lower levels of OCN than the bare OVX group; however, this was not statistically significant. In addition, serum and urinary Ca levels are associated with osteoporosis, and both have been reported to be significantly lower in osteoporotic individuals than in the non-osteoporotic group6,59. The negative association between blood calcium and osteoporosis in postmenopausal women may stem from estrogen deficiency triggering bone loss and immune changes causing chronic inflammation, alongside aging-related hormonal imbalances, including elevated parathyroid hormone levels and reduced vitamin D metabolites that disrupt calcium regulation and bone health60,61. In this study, we evaluated serum Ca levels and found that the OVX/LR/WC probiotic mixture group exhibited higher serum Ca levels than the untreated OVX group, despite all groups receiving the same diet (Fig. 7c). These findings suggest that probiotic mixtures may enhance calcium absorption in the gut and modulate the gut microbiome, ultimately improving calcium solubility.
Despite these promising outcomes, this study had some limitations. Depending on the sample size used in the study, there may be concerns regarding the statistical power to detect smaller yet clinically significant differences between the treatment groups. Larger multicenter studies with more animals per group could strengthen the findings and ensure that the observed effects are not due to random variation. This report lacks long-term efficacy data; while the current study shows promising results regarding the short-term effects of the probiotic mixture (LR/WC) on bone health in OVX rat models, the long-term impact of the probiotic mixture on bone mass, bone quality, and fracture risk remains unexplored. Another notable limitation of this study is the exclusion of fecal sample analysis following the administration of the probiotic mixture to analyze the gut microbiome. Although the observed effects on bone health suggest a potential link between probiotics and bone metabolism, the specific changes in the gut microbiome that may have contributed to these outcomes were not assessed.
Additionally, this study has limited clinical translation; it is based on an animal model of postmenopausal osteoporosis, which is based on the female sex. Therefore, it may not fully represent the complexity of osteoporotic conditions in humans, particularly in males. These findings need to be confirmed in clinical trials before any conclusions can be drawn regarding the efficacy of the probiotic mixture in the treatment of human osteoporosis. Moreover, there is a potential lack of mechanistic insight. While this study identified significant improvements in bone health, the exact mechanisms underlying the action of the probiotic mixture on bone metabolism, particularly via the gut-bone axis, have not been fully elucidated.
Based on the promising findings of this study, we suggest several important directions for future research. First, human clinical trials are required to validate the effectiveness of the probiotic mixture focusing on the safety, optimal dosage, and long-term efficacy of probiotics in managing bone health. Second, investigating the potential synergy between probiotics and other therapeutic treatments such as dietary supplements including calcium and vitamin D, and pharmacological treatments including bisphosphonates, could provide insights into how probiotics may enhance or complement existing osteoporosis therapies. Finally, further studies are needed to elucidate the specific molecular pathways through which these probiotics exert their effects on bone metabolism, particularly regarding immune modulation, gut-bone axis interactions, and osteoclast activity. Understanding the underlying mechanisms is crucial for the development of targeted probiotic therapies for osteoporosis.
Conclusion
This study demonstrated that the probiotic mixture (a combination of LR and WC) effectively attenuated bone loss in OVX-induced osteoporotic rats by preserving the bony trabeculae and suppressing osteoclastogenic and inflammatory biomarkers. These findings highlight the advantages of the probiotic mixture over single-strain treatment as a novel approach for osteoporosis management. However, limitations, such as small sample size, reliance on an animal model, and lack of mechanistic clarity, must be addressed. Future studies should define strain-specific roles, dose-response relationships, and molecular pathways to validate the translational potential. If validated in clinical trials, this probiotic blend can be developed as a dietary supplement or adjuvant therapy, offering a gut-targeted approach to enhance osteoporosis treatment with fewer side effects.
Data availability
All data needed to support the findings of this study are available within the manuscript.
References
Wehrli, F. W., Song, H. K., Saha, P. K. & Wright, A. C. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 19, 731–764. https://doi.org/10.1002/nbm.1066 (2006).
Coughlan, T. & Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. (Lond). 14, 187–191. https://doi.org/10.7861/clinmedicine.14-2-187 (2014).
Cheng, C. H., Chen, L. R. & Chen, K. H. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23031376 (2022).
Siew, K. F. S. & Satku, M. The prevalence of osteoporosis in patients older than 50 years with distal radius fractures in an institutional registry of 2,572 patients in Singapore. J. Hand Surg. Asian Pac. Vol. 27, 130–134. https://doi.org/10.1142/s2424835522500023 (2022).
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the united States, 2005–2025. J. Bone Min. Res. 22, 465–475. https://doi.org/10.1359/jbmr.061113 (2007).
