Abstract
This study aimed to quantify tumor volume reduction during radiotherapy for abdominal lymph node metastases (LNM) of hepatocellular carcinoma (HCC) and assess the potential benefits of late-course adaptive radiotherapy. Forty HCC Patients with abdominal LNM treated from January 2021 to March 2024 received radiotherapy in combination with targeted therapy or immunotherapy. Second simulation scan was performed during the fourth week of radiotherapy. Two-thirds of patients underwent redesigned radiotherapy plan as the test group, while remaining patients continued with the original plan as the control group. Dose-volume metrics for organs at risk and toxicities were compared. At the time of second scan, median radiotherapy dose administered to all patients was 38.0 Gy. Mean tumor volume reduction was 56.20cc (95% CI 35.20-77.21cc) and 29.65% (95% CI 24.49%-34.80%). Irradiation dose and volume to stomach, small intestine, colon, liver, and kidneys were significantly reduced in test group. Meanwhile, gastrointestinal toxicities were notablely decreased, including dyspepsia (P = 0.018), nausea (P = 0.013), vomiting (P = 0.041), and diarrhea (P = 0.040). Tumor regression during radiotherapy for abdominal LNM of HCC is significant. A second simulation scan performing when the irradiation dose reaches approximately 40 Gy and applying adaptive radiotherapy in the late course can enhance irradiation accuracy and reduce toxicities.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent tumors that seriously threaten human health1. China bears a disproportionately high burden of HCC, accounting for approximately half of global cases2. Over 80% of patients are diagnosed at an advanced stage, and approximately half of these have lymph node metastasis (LNM)3. Autopsy results indicate that LNM occurs in 26–37% of advanced liver cancer4. The prognosis for advanced HCC remains poor, with a median survival of less than one year. Therefore, finding new treatment methods is particularly important for advanced HCC. With advancements in radiotherapy equipment and technology, the effectiveness of radiotherapy for HCC has improved continuously. In recent years, radiotherapy has become one of the important treatment modalities for HCC5,6. There have been reports on the effectiveness and safety of radiotherapy for LNM of HCC7,8. Radiotherapy can alleviate symptoms such as pain, obstruction, compression, or bleeding, and control tumor progression, thereby improving the quality of life.
Radiotherapy planning is typically based on CT simulation scan images obtained before radiotherapy. However, with the effective implementation of radiotherapy, tumors may shrink to varying degrees and change position, resulting in changes in the dose distribution of the initial radiotherapy plan9. Therefore, quantifying changes in tumor volume from baseline to the late-course of radiotherapy and assessing whether to redesign the radiotherapy plan to improve efficacy and reduce side effects are the focus of clinical attention.
In this study, patients with abdominal LNM of HCC underwent radiotherapy were included to explore the degree of tumor volume regression during radiotherapy. The aim is to optimize radiotherapy plan during late-course radiotherapy, and discuss the potential benefit of late-course adaptive plans compared to the original plans by dose-volume relationship comparisons, providing clinical evidence for late-course adaptive radiotherapy in HCC.
Methods
Patients
Patients with abdominal LNM of HCC treated at our hospital from January 2021 to March 2024 were included. Inclusion criteria: (1) Age ≥ 18 years, male or non-pregnant, non-lactating female. (2) Performance status score of 0–2. (3) HCC diagnosed clinically or pathologically according to the Chinese National Health Commission guidelines for primary liver cancer2. (4) LNM was pathologically proven or clinically detected via radiological findings: short axis diameter of contrast-enhanced lymph node ≥ 1 cm; lymph node capsule invasion; or clustered lymph nodes fused in the lymphatic drainage area. (5) Completion of radiotherapy for LNM according to the treatment plan. Exclusion criteria: (1) History of previous radiotherapy at the same site. (2) Prior interventions such as ablation for the same LNM. (3) Incomplete pre-treatment and follow-up data. Initially, 48 patients with abdominal LNM of HCC were identified as potential candidates. Eight patients did not meet inclusion and exclusion criteria. Ultimately, 40 patients were enrolled in this study. Informed consent was obtained from all participants included in the study.
All patients underwent comprehensive pre-treatment examinations. Within one week before radiotherapy, they completed medical history collection, physical examination, performance status score, Child-Pugh score, laboratory tests. Imaging exams were conducted within one month before radiotherapy to confirm the diagnosis of HCC with LNM.
