Abstract
Sarcopenia emerges as a predominant health concern among the older adults, it makes the identification of relational factors crucial. Nut, a universally consumed dietary component, is posited to confer benefits to the musculoskeletal system. This study aimed to elucidate the association between nut consumption and sarcopenia in Chinese older adults. Data concerning nut consumption and sarcopenia were sourced from the 2018 wave of the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The analysis incorporated 14,281 participants furnishing valid responses. This research employed logistic regression to investigate the association between nut consumption and sarcopenia. A total of 14,181 older adults (mean age = 84.86 ± 11.47 years and 55.08% were female) were included in this study. This study found an inverse association between nut consumption and sarcopenia in Chinese older adults, with higher nut consumption associated with a lower prevalence of sarcopenia, even after controlling for confounders. Compared with the nut consumption group of occasionally/rarely or never, the adjusted ORs of sarcopenia for at least once per month, at least once per week, and almost every day were 0.78 (95% CI: 0.66, 0.91), 0.81 (95% CI: 0.69, 0.95), and 0.62 (95% CI: 0.51, 0.77), respectively. Moreover, the result also displayed there is a significant interaction of nut consumption with gender (P-value = 0.016). This study identified an inverse association between nut consumption and sarcopenia. Introducing nut into the dietary regimen might present an accessible approach to bolster musculoskeletal health among the older adults.
Similar content being viewed by others
Introduction
With the increase in the proportion of older adults and the improvement of people’s living standards, the expectation of healthy aging increases accordingly1. Given that China hosts the largest aging demographic in world, it inevitably amplifies concerns the health of older adults2. Sarcopenia, typified by the depletion of skeletal muscle mass, diminished muscle strength, and/or reduced physical performance, is occurred with advancing age and has become increasingly prevalent3,4,5. It represents a pressing public health challenge in today’s aging societies6.
Emerging evidence increasingly suggests that sarcopenia holds a strong association with diverse adverse outcomes such as falls, functional impairment, frailty, and mortality7,8. An epidemiological investigation from Asian nations, utilizing the Asian Working Group for Sarcopenia 2014 criteria, has reported sarcopenia prevalence rates ranging between 5.5% and 25.7% among different Asian countries4. Hence, it is more and more vital to comprehend and related factors of sarcopenia in China. Identifying the relational factors that associate with the incidence of sarcopenia can lead to marked improvements in the health and living standards of the older adults.
The factors associate with sarcopenia are multifaceted. Research has indicated that aging, suboptimal nutritional status, smoking, and a decreased BMI are among the risk factors for its onset9,10. Furthermore, numerous studies have substantiated the association between sarcopenia and diet11. Similarly, Berrazaga et al. noted that protein is positively related to sarcopenia12. A study by Remelli et al. also found that lack of vitamin D in older adults can greatly increase the risk of sarcopenia13. There was evidence to suggest that nut was rich in protein, fatty acid, fiber, vitamins, minerals, phytosterols and phenolics14. Nevertheless, in previous studies, the association between nut consumption and sarcopenia has not been specifically examined.
Consequently, we employed the 2018 dataset from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) to investigate the association between nut consumption and sarcopenia.
Methods
Study population
The CLHLS is a nationwide, community-based study designed to examine the health status and factors affecting longevity among Chines older adults (The data from CLHLS survey already obtained the ethical approval and informed consent, and was approved by research ethics committees of Peking University (IRB00001052-13074). Each participant signed an informed consent form and all methods in our study were performed in accordance with the guidelines and regulations of Declaration of Helsinki). By using a multi-stage cluster random sampling technique, the CLHLS assessed older adults across 23 of 31 provinces/autonomous regions/municipalities in mainland China. In this study, trained interviewers conducted face-to-face interviews with participants at their residences to collect data. To ensure comprehensive data collection, participants were encouraged to provide extensive responses. The CLHLS implemented a rigorous quality control system to maintain data reliability and consistency. These procedures included pretesting of survey instruments, training of survey staff, and regular monitoring of data quality15. The specifics pertaining to this survey have been previously report16.