Tang, G. et al. Low BMI, blood calcium and vitamin D, kyphosis time, and outdoor activity time are independent risk factors for osteoporosis in postmenopausal women. Front. Endocrinol. 14, 1154927. https://doi.org/10.3389/fendo.2023.1154927 (2023).
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733. https://doi.org/10.1007/s00198-006-0172-4 (2006).
Van Eck, C. F. et al. Morbidity, mortality and cost of osteoporotic fractures—should proximal humerus fractures be taken as seriously as hip fractures? Ann. Jt. 4 https://doi.org/10.21037/aoj.2019.01.01 (2019).
Nazrun, A. S., Tzar, M. N., Mokhtar, S. A. & Mohamed, I. N. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Ther. Clin. Risk Manage. 10, 937–948. https://doi.org/10.2147/tcrm.S72456 (2014).
Management of osteoporosis. In postmenopausal women: the 2021 position statement of the North American menopause society. Menopause (New York N Y). 28, 973–997. https://doi.org/10.1097/gme.0000000000001831 (2021).
Kanis, J. A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group. Osteoporos. Int. 4, 368–381. https://doi.org/10.1007/bf01622200 (1994).
Kobayakawa, T. et al. Denosumab versus Romosozumab for postmenopausal osteoporosis treatment. Sci. Rep. 11, 11801. https://doi.org/10.1038/s41598-021-91248-6 (2021).
Harahap, I. A. & Suliburska, J. Probiotics and isoflavones as a promising therapeutic for calcium status and bone health: A narrative review. Foods (Basel). 10, 2685. https://doi.org/10.3390/foods10112685 (2021).
Harahap, I. A. (ed Suliburska, J.) Can probiotics decrease the risk of postmenopausal osteoporosis in women? PharmaNutrition 24 100336 https://doi.org/10.1016/j.phanu.2023.100336 (2023).
Harahap, I. A., Schmidt, M., Pruszyńska-Oszmałek, E., Sassek, M. & Suliburska, J. Impact of Lactobacillus acidophilus and its combination with isoflavone products on calcium status, calcium transporters, and bone metabolism biomarkers in a post-menopausal osteoporotic rat model. Nutrients 16, 2524 (2024).
Hofbauer, L. C. & Heufelder, A. E. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 79, 243–253. https://doi.org/10.1007/s001090100226 (2001).
Harahap, I. A. et al. Effects of daily probiotic supplementation with Lactobacillus acidophilus on calcium status, bone metabolism biomarkers, and bone mineral density in postmenopausal women: a controlled and randomized clinical study. Front. Nutr. 11, 1401920. https://doi.org/10.3389/fnut.2024.1401920 (2024).
Harahap, I. A., Kuligowski, M., Schmidt, M., Kołodziejski, P. A. & Suliburska, J. Effects of isoflavone and probiotic intake on calcium transport and bone metabolism biomarkers in female rats. Food Sci. Nutr. 11, 6324–6335. https://doi.org/10.1002/fsn3.3571 (2023).
Simin, J., Tamimi, R., Lagergren, J., Adami, H. O. & Brusselaers, N. J. e. J. O. C. Menopausal hormone therapy and cancer risk: an O.erestimated risk? Eur. J. Cancer. 84, 60–68. https://doi.org/10.1016/j.ejca.2017.07.012 (2017).
Yu, J. et al. Anti-osteoporotic effect of Lactobacillus brevis AR281 in an ovariectomized mouse model mediated by Inhibition of osteoclast differentiation. Biology (Basel). 11 https://doi.org/10.3390/biology11030359 (2022).
McCabe, L., Britton, R. A. & Parameswaran, N. Prebiotic and probiotic regulation of bone health: role of the intestine and its Microbiome. Curr. Osteoporos. Rep. 13, 363–371. https://doi.org/10.1007/s11914-015-0292-x (2015).
Wallimann, A. et al. Butyrate inhibits osteoclast activity in vitro and regulates systemic inflammation and bone healing in a murine osteotomy model compared to antibiotic-treated mice. Mediators Inflamm. 2021, 8817421. https://doi.org/10.1155/2021/8817421 (2021).
Li, M. et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 831, 52–59. https://doi.org/10.1016/j.ejphar.2018.05.003 (2018).
Borrelli, L. et al. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci. Rep. 6, 30046. https://doi.org/10.1038/srep30046 (2016).
Bron, P. A. et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 117, 93–107. https://doi.org/10.1017/s0007114516004037 (2017).
Parvaneh, K. et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and BMP-2 genes in rats with bone loss resulting from ovariectomy. Biomed. Res. Int. 2015, 897639. https://doi.org/10.1155/2015/897639 (2015).