Treatment
All patients underwent initial enhanced CT (Siemens, Germany) simulation scan (Scan1) with a slice thickness of 5 mm. The scanned images were imported into the treatment planning system (Monaco, V5.11, Elekta, Sweden). The gross tumor volume (GTV) was defined on the enhanced CT as the visible metastatic lymph nodes. The clinical target volume (CTV) was delineated by expanding the GTV outward by 2–5 mm10, with appropriate adjustments based on the position of surrounding organs at risk (OARs), and no irradiation of lymphatic drainage areas. The planning target volume (PTV) was created by expanding the CTV outward by 5–10 mm. The prescribed radiation dose ranged from 52 to 66 Gy, administered in 20–33 fractions, depending on the tolerance of OARs. The radiotherapy plan aimed to ensure that 95% of the PTV received the prescribed dose, with dose uniformity ranging from 95 to 110%. OARs included the stomach, small intestine, colon, liver, kidneys, and spinal cord, with delineation extending 5 cm above and below the PTV. Dose constraints for OARs followed the Chinese guidelines for the diagnosis and treatment of primary liver cancer2.
The initial radiotherapy plan (Plan1) was jointly confirmed by the physicist and physician before execution. Image-guided radiotherapy (IGRT) was delivered using a linear accelerator (Elekta, Sweden) once daily, five times per week. IGRT was performed at least once a week by a cone-beam computed tomography device (Elekta, Sweden), with treatment errors kept within 5 mm.
A second simulation scan (Scan2) was performed during the fourth week of radiotherapy. The GTV, CTV, PTV, and OARs were re-delineated. Two-thirds of the patients (27 patients) were randomly selected for the redesign of the second radiotherapy plan (Plan2), while one-third (13 patients) continued with the original plan (Plan1). Radiotherapy of 27 patients was adjusted according to Plan2 until the total prescribed dose was delivered.
Targeted therapy and/or immunotherapy were administered during and after radiotherapy up to 2 years, or until intolerable adverse events, or disease progression occurred. For those patients with disease progression, systemic therapy was adjusted based on oncologist recommendations and patient preferences.
Follow up
Physical examination was conducted weekly during radiotherapy and systemic therapy, with dynamic monitoring of symptoms, signs, and treatment-related adverse events (TRAEs). Blood tests, including routine blood work, liver and kidney function tests, and coagulation function tests, were performed weekly. Tumor markers were monitored every 2–4 weeks, then every 3 months. One month after completing radiotherapy, enhanced CT or MR scan were conducted to evaluate the tumor response. Subsequently, efficacy and TRAEs were assessed every 3 months. Overall survival (OS) was calculated from the start of radiotherapy to the date of death or the last follow-up. The last follow-up data of surviving patients, death data of deceased patients, or the last available data before loss to follow-up were considered as endpoint data.
Assessments
Efficacy assessments were based on the modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The treatment response of LNM was defined as the best tumor response observed during follow-up. Results were independently interpreted by two researchers, with discrepancies resolved through discussion. Response evaluation was confirmed 4 weeks after the initial assessment. The objective response rate (ORR) was defined as the proportion of patients achieving complete remission (CR) and partial remission (PR) among the total enrolled patients. The local control rate (LCR) referred to the percentage of patients who achieved CR, PR, or stable disease (SD). Progression free survival (PFS) was defined as the time from the date of radiotherapy to the first evidence of definitive disease progression. Infield progression free survival (infield-PFS) referred to the time from the start of radiotherapy for LNM to the appearance of progressive disease or new lesions in the irradiated field, or death, whichever occurred first. TRAEs were prospectively assessed by two independent physicians using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Gastrointestinal toxicities (e.g., dyspepsia, nausea) were documented weekly during treatment and follow-up, with severity graded objectively (Grade 1–5). Acute toxicity was assessed during radiotherapy and up to 3 months after, while late toxicity was assessed from 3 months to 1 year after radiotherapy.
Statistical analysis
The data cutoff date was July 31, 2024. All statistical analyses were performed using SPSS 25.0 statistical analysis software (IBM Corp, Armonk, USA). Continuous variables were expressed as Mean ± standard deviation (SD) or median (P25, P75) and analyzed using two independent samples or paired T-tests, or rank-sum tests. Categorical variables were described as frequency (percentage) and analyzed by chi-square tests. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. All statistical tests were two-sided, with a significance level set at P < 0.05. Confidence intervals were set at 95%.