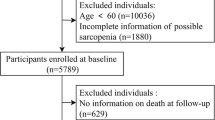
This study utilized the 2018 wave of CLHLS, which surveyed 15,874 participants. The study excluded participants who were younger than 65 years old (n = 95), missed information of sarcopenia (n = 1297), and missed information of nut consumption (n = 201). The final analysis consisted of 14,281 participants (Fig. 1).
Assessment of nut consumption
Nut consumption was assessed using a specific question, “How often do you normally eat nut?” The frequency of nut consumption was documented as “almost every day”, “not every day, but at least once a week”, “not every week, but at least once a month”, and “occasionally/rarely or never”17.
Assessment of sarcopenia
This study used the diagnostic method recommended by Asian Working Group for Sarcopenia in 2019 to define sarcopenia: low muscle mass plus low muscle strength, or low physical performance4. According to this standard, low muscle mass plus low physical performance ware used to identified sarcopenia. Low muscle mass is defined as Skeletal Muscle Mass Index (SMI) < 5.08 kg/m2 for female and < 6.88 kg/m2 for male18. SMI = Appendicular Skeletal Muscle Mass (ASM)/height (m)2; ASM = 0.193 × body weight (kg) + 0.107 × height (cm) − 4.157 × sex (male = 1; female = 2) − 0.037 × age (year) − 2.63119. Physical performance was assessed with the question “how do you stand up after sitting on a chair?” Respondents were asked to choose from three responses: “no problem,” “with problem,” and “not able to do it.” Responses of “with problem” or “not able to do it” indicated low physical performance20.
Assessments of covariates
Based on previous studies11,21,22, the covariates we included the continuous variables of age, years of education, and calf circumference; the categorical variables of gender (male vs. female), area of residence (urban vs. rural), marital status (having a spouse vs. not having a spouse), smoking status (yes vs. no), alcohol consumption (yes vs. no), annual family income (< 30000 Yuan, 30000–50000 Yuan, > 50000 Yuan) (1 Yuan = 0.15124 US dollars in 2018), and heart disease (yes vs. no).
Statistical analyses
Firstly, this study used the Kolmogorov–Smirnov test to assess the normality of continuous variables. The Q–Q plots indicated that age followed a normal distribution, while education years and calf circumference did not follow a normal distribution. Secondly, the baseline characteristics of the study participants which were stratified according to their nut consumption were presented by mean that followed a normal distribution, and by median and interquartile range (IQR) that did not follow a normal distribution (for continuous variables) or frequency distribution (for categorical variables). To analyze variations in the covariates across four nut consumption categories, the Kruskal–Wallis test was used for continuous variables, and the chi-square test for categorical ones. Thirdly, binary logistic regression analyses were used to investigate the association between nut consumption and sarcopenia. This study employed three models for binary logistic regression analysis: Model 1 was the unadjusted model; Model 2 was adjusted for gender, age, and annual family income; and Model 3 was adjusted for gender, age, annual family income, marital status, area of residence, education years, smoking status, alcohol consumption, calf circumference, and heart disease. Additionally, to explore the dose-response association between nut consumption and sarcopenia, this study performed a linear trend test using an ordinal categorical variable for nut consumption frequency. The categories included rarely or never, at least once per month, at least once per week, and almost every day. A chi-square test for trend was conducted to assess whether increasing frequency of nut consumption was associated with a linear decrease in the risk of sarcopenia. The P-value for trend was calculated for each model to test the hypothesis of a linear association. Finally, stratified and interaction analyses were carried out on categorical variables to evaluate potential effect of modifications. To account for potential inflation of type I error due to multiple subgroup comparisons, we applied the Bonferroni correction (Bonferroni correction = 0.05/number of subsamples)23. The significance threshold for interaction terms was set at P < 0.0071 (0.05/7) accordingly.