Ohlsson, C. et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 9, e92368. https://doi.org/10.1371/journal.pone.0092368 (2014).
Montazeri-Najafabady, N. et al. Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics Antimicrob. Proteins. 11, 1145–1154. https://doi.org/10.1007/s12602-018-9443-6 (2019).
Lee, S. et al. The effect of Lactobacillus gasseri BNR17 on postmenopausal symptoms in ovariectomized rats. J. Microbiol. Biotechnol. 31, 1281–1287. https://doi.org/10.4014/jmb.2105.05032 (2021).
Jin, E. S. et al. Preliminary study on effect of Lactiplantibacillus plantarum on osteoporosis in the ovariectomized rat. Food Sci. Anim. Resour. 43, 712–720. https://doi.org/10.5851/kosfa.2023.e29 (2023).
Gregori, G. et al. Limosilactobacillus reuteri 6475 and prevention of early postmenopausal bone loss: a randomized clinical trial. JAMA Netw. Open. 7, e2415455–e2415455, (2024). https://doi.org/10.1001/jamanetworkopen.2024.15455 JAMA Network Open.
Abuqwider, J., Altamimi, M. & Mauriello, G. Limosilactobacillus reuteri in health and disease. Microorganisms 10, 522. https://doi.org/10.3390/microorganisms10030522 (2022).
Gholami, A. et al. The ameliorative role of specific probiotic combinations on bone loss in the ovariectomized rat model. BMC Complement. Med. Ther. 22, 241. https://doi.org/10.1186/s12906-022-03713-y (2022).
Gregori, G. et al. Prevention of glucocorticoid-induced impairment of bone metabolism – a randomized, placebo-controlled, single centre proof-of-concept clinical trial. JBMR Plus. https://doi.org/10.1093/jbmrpl/ziaf031 (2025).
Yun, T. J., Kim, Y., Lee, J. J., Park, J. Y. & Kim, J. H. Protective effects of probiotics against menopausal symptoms in ovariectomized mice. Food Biosci. 61, 104611. https://doi.org/10.1016/j.fbio.2024.104611 (2024).
Ribeiro, J. L. et al. Heat-killed Limosilactobacillus reuteri ATCC PTA 6475 prevents bone loss in ovariectomized mice: A preliminary study. PLoS One. 19, e0304358. https://doi.org/10.1371/journal.pone.0304358 (2024).
Britton, R. A. et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 229, 1822–1830. https://doi.org/10.1002/jcp.24636 (2014).
Kim, M. J., You, Y. O., Kang, J. Y., Kim, H. J. & Kang, M. S. Weissella cibaria CMU exerts an anti–inflammatory effect by inhibiting Aggregatibacter actinomycetemcomitans–induced NF–κB activation in macrophages. Mol. Med. Rep. 22, 4143–4150. https://doi.org/10.3892/mmr.2020.11512 (2020).
Park, G. Y., Park, J. A. & Kang, M. S. In vitro effects of Weissella cibaria CMU and CMS1 on receptor activator of NF-κB ligand (RANKL)-induced osteoclast differentiation. J. Funct. Biomater. 15. https://doi.org/10.3390/jfb15030065 (2024).
Kim, J. W. et al. Effect of Weissella cibaria on the reduction of periodontal tissue destruction in mice. J. Periodontol. 91, 1367–1374. https://doi.org/10.1002/jper.19-0288 (2020).
Jin, E. S. et al. The effect of genetically modified Lactobacillus plantarum carrying bone morphogenetic protein 2 gene on an ovariectomized rat. J. Korean Neurosurg. Soc. 65, 204–214. https://doi.org/10.3340/jkns.2021.0098 (2022).
Myeong, J. Y. et al. Protective effects of the postbiotic Lactobacillus plantarum MD35 on bone loss in an ovariectomized mice model. Probiotics Antimicrob. Proteins. 16, 541–551. https://doi.org/10.1007/s12602-023-10065-7 (2024).
Cegieła, U., Londzin, P., Janas, A., Pytlik, M. & Folwarczna, J. Effect of administration of Azithromycin and/or probiotic bacteria on bones of estrogen-deficient rats. Pharmaceuticals 15. https://doi.org/10.3390/ph15080915 (2022).
Demontiero, O., Vidal, C. & Duque, G. Aging and bone loss: new insights for the clinician. Ther. Adv. Musculoskelet. Dis. 4, 61–76. https://doi.org/10.1177/1759720x11430858 (2012).
Lee, C. S. et al. Lactobacillus-fermented milk products attenuate bone loss in an experimental rat model of ovariectomy-induced post-menopausal primary osteoporosis. J. Appl. Microbiol. 130, 2041–2062. https://doi.org/10.1111/jam.14852 (2021).