Results
Patient characteristics
The clinical characteristics of 40 patients at baseline were listed in Table 1. The whole cohort consisted 35 males (87.5%) and 5 females (12.5%), with an average age of 54.58 ± 10.94 years (range 29–79). Of these patients, 92.5% (37/40) patients had a history of hepatitis B, while none had a history of hepatitis C. One patient (2.5%) had a history of parasitic infection, 8 patients (20.0%) had a family history of cancer, 9 patients (22.5%) had a history of alcohol consumption, and 16 patients (40.0%) had a history of smoking. Child-Pugh Class A and B patients accounted for 82.5% (33/40) and 17.5% (7/40) of the cohort, respectively. All patients were classified as BCLC Stage C for HCC.
Tumor volume reduction
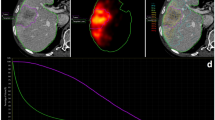
In the initial simulation scan (Scan1) prior to radiotherapy, the average GTV1 and PTV1 were 191.64 ± 152.90 cc and 423.20 ± 235.77 cc, respectively (Table 2). The second simulation scan (Scan2) conducted during the fourth week of radiotherapy, with a median irradiation dose of 38.0 Gy (range 30.0–44.0 Gy), and a median biologically effective dose (BED) of 45.6 Gy (range 36.0–52.8 Gy). In Scan2 during radiotherapy, the average GTV2 and PTV2 were 135.44 ± 128.17 cc and 324.05 ± 200.14 cc, respectively. Rescan results showed a significant regression in the volume of metastatic lymph nodes (P < 0.001), as shown in Table 2. Figure 1 illustrated the reduction of GTV. ∆GTV represented the absolute volume difference between GTV1 and GTV2, and ∆GTV% represented the percentage reduction in GTV, which was calculated as ∆GTV% = (GTV1 – GTV2)/GTV1. The mean ∆GTV was 56.20 cc (95% CI 35.20–77.21 cc), ranging from 1.53 to 337.10 cc; and the average ∆GTV% was 29.65% (95% CI 24.49%−34.80%). Patients received a median total irradiation dose of 60.0 Gy (range 52.0–60.0 Gy), and a median total BED of 72.0 Gy (range 62.4–75.0 Gy).
Comparison of dose-volume relationship of OARs
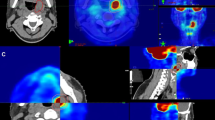
Twenty-seven patients were randomly selected to form the test group, where their radiotherapy plans were redesigned. The dose-volume relationships of OARs in the initial radiotherapy plan (Plan1) and the second radiotherapy plan (Plan2) for these 27 patients were recorded. The remaining 13 patients constituted the control group. There was no difference in baseline clinical characteristics between two groups, as shown in Table 1. The average GTV1 of the test group was greater than that of the control group, 219.13 ± 167.73 cc versus 134.18 ± 98.61 cc, but the difference was not statistically significant (P = 0.100). Tumor reduction was more pronounced in the test group compared to the control group, with an average ∆GTV of 72.73 ± 73.18 cc versus 21.87 ± 22.92 cc (P = 0.020), and an average ∆GTV% of 34.10% ±15.71% versus 20.41% ±13.14% (P = 0.010). Figure 2 showed a patient who underwent a redesigned radiotherapy plan with significant lymph node shrinkage following 40 Gy/20 fractions of radiotherapy.
The dose-volume difference of OARs between Plan1 and Plan2 in test group was analyzed using a paired design T-test. V20 represents the volume that received irradiation dose of 20 Gy. Statistics show that the volume of OARs received irradiation dose in Plan2 have all decreased to varying degrees, as shown in Table 3. Specifically, for the stomach, significant reductions were observed in the maximum irradiation dose (Dmax) (P = 0.025), V20 (P = 0.004), V30 (P = 0.011), V45 (P = 0.001), and V50 (P = 0.001). For the small intestine, reductions were seen in Dmax (P = 0.041), V30 (P = 0.006), V45 (P = 0.004), and V50 (P = 0.004). For the colon, significant reductions were noted in Dmax (P = 0.001), V20 (P < 0.001), V30 (P < 0.001), V45 (P = 0.007), and V50 (P = 0.017). For the left kidney, there were decreases in mean irradiation dose (Dmean) (P = 0.003) and V20 (P = 0.007), while for the right kidney, reductions were observed in Dmean (P = 0.006), V20 (P = 0.012), and V30 (P = 0.022). For the liver, Dmean (P = 0.002), V20 (P = 0.002), and V30 (P = 0.003) all decreased.