In addition, a sensitivity analyse was performed. Missing values were multiply-imputed using the “chained equations” method. Five imputations were performed. The imputation models included all variables used in the final analytical model, including: age, gender, annual family income, marital status, years of education, area of residence, smoking status, alcohol consumption, calf circumference, and heart disease status.
This study uses double tailed P-value < 0.05 as the standard for statistical significance. The statistical software used in this study are R statistical package (http://www.R-project.org; version3.6.3), Empower (R) software (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA), and STATA version 17.0 (Stata Corp, College Station, TX, USA).
Result
Characteristics of participants
Among the 14,281 participants, 8518 participants had sarcopenia. The mean age of participants is 84.86 years old. Of the participants, 55.08% were female and 83.60% resided in urban. Participants consuming nut almost everyday were more frequently observed to be advanced age, live in urban areas, have a spouse, and have a long education years (Table 1).
Association between nut consumption and sarcopenia
In Model 1, participants consuming nut almost everyday or at least once per week showed reduced likelihood of sarcopenia: at least once per week, OR = 0.47 (95% CI: 0.43, 0.52); almost everyday, OR = 0.36 (95% CI: 0.31, 0.41). In Model 2, the association was also existent: at least once a week, OR = 0.74 (95% CI: 0.65, 0.85); almost every day, OR = 0.57 (95% CI: 0.47, 0.68). In Model 3 when adjusting for all the study potential confounding risk factors, OR = 0.62 (95% CI:0.51, 0.77) (Table 2).
The results of the chi-square test for trend revealed a significant linear association between increasing frequency of nut consumption and a decreased risk of sarcopenia. The P-values for the trend tests were all less than 0.001 across all models, as nut consumption increased, the odds of developing sarcopenia significantly decreased, with the trend remaining highly significant (P-for-trend < 0.001) in each model. These results support a dose-response association between nut consumption and sarcopenia risk.
Stratified and interaction analyses
Subgroup analyses were conducted using nine categorical variables (gender, marital status, area of residence, smoking status, alcohol consumption, annual family income, and heart disease) (Supplementary Table 1). The positive association between nut consumption and sarcopenia was consistent across these subgroups. The interaction results showed that gender had significant interactions with nut consumption on sarcopenia (P-value for interaction = 0.016). However, after Bonferroni correction for multiple comparisons, the interaction between nut consumption and gender, which was initially statistically significant (p = 0.016), no longer met the corrected threshold (adjusted p = 0.112). Similar results were observed for other subgroup interactions. (Supplementary Table 1).
Sensitivity analysis
In the sensitivity analysis, we imputed the missing covariates for participants. The association of nut consumption and sarcopenia remained conherent (all P-values < 0.05) (Supplementary Table 2).
Discussion
This study is the first population-based study that explores the associations of nut consumption with sarcopenia. Our findings indicated a negative association between nut consumption and sarcopenia, with the association being significantly pronounced in male, suggesting a potential interaction between nut consumption and gender in the older adults.
Nut was rich of protein, fatty acid, fiber, vitamins, minerals, phytosterols and phenolics14. Previous studies have confirmed there is a negative association between protein and sarcopenia24. Reduced protein intake is correlate with the decreased muscle strength25. In addition, a low-quality restricted diet system of protein supply can exacerbates muscle function in older sarcopenic adults24. The possible reason for these results is that protein offers the amino acids required for muscle compound, so when protein intake decreases, the quantity and quality of muscle synthesis also decrease6. The nutrients contained in nut are not just proteins, previous studies have also shown that some components in nut such as vitamins, omega-3 fatty acids, and mineral are also associated with sarcopenia26,27.
