Abstract
This study aimed to evaluate the risk of secondary primary urinary system neoplasms (SPUNs) in patients undergoing different treatment strategies during the perioperative period of rectal cancer (RC) and evalue survial prognostsis of SPUNs. The data were sourced from the US SEER database from 1975 to 2020. A total of 85,799 RC patients who underwent surgical treatment were included and divided into four groups: surgery alone (S), surgery plus chemoradiotherapy (SCR), surgery plus radiotherapy (SR), and surgery plus chemotherapy (SC). The results showed significant differences in the cumulative incidence of SPUNs among the four groups, with higher rates in patients receiving radiotherapy and chemotherapy. Both chemotherapy and radiotherapy increased the incidence of secondary primary bladder cancer (SPBC), and radiotherapy also elevated the incidence of secondary primary urethral cancer (SPUaC). However, there was no obvious association between chemoradiotherapy and the incidence of secondary primary kidney cancer (SPKC) or secondary primary ureteral cancer (SPUrC). Risk analysis indicated that the independent risk factors for SPBC were age, year of diagnosis, and postoperative radiotherapy, while race was a protective factor. For SPKC, the independent risk factors were age and year of diagnosis. No significant influencing factors were found for SPUrC, and postoperative radiotherapy was an independent risk factor for SPUaC. The relative risk (RR) of SPBC increased with the prolongation of the latency period after chemoradiotherapy. The RR value of radiotherapy was related to the year of diagnosis, reaching the highest from 1975 to 1984, and was the highest in the 18–50 age group after radiotherapy. Survival analysis revealed differences in cancer-specific survival (CSS) and overall survival (OS) among the four groups, with lower survival rates in patients receiving postoperative radiotherapy. After propensity score matching (PSM), only the overall survival rate of SPUNs patients in the postoperative radiotherapy group was lower than that of patients with original primary urological neoplasms (OPUNs). In conclusion, chemoradiotherapy during the perioperative period of RC is associated with the risk of SPUNs, and its benefits and risks need to be weighed in clinical decision-making.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most prevalent malignant tumor globally, accounting for 9.6% of newly diagnosed cancer cases worldwide and ranking as the second leading cause of cancer - related deaths1,2.
With the aging of the population and the advancement of early detection and treatment technologies, the number of cancer survivors is likely to continue increasing in the future. As of January 1, 2022, more than 18 million people had a history of cancer3,4. This trend brings new challenges in aspects such as medical services and social support for cancer survivors.
Clinical studies have demonstrated that adjuvant chemoradiotherapy plays a significant role in controlling localized and regionally metastatic tumors of rectal cancer (RC)5,6,7. However, some research has found that chemoradiotherapy may be one of the risk factors for second primary cancer (SPC), a rare complication of cancer treatment8,9.
Currently, there is still controversy regarding whether adjuvant chemoradiotherapy in the treatment of rectal cancer could induce SPC. Martling et al. studied the risk of second primary cancer in rectal cancer patients after radiotherapy and found that within 20 years of follow-up, the risk of SPC both within and outside the irradiated area did not increase after radiotherapy for rectal cancer10. Wang et al. conducted a similar study using the Taiwan National Health Insurance Research Database, identifying rectal cancer patients diagnosed between 1996 and 2011. Their results showed that rectal cancer patients receiving pelvic radiotherapy did not have an increased risk of SPC, and the risk was the same across different radiotherapy regimens11. Conversely, Rombouts et al. obtained data from the Dutch national cancer registry and analyzed multiple sites, finding that radiotherapy could increase the incidence of gynecological tumors12. Other studies have shown that the risk assessment of SPC associated with radiotherapy for pelvic cancers13(such as prostate cancer14,15, gynecological cancers16,17, and bladder cancer18,19 suggests that patients receiving pelvic radiotherapy have a higher risk of SPC. Regarding the overall impact of radiotherapy on the urinary system, radiation cystitis is the most common complication, followed by urethral stricture, ureteral stricture, urinary fistula, and secondary cancer. Although the risk of radiotherapy-induced second primary cancer is low, the latency period is prolonged, and the risk is significantly higher after radiotherapy for pelvic malignancies. For example, radiotherapy may increase the risk of bladder cancer, but the results after radiotherapy for rectal cancer are still unclear20,21. Chemotherapy is also significantly associated with the occurrence of various SPC22. For instance, in pediatric cancer research, certain chemotherapeutic drugs (such as topoisomerase II inhibitors) are associated with an increased risk of leukemia23. In breast cancer patients, chemotherapy is associated with an increased risk of SPC at sites other than lymphoma, myeloma, and chronic lymphocytic leukemia, especially acute myeloid leukemia24. In addition, in patients with multiple cancer survivorship, radiotherapy or chemotherapy before the age of 35 is associated with an increased incidence of second primary thyroid cancer25. In rectal cancer patients, chemotherapy is significantly associated with an increased risk of second primary endometrial cancer and is an important risk factor for second primary endometrial cancer in female rectal cancer patients26. Previous studies have shown that chemotherapeutic drugs such as cisplatin, methotrexate, ifosfamide, docetaxel, and 5 - fluorouracil can cause toxic reactions, including renal tubular damage and changes in glomerular filtration function27,28,29.