Toxicity
Treatment-related toxicities emerged post-treatment, and overall tolerance was favorable. TRAEs occurring during and after radiotherapy were summarized in Supplementary Tables 1 and Table 4, recorded according to the most severe TRAE and categorized by severity. The primary TRAEs included hematologic toxicities such as neutropenia, lymphocytopenia, thrombocytopenia, and anemia. Hepatic impairment, marked by elevated alanine aminotransferase, aspartate aminotransferase, total bilirubin, and hypoproteinemia, was the second most common TRAE. The third group of TRAEs comprised radiation-induced gastrointestinal toxicities including dysphagia, dyspepsia, nausea, vomiting, diarrhea, abdominal distension, abdominal pain, gastroduodenal ulcer, and gastrointestinal hemorrhage. Most gastrointestinal toxicities were mild to moderate.
Compared to the control group, the test group exhibited no significant differences in hematologic toxicity and hepatic impairment (Supplementary Table 1). However, there was a significant reduction in gastrointestinal toxicities, including dyspepsia (P = 0.018), nausea (P = 0.013), vomiting (P = 0.041), and diarrhea (P = 0.040), as shown in Table 4. A decreasing trend was observed for dysphagia and gastroduodenal ulcers, though these differences were not statistically significant (P = 0.056 for both).
Tumor response and survival analyses
With a median follow-up time of 12.0 months (range 4.0–37.0 months), CR, PR, and SD were achieved in 7 (17.5%) patients, 28 (70.0%) patients, and 3 (7.5%) patients, respectively. For the entire cohort, the ORR was 87.5% (35/40) and the LCR was 95.0% (38/40). The median OS and PFS were 16.0 months (95% CI 10.2–21.8) and 15.0 months (95% CI 9.6–20.4), respectively. The median infield-PFS was not reached.
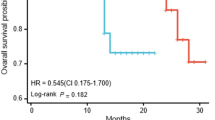
Subgroup analysis revealed that patients with ∆GTV% > 30% (median PFS not reached) had a longer PFS (P = 0.047) compared to those with ∆GTV% ≤ 30% [median PFS of 12.0 months (95% CI 6.7–17.3 months)], as shown in Fig. 3A. PFS was not associated with GTV1 (P = 0.454), GTV2 (P = 0.254) and ∆GTV (P = 0.995). OS showed no correlation with GTV1 (P = 0.322), GTV2 (P = 0.173), ∆GTV (P = 0.403), or ∆GTV% (P = 0.623).
Although ∆GTV% was more pronounced in the test group compared to the control group, and the survival data (OS and PFS) in the test group were better, these differences were not statistically significant. The median OS was 19.0 months (95% CI 1.8–36.3 months) in the test group versus 16.0 months (95% CI 10.5–20.5 months) in the control group (P = 0.897). The median PFS was 15.0 months (95% CI 7.7–22.3 months) versus 12.0 months (95% CI 7.8–16.2 months) (P = 0.833). Neither group reached the median infield-PFS, as illustrated in Fig. 3B and C, and 3D.
Discussion
Lymph node metastasis in HCC presents significant treatment challenges, often resulting in poor prognosis and short survival times. While local resection of a single metastatic lymph node can improve survival rates, surgical resection remains technically demanding, particularly when multiple metastatic lymph nodes are located in the abdominal cavity or retroperitoneum, where complete removal is difficult4. Radiotherapy offers notable advantages due to its non-invasive nature and reduced impact on vital organs, making it particularly suitable for metastatic lymph nodes in challenging locations such as the abdominal cavity, retroperitoneum, or near the diaphragm, where surgery or ablation may not be feasible11,12.
Advancements in radiotherapy technology have demonstrated substantial benefits for treating HCC with LNM, including improved LCR and survival outcomes, along with reduced adverse events. Lee et al.13 reported that intensity-modulated radiation therapy for HCC with abdominal LNM achieved a median OS of 8.1 months. Zhang et al.7 found that IGRT resulted in a median OS of 15.3 months, a one-year OS rate of 69%, and relatively mild toxicity. Since 2020, Asian researchers have extensively studied the role of stereotactic body radiotherapy for metastatic HCC8,14, with one-year PFS rates ranging from 22 to 47%, two-year OS rates from 29 to 67%, and two-year LCR from 90 to 91%, Acute toxicities of grade 2 or higher ranged from 20 to 26%. A series of studies indicated that radiotherapy is an effective treatment option for patients with LNM of HCC. In our study, IGRT was used to treat abdominal LNM of HCC, achieved an ORR of 87.5%, an LCR of 95.0%, and median OS and PFS of 16.0 and 15.0 months, respectively, with predominantly grade 1–2 gastrointestinal toxicities, demonstrating effective tumor control and safety.