Previous studies have shown that have found low vitamin D levels can diminish muscle strength26. A study by Remelli et al. has proven that low levels of vitamin D intake are believed to reduce skeletal muscle mass, leading to the development of skeletal muscle atrophy13. In addition, frailty (a disease that overlaps with some skeletal muscle wasting disorders) is also associated with vitamin D absence28. Previous studies have revealed that vitamin D can be localized in the nucleus of human muscle cell lines, myoblasts29, and adult skeletal muscle30,31, which can promote muscle growth. Furthermore, chronic inflammation is a common cause of sarcopenia32, however, the omega-3 fatty acids as a class of long-chain fatty acids in nut, they have long been hailed as having anti-inflammatory effects27. Smith et al. has also found omega-3 fatty acids significantly improving the efficiency of muscle synthesis rate induced by hyperaminoacidemia–hyperaminoacidemia33. Additionally, some minerals in nuts, such as phosphorus, zinc, have also been proven to contribute to reduce the incidence rate of sarcopenia27.
From another perspective, frequent nut consumption may reflect a generally healthier dietary pattern. Previous studies have shown that individuals who consume more nuts also tend to have higher intakes of vegetables, fruits, fish, whole grains, and other high-quality foods34,35, all of which are beneficial for muscle health and may contribute to a lower prevalence of sarcopenia36. In addition, people who frequently consume nuts may have greater health consciousness, as reflected in more regular physical activity and fewer unhealthy lifestyle behaviors such as smoking and excessive alcohol consumption, which may also jointly affect muscle health37.Therefore, the observed association between nut consumption and sarcopenia may not be entirely attributable to the nutrients in nuts alone, but rather to a combination of dietary and lifestyle factors. Future studies should aim to adjust for overall dietary patterns and incorporate comprehensive lifestyle assessments to further disentangle the independent effects of nut consumption from those of broader health behaviors.
Several biological mechanisms have been proposed to clarify the association between nut consumption and sarcopenia. A potential mechanism is that essential amino acids are the most principal nutritional imports in muscle protein synthesis. In this regard, leucine in nut protein is supposed to a major nutritional modifier of muscle protein synthesis and metabolism, which can touch off the mammalian rapamycin target protein pathway and inhibit proteasomes, it can improve the quantity and quality of muscles38. Additionally, Vitamin D promotes cell proliferation by up-regulating follistatin and insulin-like growth factor 2. It influences cell differentiation by inducing multiple myogenic transcription factors, including fetal myosin, neural cell adhesion molecule, B-cell lymphoma 2, insulin-like growth factor-i, fibroblast growth factor, retinoblastoma protein, and myogenic differentiation protein 139,40. Furthermore, Vitamin D initiates muscle regeneration, increasing the cross-sectional area of skeletal muscle fibers through generation cycle retardant, it is a crucial phase for myogenic onset39,40. Moreover, Vitamin D inhibits myostatin expression, a detractor of muscle growth, thereby preventing muscle deterioration and enhancing contractile filament functionality and muscle strength41. Another possible mechanism is omega-3 fatty acids can increase nerve conduction and muscle activation, then improve mitochondrial function (energy production) or compose a larger myofiber size42. In addition, trace element zinc has been proven to promote protein synthesis and slow down protein degradation27.