So far, relevant scholars have investigated the impact of radiotherapy or chemotherapy on the risk of second primary gynecological tumors, prostate tumors, thyroid tumors, and hematological tumors. However, the urinary system, as an important excretory and regulatory system in the human body, undertakes crucial functions such as excreting metabolic waste, regulating fluid and electrolyte balance, maintaining acid - base balance, and performing endocrine functions. It is vital for the stability of the human body’s internal environment and overall health. Meanwhile, the urinary system is involved in both radiotherapy and chemotherapy for rectal cancer. Nevertheless, there are still relatively few relevant studies.
This study aims to evaluate the risk of second primary urinary system neoplasms(SPUNs) in patients undergoing different treatment strategies during the perioperative period of rectal cancer and conduct a prognostic analysis of SPUNs.
Methods
Database and participants
The data were derived from the Surveillance, Epidemiology, and End Results (SEER) database in the United States, covering eight registries from 1975 to 2020. A total of 137,443 patients diagnosed with rectal cancer or cancer of the rectosigmoid junction were initially identified. Ultimately, 85,799 patients aged 18 years or older who underwent surgical treatment for localized or regionally metastatic rectal cancer were included in the study (Fig. 1).
The primary cancer sites were coded according to the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3). The included patients were pathologically diagnosed with rectal cancer (C19.9) or cancer of the rectosigmoid junction (C20.9). The exclusion criteria were as follows:
-
a.
patients under 18 years of age;
-
b.
patients with unknown race;
-
c.
patients who did not undergo surgery or had missing surgical information;
-
d.
patients with distant metastasis.
Main workflow of the study. Abbreviations: SEER, Surveillance, Epidemiology, and End Results. S, Surgery alone. SR, Surgery plus Radiotherapy. SC, Surgery plus Chemotherapy. SCR, Surgery plus ChemoRadiotherapy. SPUNs, Second Primary Urinary System Neoplasms. OPUNs, Original Primary Urological Neoplasms. PSM, Propensity Score Matching.
Therapeutic interventions for RC
Patients were divided into four groups according to different postoperative adjuvant treatment modalities: surgery alone group (S), surgery plus chemoradiotherapy group (SCR), surgery plus radiotherapy group (SR), and surgery plus chemotherapy group (SC). The surgical grouping was based on the “Reason no cancer-directed surgery” variable in the SEER database. Patients with “Surgery performed” were included, while those who did not undergo colorectal cancer surgery were excluded to avoid survival bias. Whether a patient received chemotherapy was determined by referring to the “Chemotherapy recode (yes, no/unk)” variable in the SEER database, and radiotherapy was judged according to the “Radiation recode” variable. The S group served as the reference group, while the other three groups were the observation groups. The aim was to analyze the impact of postoperative chemotherapy, radiotherapy, and chemoradiotherapy on the development of SPUNs in patients with RC. The SEER database only collects data on the initial cancer treatment process, and the dose of radiotherapy and the specific drugs used in chemotherapy are not registered in the SEER database. Considering the significant impact of radiotherapy doses and specific chemotherapy drugs on carcinogenesis, this issue will be explored in depth in the subsequent discussion.
Definition and Follow-up of Spuns
In accordance with the 5 th revision of the Warren and Gates criteria by the National Cancer Institute of the United States, a second primary cancer is defined as a metachronous invasive solid cancer that occurs ≥ 6 months after the primary cancer30.