Radiotherapy is a crucial treatment modality for malignant tumors; however, surrounding organs inevitably receive radiation, leading to potential damage. Radiation-induced gastrointestinal, liver, and kidney injuries are the significant factors limiting the irradiation dose for abdominal tumors, impacting patient quality of life and treatment progress. Most abdominal metastatic lymph nodes of HCC are adjacent to the gastrointestinal tract. Previous studies using 3D-conformal radiotherapy often failed to adequately protect surrounding OARs, resulting in high incidences of gastrointestinal complications. It has been reported that the incidence of radiotherapy-induced gastroduodenal ulcers and gastrointestinal bleeding can be as high as 22–30.7% and 20–22%, respectively15,16.
Radiotherapy plans are typically based on pre-treatment CT simulation scans, which may not account for tumor shrinkage or positional shifts during the 6- to 7-week treatment course. Tumor volume and ___location changes can lead to dose distribution deviations. Research indicates that tumor volumes can significantly shrink and shift, particularly when doses exceed 40 Gy17 during the entire radiotherapy process. Continuing treatment based on pre-radiotherapy plans may lead to substantial deviations in the actual dose received by the tumor18. Therefore, it is essential to adjust the radiotherapy plan according to the tumor regression pattern during the radiotherapy process. Timely adjustment of the radiotherapy plan is an important means to ensure the optimization of treatment. Currently, there is increasing research on adjusting the radiotherapy plan based on tumor regression patterns, but it has mainly focused on thoracic tumors such as lung cancer and esophageal cancer, with limited data on HCC.
Ostheimer et al.19 reported that an average tumor volume reduction of 48.2 cc when re-planning after administering a radiation dose of 40–50 Gy for non-small cell lung cancer (NSCLC). Ding et al.20 found that after 40 Gy irradiation for stage III NSCLC, tumor volumes regressed in 84 (96.6%) patients and increased in 3 (3.4%) patients. The mean GTV and PTV reduction was 38% (range − 13%~95%) and 30% (range − 5%~95%). Fox et al.21 quantified the tumor volume reduction occurring during a course of radiotherapy for NSCLC. Two repeat CT scans were performed at a nominal dose of 30 Gy and 50 Gy. The median GTV reduction was 24.7% (range − 0.3%~61.7%) at the first repeat scan and 44.3% (range 0.2%~81.6%) at the second repeat scan. These results demonstrate that substantial tumor regression occurs at irradiation doses approximating 40 Gy, validating the rationale for adaptive replanning at this threshold. HCC was known to be a radiation-sensitive tumor22, and tumor may also significantly shrink during radiotherapy for LNM, although relevant studies are scarce. According to the research experience from shrink field radiotherapy studies of NSCLC, our study conducted a second CT scan during the fourth week of radiotherapy course. The median radiation dose was 38.0 Gy, and the average absolute tumor volume reduction (∆GTV) was 56.20 cc (95% CI 35.20–77.21), while the relative tumor volume reduction (∆GTV %) was 29.65% (95% CI 24.49%−34.80%). These results were similar to those found in other tumors and demonstrate the necessity of late-course shrink field adaptive radiotherapy for HCC. This is the first study to support the practice of performing repeat CT scans when the irradiation dose reached around 40 Gy during radiotherapy for abdominal LNM of HCC, to evaluate changes in tumor volume.