The results of the interaction showed that gender had interactions with nut consumption on sarcopenia, significant association was observed among the male in older adults. Several studies had suggested that the absolute muscle loss rate in males was higher than that in females, so they are more likely to suffer from muscle-related diseases43. A study has shown that testosterone can interacts with androgen receptors in skeletal muscle, promoting the synthesis of skeletal muscle and thereby improving the quantity and quality of muscle44. However, Testosterone in older male decreases with age45. Therefore, the decrease of testosterone can lead to muscle dysfunction in older male. A study by Peng et al. also attested that among older adults, low serum myostatin levels only reduced the low skeletal muscle mass of male, but did not affect female46. There are significant differences in skeletal muscle composition between males and females. Studies have shown that male myofibers generally have a larger cross-sectional area than those in females, with this difference being particularly prominent in type II (fast-twitch) fibers47. Type II fibers play a critical role in rapid contraction and high-intensity activities, and they are more susceptible to age-related atrophy48. During the aging process, the accelerated degeneration of these fibers may render males more vulnerable to muscle deterioration and functional decline. In this physiological context, nut, which are rich in high-quality nutrients such as protein, unsaturated fatty acids, vitamin E, and antioxidants, may play a more important role in maintaining type II fibers health in males, thereby presenting a significant association between nut consumption and sarcopenia. Furthermore, Estrogen plays a vital role in muscle metabolism and repair in females. It possesses antioxidant properties that help reduce oxidative stress in muscle cells and protect myofibers from damage49. In addition, estrogen promotes muscle regeneration by enhancing the activity of satellite cells and stabilizing the muscle cell membrane after injury50. Moreover, estrogen regulates the balance of muscle protein synthesis and degradation through pathways such as Akt/mTOR, thereby delaying muscle atrophy51. Therefore, the protective effects of estrogen may, to some extent, attenuate the significant association between nut consumption and sarcopenia in females. In contrast, due to the lack of similar hormonal protection in males, nut consumption may play a more critical role in maintaining muscle health, thereby exhibiting a stronger association. These sex-specific physiological differences underscored the importance of nut supplementation in men should be emphasized, while also considering the investigation of other dietary options that can help women increase muscle mass through nutrition.
Although subgroup analyses suggested potential heterogeneity in the association between nut consumption and sarcopenia across gender and other sociodemographic factors, these interaction effects were not statistically significant after Bonferroni correction for multiple comparisons. The results before and after Bonferroni correction were inconsistent, however, the non-significant findings after correction may reflect statistical outcomes rather than true null associations. Therefore, these results should not be totally interpreted as evidence negating potential gender differences. Previous studies have also pointed out that the Bonferroni correction imposes a stringent penalty during multiple comparisons, which may obscure potentially meaningful findings52,53. Thus, further investigation is warranted to explore these potential interactions in greater depth.
This study possesses several limitations that warrant acknowledgment. Firstly, due to the limitations of cross-sectional studies, causal association cannot be determined, therefore we cannot infer a causal relationship between nut consumption and sarcopenia. Hence, subsequent longitudinal studies are imperative to elucidate the relationship in question. Secondly, given that this study relies on self-reported data collection, there are certain limitations in the dietary frequency self-report, as it uses vague frequency descriptions such as “occasionally/rarely,” which may lead to recall bias. Therefore, future studies will adopt more objective and systematic dietary assessment tools, such as 24-hour dietary recall or dietary frequency questionnaires. Thirdly, there are some confounding factors that have not been taken into account, such as physical activity, total dietary protein, dental health, esophageal and gastrointestinal digestive problems, which may associated with nut consumption. Future research will focus on collecting and adjusting for these related factors to more comprehensively assess the association between nut consumption and sarcopenia. Fourthly, in accordance with previous research, this study employed a combining “muscle mass and chair-stand performance” as the diagnostic criteria for analysis. However, prior studies have indicated that handgrip strength is also a widely accepted indicator for the assessment of sarcopenia. Future research may consider integrating multiple measurement methods, including handgrip strength, to validate and expand upon the current findings, thereby enhancing the robustness of the evidence and supporting further advancements in this field. Finally, although this study identified a significant inverse association between nut consumption and the risk of sarcopenia, practical implementation should consider the specific needs of older adults. For some individuals, especially those with chewing difficulties or dysphagia, consuming whole nuts may not be appropriate. In such cases, softer and safer alternatives are recommended, such as nut-based beverages (e.g., almond milk, walnut milk, peanut milk) or nut-based soft foods (e.g., almond tofu, peanut tofu). These options are easier to swallow and still provide similar nutritional benefits. Additionally, for individuals with nut allergies, consuming nuts or nut-based products may pose health risks. For them, other nutrient-rich substitutes such as avocado, soft tofu, or soft-textured fatty fish like salmon can be considered. These foods are also rich in unsaturated fatty acids and high-quality protein. Personalized dietary recommendations should account for swallowing ability and allergy history to maximize the preventive potential of nutritional strategies against sarcopenia.