There is yet to be a unified standard in oncology research for defining the time interval between the first primary cancer and the second primary cancer. Different researchers hold varying viewpoints. For instance, some researchers claim that a new cancer that emerges six months after the initial cancer diagnosis can be regarded as a second primary cancer. Others believe that this time interval should be one year. Still, some researchers take a more cautious approach and set the cut-off at five years. If the interval is too short, for example, within one year, it is necessary to reflect on whether previous treatments for the primary cancer, such as radiotherapy and chemotherapy, have played a negative role in inducing new cancers. On the other hand, a relatively long time interval may blur the etiological links between the primary cancer and the second primary cancer, which is not conducive to accurately identifying the carcinogenic factors. After comprehensive consideration, this study included patients who developed any urinary tract tumor three years after the diagnosis of RC in the trial.
Meanwhile, the SEER program adheres to the ICD-O-3 guidelines to differentiate SPUNs from recurrent diseases. To obtain a comprehensive estimate of the risk of SPUNs, we first estimated the risk of SPUNs across all type combinations. Then, we separately estimated the risks of different types of urinary tract tumors, including bladder cancer (C67); kidney cancer (C64, C65); cancer of the ureter (C66); and urethral cancer (C68).
The follow-up for SPUNs commenced three years after the diagnosis of RC and concluded upon the diagnosis of any SPUNs, all-cause death, or 500 months of follow-up, whichever occurred first. The follow-up cut-off point was designated as January 1, 2020 (the SEER data was released in April 2023).
Statistical methods
The chi-square test is used to compare categorical data. For continuous factors with a normal distribution, the t-test or analysis of variance is employed. For continuous factors with a non-normal distribution, the Mann-Whitney U test is adopted. COX regression analysis, including univariate and multivariate analyses, is used to evaluate the risk factors for developing SPUNs. Poisson regression is employed to dynamically assess the impact of radiotherapy and chemotherapy on the relative risk RR of bladder cancer, based on the patient’s latency period, year of diagnosis, and age. The Kaplan-Meier method is used to analyze the overall survival rate (OS) and cancer-specific survival rate (CSS) of patients with second primary urinary system tumors after rectal cancer, under different treatment strategies. Propensity score matching (PSM) is performed between SPUNs and other primary urinary neoplasms (OPUNs), and then the Kaplan-Meier method is used to analyze the overall survival rates of the two groups.
Ethical statement
This study adhered to relevant guidelines and regulations. Since all data used in this study were derived from the publicly accessible SEER database in the United States, informed consent from research subjects and their legal representatives was not required. Therefore, this study was deemed exempt from ethical review due to the use of publicly available data31,32.
Results
General clinical information of patients
A total of 137,443 patients with RC were identified (Fig. 1). According to the inclusion and exclusion criteria, a total of 85,799 patients were determined to be included in the final cohort. There were 54,617 patients (63.7%) in S, 19,734 patients (23%) in SCR, 6,697 patients (7.8%) in SR, and 4,751 patients (5.5%) in SC. The baseline characteristics of RC patients with different treatment modalities are shown in Table 1. After a 3-year latency period, a total of 766 patients developed SPUNs among 56,250 patients. Among them, the incidence of SPUNs was 0.84% (458/54,617) in S, 1.04% (206/19,734) in SCR, 1.05% (70/6,697) in SR, and 0.02% (32/4,751) in SC. Classified by organs, 531 patients had second primary bladder cancer (SPBC), 182 had second primary kidney cancer (SPKC), 37 had second primary urethral cancer (SPUaC), and 16 had secondary primary ureteral cancer (SPUrC). The baseline characteristics of patients with SPUNs are shown in Table S1 in the supplementary materials.