Changes in the shape and size of the tumor target area during radiotherapy inevitably affect the dose distribution to the surrounding OARs. Xiao et al.23 reported that the dose volume of lung, esophagus, heart and spinal cord could be effectively reduced by re-designing plan at approximately two-thirds of the total dose (approximately 40 Gy) during radiotherapy for NSCLC. They considered that late- course adaptive radiotherapy may be an effective way to reduce the dose volume to the OARs, thus reducing radiation toxicity in patients with NSCLC. Ramsey et al.24 showed that during the course of radiotherapy for NSCLC, the V20 of the ipsilateral lung could be reduced by 17–23% after the adaptive radiotherapy plan. Zhang et al.25 reported that repeat CT scan and re-designing plan after the initial radiotherapy plan was implemented to 40 Gy/20 Fx for esophageal cancer, contributed to lower irradiation dose and volumes for the V20 of lung, V30 of heart, and Dmax of spinal cord. In this study, the changes of dose-volume relationship of major OARs including stomach, small intestine, colon, liver and kidney were analyzed after re-scanning and re-planning during late-course radiotherapy of abdominal LNM in HCC. The results showed a significant reduction in the irradiation dose to the gastrointestinal tract, liver, and kidneys. This indicates that late-course shrink field adaptive radiotherapy for abdominal LNM of HCC would better protect OARs, compared to initial radiotherapy, which preliminarily confirming its clinical application value. Follow-up observations after radiotherapy revealed a significant reduction in gastrointestinal toxicities, including dyspepsia (P = 0.018), nausea (P = 0.013), vomiting (P = 0.041), and diarrhea (P = 0.040). This further proved that re-designing plan after tumor shrinkage during radiotherapy for abdominal LNM of HCC could reduce the irradiation dose to OARs and subsequently decrease the occurrence of radiation-induced toxicities.
We found that ∆GTV% was associated with PFS. Additionally, compared to control group, patients in the test group who underwent late-course shrink field adaptive radiotherapy did not experience a worse prognosis of OS, PFS, and infield-PFS. Previous study indicated that irradiation dose was related to prognosis26. In the future, we will conduct prospective controlled studies with larger sample size to further confirm whether late-course adaptive radiotherapy of HCC could escalate the irradiation dose to the target area, subsequently enhance local control of tumor, and consequently prolong survival, in addition to reduce toxicities.
Conclusions
Tumor volume may significantly shrink during radiotherapy for abdominal LNM of HCC. Performing a second CT scan when the irradiation dose reaches around 40 Gy and implementing shrink field adaptive radiotherapy during the late-course can addresses the issues of tumor shrinkage and displacement, improve the irradiation accuracy, meanwhile significantly reduce the irradiation dose and volume of OARs, and consequently minimize radiation-induced toxicities.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- LNM:
-
Lymph node metastasis
- GTV:
-
Gross tumor volume
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- OARs:
-
Organs at risk
- IGRT:
-
Image-guided radiotherapy
- BED:
-
Biologically effective dose
- ORR:
-
Objective response rate
- LCR:
-
Local control rate
- CR:
-
Complete remission
- PR:
-
Partial remission
- SD:
-
Stable disease
- PD:
-
Progressive disease
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- TRAEs:
-
Treatment-related adverse events
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263 (2024).
National Health Commission of the People’s Republic of China. Standardization for diagnosis and treatment of primary hepatic carcinoma (2022 Edition). Cancer Res. Prev. Treat. 49 (3), 251–276 (2022).
Xia, F. et al. Positive lymph node metastasis has a marked impact on the long-term survival of patients with hepatocellular carcinoma with extrahepatic metastasis. PLoS One. 9 (4), e95889 (2014).
Yang, A. et al. Prevalence and clinical significance of regional lymphadenectomy in patients with hepatocellular carcinoma. ANZ J. Surg. 89 (4), 393–398 (2019).
Robbins, J. R., Schmid, R. K., Hammad, A. Y., Gamblin, T. C. & Erickson, B. A. Stereotactic body radiation therapy for hepatocellular carcinoma: practice patterns, dose selection and factors impacting survival. Cancer Med. 8 (3), 928–938 (2019).
Lazarev, S., Hardy-Abeloos, C., Factor, O., Rosenzweig, K. & Buckstein, M. Stereotactic body radiation therapy for centrally located hepatocellular carcinoma: outcomes and toxicities. J. Cancer Res. Clin. Oncol. 144 (10), 2077–2083 (2018).
Zhang, H. et al. Image-guided intensity-modulated radiotherapy improves short-term survival for abdominal lymph node metastases from hepatocellular carcinoma. Ann. Palliat. Med. 8 (5), 717–727 (2019).
Matoba, M., Tsuchiya, H., Kondo, T. & Ota, K. Stereotactic body radiotherapy delivered with IMRT for oligometastatic regional lymph node metastases in hepatocellular carcinoma: a single-institutional study. J. Radiat. Res. 61 (5), 776–783 (2020).