Conclusions
This investigation centered on the association between nut consumption and sarcopenia in Chinese older adults, our findings provide significant empirical support for the protective role of nut in sarcopenia among older adults. The routine integration of nut into the diets of older adults might serve as a pragmatic and accessible approach to benefit musculoskeletal well-being.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://opendata.pku.edu.cn/dataverse/CHADS.
References
WPP2022_Summary-of-Results.pdf.
Chen, X. et al. The path to healthy ageing in China: a Peking University-Lancet commission. Lancet 400, 1967–2006. https://doi.org/10.1016/s0140-6736(22)01546-x (2022).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646 https://doi.org/10.1016/s0140-6736(19)31138-9 (2019).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300–307. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Gao, K. et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine 44, 101264. https://doi.org/10.1016/j.eclinm.2021.101264 (2022).
Papadopoulou, S. K. & Sarcopenia A contemporary health problem among older adult populations. Nutrients 12, 93. https://doi.org/10.3390/nu12051293 (2020).
Cho, M. R., Lee, S. & Song, S. K. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci. 37, e146. https://doi.org/10.3346/jkms.2022.37.e146 (2022).
Dent, E. et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J. Nutr. Health Aging. 22, 1148–1161. https://doi.org/10.1007/s12603-018-1139-9 (2018).
Shimokata, H. & Ando, F. Sarcopenia and its risk factors in epidemiological study. Nihon Ronen Igakkai Zasshi. 49, 721–725. https://doi.org/10.3143/geriatrics.49.721 (2012).
Dodds, R. M. et al. Prevalence and incidence of sarcopenia in the very old: findings from the Newcastle 85 + study. J. Cachexia Sarcopenia Muscle. 8, 229–237. https://doi.org/10.1002/jcsm.12157 (2017).
Du, Q. et al. Dietary diversity and possible sarcopenia among older people in China: a nationwide population-based study. Front. Nutr. 10, 1218453. https://doi.org/10.3389/fnut.2023.1218453 (2023).
Berrazaga, I., Micard, V., Gueugneau, M. & Walrand, S. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: a critical review. Nutrients 11, 825. https://doi.org/10.3390/nu11081825 (2019).
Remelli, F., Vitali, A., Zurlo, A. & Volpato, S. Vitamin D deficiency and sarcopenia in older persons. Nutrients 11, 61. https://doi.org/10.3390/nu11122861 (2019).
Lamuel-Raventos, R. M. & Onge, M. S. Prebiotic nut compounds and human microbiota. Crit. Rev. Food Sci. Nutr. 57, 3154–3163. https://doi.org/10.1080/10408398.2015.1096763 (2017).
Zeng, Y., Feng, Q., Hesketh, T., Christensen, K. & Vaupel, J. W. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet 389, 1619–1629. https://doi.org/10.1016/s0140-6736(17)30548-2 (2017).
Zeng, Y. et al. Health consequences of Familial longevity influence among the Chinese elderly. J. Gerontol. Biol. Sci. Med. Sci. 68, 473–482. https://doi.org/10.1093/gerona/gls203 (2013).
He, D., Huangfu, Z. & Pan, M. Association between nut consumption and mortality among Chinese older people: A National cohort study based on CLHLS from 2008 to 2018. Front. Nutr. 9, 1080714. https://doi.org/10.3389/fnut.2022.1080714 (2022).
Wu, X. et al. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China health and retirement longitudinal study. PLoS ONE. 16, e0247617. https://doi.org/10.1371/journal.pone.0247617 (2021).
Rosenberg, I. H. Sarcopenia: origins and clinical relevance. J. Nutr. 127, 990s–991s. https://doi.org/10.1093/jn/127.5.990S (1997).