Cumulative incidence of Spuns
Firstly, we analyzed the differences in the cumulative incidence of SPUNs among four groups of patients.Significant differences were observed in the cumulative incidence of SPUNs among these groups (at 300 months of follow-up, the incidence was 2.85% in the surgery-only group, 4.7% in the surgery combined with chemoradiotherapy group, 3.29% in the surgery combined with chemotherapy group, and 3.88% in the surgery combined with radiotherapy group; P = 2.8 × 10⁻⁴) (Fig. 2 A). To more precisely evaluate the impact of chemotherapy and radiotherapy on SPUNs, we further defined separate chemotherapy and radiotherapy groups and monitored the cumulative incidence of SPUNs in these groups.The results showed that the cumulative incidence of SPUNs was significantly higher in patients who received chemotherapy. Specifically, around the 400 th month after chemotherapy, the cumulative incidence peaked. By the 500th month after chemotherapy, the cumulative incidence was roughly equivalent to that of patients who did not receive chemotherapy (at 300 months of follow-up, the cumulative incidences were 4.42% and 2.93%, respectively; P = 3.8 × 10⁻³) (Fig. 2B). Similarly, compared with patients who did not receive radiotherapy, the incidence of SPUNs in patients who received radiotherapy after rectal cancer surgery was significantly higher (at 300 months of follow-up, the cumulative incidences were 4.33% and 2.85%, respectively; P = 2.56 × 10⁻⁶) (Fig. 2 C).
We further divided urinary system tumors into bladder cancer, kidney cancer, ureteral cancer, and urethral cancer for in-depth analysis. Significant differences were observed in the cumulative incidence of SPBC among the four groups (at 300 months of follow-up, the cumulative incidence was 1.99% in the surgery-only group, 3.61% in the surgery combined with chemoradiotherapy group, 2.87% in the surgery combined with radiotherapy group, and 2.03% in the surgery combined with chemotherapy group; P = 5 × 10⁻⁵) (Fig. 2 D). Chemotherapy increased the incidence of SPBC (after 300 months of follow-up, the cumulative incidence was 3.15% in the chemotherapy group and 2.06% in the non-chemotherapy group; P = 0.01) (Fig. 2 E). Radiotherapy also increased the incidence of SPBC (at 300 months of follow-up, the cumulative incidence was 3.25% in the radiotherapy group and 1.99% in the non-radiotherapy group; P = 2 × 10⁻⁵) (Fig. 2 F). Moreover, significant differences were observed in the cumulative incidence of SPUaC among the four treatment modalities (at 300 months of follow-up, the cumulative incidence was 0.129% in the surgery-only group, 0.131% in the surgery combined with chemoradiotherapy group, 0.189% in the surgery combined with chemotherapy group, and 0.792% in the surgery combined with radiotherapy group; P = 1.4 × 10⁻⁴) (Fig. 2 G). Radiotherapy was associated with an increased incidence of SPUaC (at 300 months of follow-up, the cumulative incidence was 0.401% in the radiotherapy group and 0.129% in the non-radiotherapy group; P = 5.1 × 10⁻³) (Fig. 2 I). However, no significant association was observed between chemotherapy and increased incidence of SPUaC (at 300 months of follow-up, the cumulative incidence was 0.14% in the chemotherapy group and 0.16% in the non-chemotherapy group; P = 0.28) (Fig. 2 H).
In addition, the study showed that the four different treatment modalities were not associated with the incidence of SPKC or SPUrC (Figs. 3 A, D).For patients who received combined chemotherapy after rectal cancer surgery, the risks of developing SPKC (Fig. 3 B), SPUrC (Fig. 3 E), and SPUaC did not increase significantly. This suggests that within the scope of the current study, chemotherapy drugs may not have a significant impact on the carcinogenic risk of these urinary system organs. However, further in-depth research is still needed to comprehensively evaluate potential long-term effects. On the other hand, combined radiotherapy after rectal cancer surgery was not associated with the risk of developing SPKC (Fig. 3 C) or SPUrC (Fig. 3 F). From an anatomical perspective, the special positions of the kidneys and ureters may constitute a natural protective mechanism, thus shielding them from the potential effects of radiotherapy.
(A) Comparison of SPUNs cumulative incidence among 4 groups of patients. (B) Comparison of SPUNs cumulative incidence between chemo-treated and non-chemo-treated patients. (C) Comparison of SPUNs cumulative incidence between radio-treated and non-radio-treated patients. (D) Comparison of SPBC cumulative incidence among 4 groups of patients. (E) Comparison of SPBC cumulative incidence between chemo-treated and non-chemo-treated patients. (F) Comparison of SPBC cumulative incidence between radio-treated and non-radio-treated patients. (G) Comparison of SPUaC cumulative incidence among 4 groups of patients. (H) Comparison of SPUaC cumulative incidence between chemo-treated and non-chemo-treated patients. (I) Comparison of SPUaC cumulative incidence between radio-treated and non-radio-treated patients.