Yap, M. L. et al. Adaptive dose escalation using serial Four-dimensional positron emission tomography/computed tomography scans during radiotherapy for locally advanced Non-small cell lung Cancer. Clin. Oncol. (R Coll. Radiol). 28 (12), e199–e205 (2016).
Yan, H. et al. Microinvasion in hepatocellular carcinoma: predictive factor and application for definition of clinical target volume for radiotherapy. World J. Surg. Oncol. 22 (1), 125 (2024).
Palma, D. A. et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-Term results of the journal Pre-proo26 SABR-COMET phase II randomized trial. J. Clin. Oncol. 38, 2830–2838 (2020).
Harrow, S. et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR-COMET): extended Long-Term outcomes. Int. J. Radiat. Oncol. Biol. Phys. 114, 611–616 (2022).
Lee, D. Y. et al. Prognostic indicators for radiotherapy of abdominal lymph node metastases from hepatocellular carcinoma [J]. Strahlenther Onkol. 191 (11), 835–844 (2015).
Jo, I. Y. et al. Stereotactic ablative radiotherapy for pulmonary oligometastases from primary hepatocellular carcinoma: a multicenter and retrospective analysis (KROG 17 – 08). Jpn J. Clin. Oncol. 52, 616–622 (2022).
Zeng, Z. C. et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int. J. Radiat. Oncol. Biol. Phys. 63 (4), 1067–1076 (2005).
Park, Y. J. et al. Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J. Gastroenterol. 41 (11), 1099–1106 (2006).
Welsh, J. et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer 118 (10), 2632–2640 (2012).
Yamashita, H., Abe, O. & Nakagawa, K. Involved-field irradiation concurrently combined with nedaplatin/5-fluorouracil for inoperable esophageal cancer on basis of 18FDG-PET scans: A long follow-up results of phase II study. Radiother Oncol. 123 (3), 488 (2017).
Ostheimer, C. et al. Correction to: prognostic impact of gross tumor volume during radical radiochemotherapy of locally advanced non-small cell lung cancer-results from the NCT03055715 multicenter cohort study of the young DEGRO trial group. Strahlenther Onkol. 197 (6), 560–561 (2021).
Ding, X. et al. A clinical study of shrinking field radiation therapy based on (18)F-FDG PET/CT for stage III non-small cell lung cancer. Technol. Cancer Res. Treat. 12 (3), 251–257 (2013).
Fox, J. et al. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 74 (2), 341–348 (2009).
Yang, G. et al. Advancements in Understanding mechanisms of hepatocellular carcinoma radiosensitivity: A comprehensive review. Chin. J. Cancer Res. 35 (3), 266–282 (2023).
Xiao, L. et al. Late-Course adaptive adjustment based on metabolic tumor volume changes during radiotherapy May reduce radiation toxicity in patients with Non-Small cell lung Cancer. PLoS One. 12 (1), e0170901 (2017). Published 2017 Jan 26.
Ramsey, C. R. et al. A technique for adaptive image-guided helical tomotherapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 64 (4), 1237–1244 (2006).
Zhang Zhu ling-xiao, Shi xue-bing. Dosimetric study of reducing IMRT field by secondary positioning adjustment in esophageal carcinoma. Chin. J. Clin. Res. Jul;31 (7), 970–972 (2018).
Wang, Y. et al. Efficacy and Dose-Response relationship of stereotactic body radiotherapy for abdominal lymph node metastases from hepatocellular carcinoma. Oncologist 28 (6), e369–e378 (2023).
Acknowledgements
Not applicable.
Funding
This work was supported by the Third Affiliated Hospital of Sun Yat-sen University, Clinical Research Program (No. YHJH201908).
Author information
Authors and Affiliations
Contributions
Project administration, writing - original draft: Yan H. Software,formal analysis: Li Z. Data curation: Li N. Investigation: Yan H, Guo X. Supervision: Guo X. Methodology: Wu M. Validation: Kong F. Visualization: Dong J. Resources: Deng M. Funding acquisition: Xu X. Writing - review & editing: Xu X, Deng M. All authors participated in data interpretation, drafting, and finalizing the report. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University, Approval No. II2024-040-01.
Patient consent statement
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, H., Li, Z., Li, N. et al. Late course adaptive radiotherapy based on tumor volume reduction decreases Gastrointestinal toxicity for abdominal lymph node metastasis of hepatocellular carcinoma. Sci Rep 15, 17477 (2025). https://doi.org/10.1038/s41598-025-02363-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02363-7