Tan, J. Y., Zeng, Q. L., Ni, M., Zhang, Y. X. & Qiu, T. Association among calf circumference, physical performance, and depression in the elderly Chinese population: a cross-sectional study. BMC Psychiatry. 22, 278. https://doi.org/10.1186/s12888-022-03925-z (2022).
Wang, X. et al. Associations of lifestyle with mental health and well-being in Chinese adults: a nationwide study. Front. Nutr. 10, 1198796. https://doi.org/10.3389/fnut.2023.1198796 (2023).
Du, H. et al. Association between sarcopenia and cognitive function in older Chinese adults: evidence from the China health and retirement longitudinal study. Front. Public. Health. 10, 1078304. https://doi.org/10.3389/fpubh.2022.1078304 (2022).
Petrie, A. & Sabin, C. Medical Statistics at a Glance (Wiley, 2019).
Yu, R. et al. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr. Gerontol. Int. 14 (Suppl 1), 15–28. https://doi.org/10.1111/ggi.12220 (2014).
Hashemi, R. et al. Sarcopenia and its associated factors in Iranian older individuals: results of SARIR study. Arch. Gerontol. Geriatr. 66, 18–22. https://doi.org/10.1016/j.archger.2016.04.016 (2016).
Tay, L. et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr). 37, 121. https://doi.org/10.1007/s11357-015-9860-3 (2015).
Ganapathy, A. & Nieves, J. W. Nutrition and sarcopenia-what do we know? Nutrients 12, 755. https://doi.org/10.3390/nu12061755 (2020).
Wilhelm-Leen, E. R., Hall, Y. N., Deboer, I. H. & Chertow, G. M. Vitamin D deficiency and frailty in older Americans. J. Intern. Med. 268, 171–180. https://doi.org/10.1111/j.1365-2796.2010.02248.x (2010).
Olsson, K. et al. Evidence for vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 157, 98–111. https://doi.org/10.1210/en.2015-1685 (2016).
Bischoff, H. A. et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 33, 19–24. https://doi.org/10.1023/a:1017535728844 (2001).
Hassan-Smith, Z. K. et al. 25-Hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE. 12, e0170665. https://doi.org/10.1371/journal.pone.0170665 (2017).
Dupont, J., Dedeyne, L., Dalle, S., Koppo, K. & Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 31, 825–836. https://doi.org/10.1007/s40520-019-01146-1 (2019).
Smith, G. I. et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am. J. Clin. Nutr. 93, 402–412. https://doi.org/10.3945/ajcn.110.005611 (2011).
Hosseini-Esfahani, F. et al. Nutrition and cardio-metabolic risk factors: findings from 20 years of the Tehran lipid and glucose study. Int. J. Endocrinol. Metab. 16, e84772. https://doi.org/10.5812/ijem.84772 (2018).
Hu, F. B. Diet and lifestyle influences on risk of coronary heart disease. Curr. Atheroscler Rep. 11, 257–263. https://doi.org/10.1007/s11883-009-0040-8 (2009).
Jeromson, S., Gallagher, I. J., Galloway, S. D. & Hamilton, D. L. Omega-3 fatty acids and skeletal muscle health. Mar. Drugs. 13, 6977–7004. https://doi.org/10.3390/md13116977 (2015).
Wieczorek, M. et al. Smoking, alcohol consumption and disease-specific outcomes in rheumatic and musculoskeletal diseases (RMDs): systematic reviews informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open. 8, 70. https://doi.org/10.1136/rmdopen-2021-002170 (2022).
Martone, A. M. et al. Exercise and protein intake: A synergistic approach against sarcopenia. Biomed. Res. Int. 2017, 2672435. https://doi.org/10.1155/2017/2672435 (2017).
Shinchuk, L. M. & Holick, M. F. Vitamin d and rehabilitation: improving functional outcomes. Nutr. Clin. Pract. 22, 297–304. https://doi.org/10.1177/0115426507022003297 (2007).