Cumulative Incidence. (A) Comparison of SPKC cumulative incidence among 4 groups of patients. (B) Comparison of SPKC cumulative incidence between chemo-treated and non-chemo-treated patients. (C) Comparison of SPKC cumulative incidence between radio-treated and non-radio-treated patients. (D) Comparison of SPUrC cumulative incidence among 4 groups of patients. (E) Comparison of SPUrC cumulative incidence between chemo-treated and non-chemo-treated patients. (F) Comparison of SPUrC cumulative incidence between radio-treated and non-radio-treated patients.
Risk analysis of Spuns in RC survivors
Using univariate and multivariate COX regression analysis methods, we deeply explored the risk factors for RC patients developing bladder cancer, kidney cancer, ureteral cancer, and urethral cancer. For each of the factors analyzed, including demographic factors (age, gender, race), temporal factors (year of diagnosis), and treatment modalities (radiotherapy and chemotherapy), among others, we calculated the hazard ratio (HR) and its corresponding 95% confidence interval (95% CI) to comprehensively assess their associations with the development of these secondary primary cancers.
Secondary Primary Bladder Cancer:
Univariate COX regression analysis: The results showed that elderly male RC patients had a higher risk of developing SPBC (for those aged 50–70 years, HR: 3.50, 95% CI: 2.47–4.95, P = 1.81 × 10⁻¹²; for those over 70 years old, HR: 11.58, 95% CI: 7.99–17.08, P = 7.32 × 10⁻³⁷). Male patients had a higher risk than female patients (HR: 3.99, 95% CI: 3.21–4.97, P = 2.54 × 10⁻³⁵). Compared with White patients, patients of Black and other races had a lower risk of SPBC (for Black patients, HR: 0.42, 95% CI: 0.21–0.81, P = 8.94 × 10⁻³; for other races, HR: 0.42, 95% CI: 0.29–0.61, P = 4.62 × 10⁻⁶). The risk of SPBC in RC patients gradually increased from 1975 to 2020 (from 1995 to 2004, HR: 1.88, 95% CI: 1.43–2.48, P = 1.08 × 10⁻⁵; from 2005 to 2020, HR: 2.46, 95% CI: 1.70–3.56, P = 1.69 × 10⁻⁶). Additionally, patients who received surgery combined with radiotherapy had a higher risk than those who did not receive radiotherapy (HR: 1.51, 95% CI: 1.12–2.04, P = 7.7 × 10⁻³) (Fig. 4 A). (HR: 1.51, 95% CI: 1.12–2.04, P = 7.7 × 10⁻³) (Fig. 4 A).Multivariate COX regression analysis: The results indicated that age (for those aged 50–70 years, HR: 3.47, 95% CI: 2.46–4.89, P = 1.48 × 10⁻¹²; for those over 70 years old, HR: 10.18, 95% CI: 7.00–14.81, P = 7.28 × 10⁻³⁴), year of diagnosis (from 1995 to 2004, HR: 2.00, 95% CI: 1.52–2.64, P = 9.95 × 10⁻⁷; from 2005 to 2020, HR: 2.58, 95% CI: 1.79–3.73, P = 4.63 × 10⁻⁷), and combined radiotherapy after rectal cancer surgery (HR: 1.62, 95% CI: 1.20–2.19, P = 1.59 × 10⁻³) were independent risk factors for RC patients developing SPBC. Conversely, race (for Black patients, HR: 0.39, 95% CI: 0.20–0.65, P = 5.65 × 10⁻³; for other races, HR: 0.45, 95% CI: 0.31–0.65, P = 1.88 × 10⁻⁵) was an independent protective factor against SPBC development (Fig. 4 B).
COX regression analyses of the risk factors for RC patients developing SPBC. (A) Univariate COX regression analysis. (B) Multivariate COX regression analysis. Abbreviation: Period, The year of RC diagnosis. Origin, Origin recode NHIA (Hispanic, Non-Hisp). Non-SHL, Non-Spanish-Hispanic-Latino. SHL, Spanish-Hispanic-Latino.