Collins, F. L., Rios-Arce, N. D., Schepper, J. D., Parameswaran, N. & McCabe, L. R. The potential of probiotics as a therapy for osteoporosis. Microbiol. Spectr. 5. https://doi.org/10.1128/microbiolspec.BAD-0015-2016 (2017).
Crittenden, R. G., Martinez, N. R. & Playne, M. J. Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. Int. J. Food Microbiol. 80, 217–222. https://doi.org/10.1016/s0168-1605(02)00170-8 (2003).
Campbell, J. M., Fahey, G. C. Jr. & Wolf, B. W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 127, 130–136. https://doi.org/10.1093/jn/127.1.130 (1997).
Li, J. Y. et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 126, 2049–2063. https://doi.org/10.1172/jci86062 (2016).
Sapra, L. et al. Bifidobacterium longum ameliorates ovariectomy-induced bone loss via enhancing anti-osteoclastogenic and Immunomodulatory potential of regulatory B cells (bregs). Front. Immunol. 13, 875788. https://doi.org/10.3389/fimmu.2022.875788 (2022).
Yang, L. C. et al. Lactobacillus plantarum GKM3 and Lactobacillus paracasei GKS6 supplementation ameliorates bone loss in ovariectomized mice by promoting osteoblast differentiation and inhibiting osteoclast formation. Nutrients 12. https://doi.org/10.3390/nu12071914 (2020).
Zhang, C., Yu, H., Miao, Y. & Wei, B. Causal relationship between osteoporosis, bone mineral density, and osteonecrosis: A bidirectional two-sample Mendelian randomization study. J. Transl Med. 23, 226. https://doi.org/10.1186/s12967-024-06030-9 (2025).
Nazarian, A., von Stechow, D., Zurakowski, D., Müller, R. & Snyder, B. D. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif Tissue Int. 83, 368–379. https://doi.org/10.1007/s00223-008-9174-x (2008).
Yatsonsky Ii, D., Pan, K., Shendge, V. B., Liu, J. & Ebraheim, N. A. Linkage of microbiota and osteoporosis: A mini literature review. World J. Orthop. 10, 123–127. https://doi.org/10.5312/wjo.v10.i3.123 (2019).
de Moraes, M., de Lucena, H. F., de Azevedo, P. R. & Queiroz, L. M. Costa Ade, L. Comparative immunohistochemical expression of RANK, RANKL and OPG in radicular and dentigerous cysts. Arch. Oral Biol. 56, 1256–1263. https://doi.org/10.1016/j.archoralbio.2011.05.009 (2011).
Liao, F. et al. Human osteoprotegerin inhibits osteoclasts and promotes hydroxyapatite to repair the mandibular defects in ovariectomized rats. Hua Xi Kou Qiang Yi Xue Za zhi = huaxi Kouqiang Yixue Zazhi = West. China J. Stomatology. 36, 367–371. https://doi.org/10.7518/hxkq.2018.04.004 (2018).
Brennan-Speranza, T. C. & Conigrave, A. D. Osteocalcin: an osteoblast-derived polypeptide hormone that modulates whole body energy metabolism. Calcif Tissue Int. 96, 1–10. https://doi.org/10.1007/s00223-014-9931-y (2015).
Garnero, P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol. Diagn. Ther. 12, 157–170. https://doi.org/10.1007/bf03256280 (2008).
Reid, I. R. & Bristow, S. M. Calcium and bone. Handb. Exp. Pharmacol. 262, 259–280. https://doi.org/10.1007/164_2019_324 (2020).
Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell. Dev. Biol. 123, 14–21. https://doi.org/10.1016/j.semcdb.2021.05.014 (2022).
López-Baena, M. T. et al. menopause, and aging: quo vadis? Climacteric 23, 123–129. https://doi.org/10.1080/13697137.2019.1682543 (2020).
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R1A6A1A03046418 and NRF-2020R1F1A1069896) and was supported by the Soonchunhyang University Research Fund.
Author information
Authors and Affiliations
Contributions
M.H. contributed to conception, design and methodology, data acquisition, analysis, interpretation, and wrote and edited the manuscript; T.S. investigated the study and assisted in animal experiments and data analysis. J.E.M. performed statistical analyses and interpreted data; J.H.J. and G.S.M. contributed to conception, design and methodology, funding acquisition, validation, edited the manuscript and supervised the study. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conformed to the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hossain, M., Sultana, T., Moon, J.E. et al. Anti-osteoporotic potential of a probiotic mixture containing Limosilactobacillus reuteri and Weissella cibaria in ovariectomized rats. Sci Rep 15, 18586 (2025). https://doi.org/10.1038/s41598-025-02089-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02089-6