Garcia, L. A., King, K. K., Ferrini, M. G., Norris, K. C. & Artaza, J. N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152, 2976–2986. https://doi.org/10.1210/en.2011-0159 (2011).
Birge, S. J. & Haddad, J. G. 25-hydroxycholecalciferol stimulation of muscle metabolism. J. Clin. Investig. 56, 1100–1107. https://doi.org/10.1172/jci108184 (1975).
Gray, S. R. & Mittendorfer, B. Fish oil-derived n-3 polyunsaturated fatty acids for the prevention and treatment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 21, 104–109. https://doi.org/10.1097/mco.0000000000000441 (2018).
Payette, H. et al. Insulin-like growth factor-1 and Interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham heart study. J. Am. Geriatr. Soc. 51, 1237–1243. https://doi.org/10.1046/j.1532-5415.2003.51407.x (2003).
Kraemer, W. J., Ratamess, N. A., Hymer, W. C., Nindl, B. C. & Fragala, M. S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front. Endocrinol. (Lausanne). 11, 33. https://doi.org/10.3389/fendo.2020.00033 (2020).
Bhasin, S. et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy Nonobese young men in the Framingham heart study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 96, 2430–2439. https://doi.org/10.1210/jc.2010-3012 (2011).
Peng, L. N., Lee, W. J., Liu, L. K., Lin, M. H. & Chen, L. K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J. Cachexia Sarcopenia Muscle. 9, 635–642. https://doi.org/10.1002/jcsm.12302 (2018).
Miller, A. E., MacDougall, J. D., Tarnopolsky, M. A. & Sale, D. G. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. Occup. Physiol. 66, 254–262. https://doi.org/10.1007/bf00235103 (1993).
Brunner, F. et al. Effects of aging on type II muscle fibers: a systematic review of the literature. J. Aging Phys. Act. 15, 336–348 (2007).
Liehr, J. G. & Roy, D. Pro-oxidant and antioxidant effects of estrogens. Methods Mol. Biol. 108, 425–435. https://doi.org/10.1385/0-89603-472-0:425 (1998).
Pellegrino, A., Tiidus, P. M. & Vandenboom, R. Mechanisms of Estrogen influence on skeletal muscle: mass, regeneration, and mitochondrial function. Sports Med. 52, 2853–2869. https://doi.org/10.1007/s40279-022-01733-9 (2022).
Hansen, M. Female hormones: do they influence muscle and tendon protein metabolism? Proc. Nutr. Soc. 77, 32–41. https://doi.org/10.1017/s0029665117001951 (2018).
White, T., van der Ende, J. & Nichols, T. E. Beyond bonferroni revisited: concerns over inflated false positive research findings in the fields of conservation genetics, biology, and medicine. Conserv. Genet. 20, 927–937 (2019).
Etymologia. Bonferroni correction. Emerg. Infect. Dis. 21, 289. https://doi.org/10.3201/eid2102.et2102 (2015).
Author information
Authors and Affiliations
Contributions
S.Z. and D.L. are the corresponding authors of this work. Conceptualization: J.X. and D.L. Methodology: J.X., D.L. Data validation and analyses: J.X. Data interpretation: J.X., D.L., K.P., W.S., C.P., and S.Z. Writing—original draft preparation: J.X. Writing—review and editing: J.X., D.L., K.P., W.S., C.P., and S.Z. Supervision: J.X., D.L., S.Z., K.P., W.S., C.P., Final approval of manuscript: J.X., D.L., K.P., W.S., C.P., and S.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The data from CLHLS survey already obtained the ethical approval and informed consent, and was approved by research ethics committees of Peking University (IRB00001052-13074).
Informed consent
Each participant signed an informed consent form and all methods in our study were performed in accordance with the guidelines and regulations of Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, J., Pu, K., Sun, W. et al. Association of nut consumption and sarcopenia in Chinese older adults. Sci Rep 15, 17598 (2025). https://doi.org/10.1038/s41598-025-02389-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02389-x