Secondary Primary Kidney Cancer:
Univariate analysis: The analysis demonstrated that the risk of developing SPKC in RC patients increased with age (for those aged 50–70 years, HR: 2.26, 95% CI: 1.34–3.82, P = 2.31 × 10⁻³; for those over 70 years old, HR: 4.83, 95% CI: 2.67–8.72, P = 1.81 × 10⁻⁷). Male RC patients had a higher risk of SPKC compared with female patients (male, HR: 2.88, 95% CI: 2.03–4.10, P = 4.3 × 10⁻⁴). Additionally, the risk of developing SPKC was higher in patients diagnosed with rectal cancer from 2005 to 2020 compared with other years (2005–2020, HR: 1.81, 95% CI: 1.02–3.23, P = 0.043) (Fig. 5 A). Multivariate analysis: The analysis revealed that age (for those aged 50–70 years, HR: 2.27, 95% CI: 1.35–3.82, P = 2.00 × 10⁻³; for those over 70 years old, HR: 4.31, 95% CI: 2.40–7.74, P = 1.03 × 10⁻⁶) and year of diagnosis (2005–2020, HR: 1.91, 95% CI: 1.08–3.40, P = 0.027) were independent risk factors for RC patients developing SPKC (Fig. 5 B).
Secondary Primary Ureteral Cancer:
Univariate COX regression analysis: The results suggested that male rectal cancer patients had a higher risk of developing SPUrC compared with female patients (male, HR: 3.77, 95% CI: 1.06–13.42, P = 0.04) (Fig. 6 A). Multivariate COX regression analysis: After adjusting for age at diagnosis and treatment methods, no significant statistical differences were found (Fig. 6 B).
Secondary Primary Urethral Cancer:
Univariate COX regression analysis: It highlighted that the development of SPUaC after rectal cancer was associated with gender and radiotherapy. Male patients had a higher risk of the disease than female patients (for male patients, HR: 3.20, 95% CI: 1.39–7.39, P = 6.42 × 10⁻³). Patients who received combined radiotherapy after rectal cancer surgery had a relatively high risk of developing SPUaC (for radiotherapy patients, HR: 4.25, 95% CI: 1.78–10.11, P = 1.1 × 10⁻³) (Fig. 7 A). Multivariate COX regression analysis: It was found that combined radiotherapy after surgery (for radiotherapy patients, HR: 4.66, 95% CI: 1.96–11.06, P = 4.88 × 10⁻⁴) was an independent risk factor for rectal cancer patients to develop SPUaC (Fig. 7 B).
Evaluation of the dynamic risk and incidence of spuns
The relative risk (RR) of developing SPBC after chemotherapy increased with the prolongation of the latency period (from 0 to 100 months, RR: 0.869, 95% CI: 0.553–1.35, P = 0.6004; from 100 to 200 months, RR: 1.323, 95% CI: 1.136–2.065, P = 0.198; from 200 to 300 months, RR: 1.535, 95% CI: 1.136–2.065, P = 0.0182; after 300 months, RR: 1.918, 95% CI: 1.116–2.433, P = 0.0127) (Fig. 8 A).
The RR of developing SPBC after radiotherapy also increased with the prolongation of the latency period (from 0 to 100 months, RR: 0.667, 95% CI: 0.423–1.027, P = 0.1317; from 100 to 200 months, RR: 1.323, 95% CI: 1.176–1.937, P = 6.39 × 10⁻³; from 200 to 300 months, RR: 1.767, 95% CI: 1.331–2.34, P = 8.83 × 10⁻⁵; after 300 months, RR: 1.777, 95% CI: 1.116–2, P = 0.0107) (Fig. 8 B).
The study also found that there was no association between the RR of developing SPBC after chemotherapy and the year of RC diagnosis (from 1975 to 1984, RR: 0.67, 95% CI: 0.4–1.7, P = 0.78; from 1985 to 1994, RR: 0.36, 95% CI: 1–1.9, P = 0.068; from 1995 to 2004, RR: 0.35, 95% CI: 1–1.6, P = 0.181; from 2005 to 2020, RR: 0.7, 95% CI: 0.7–1.7, P = 0.642) (Fig. 8 C).
The RR of developing SPBC after radiotherapy was associated with the year of RC diagnosis. In particular, the RR value reached the highest and showed a significant difference during 1975–1984 (from 1975 to 1984, RR: 1.75, 95% CI: 1.21–2.46, P = 0.009; from 1985 to 1994, RR: 1.15, 95% CI: 0.87–1.53, P = 0.404; from 1995 to 2004, RR: 1.32, 95% CI: 1.02–1.69, P = 0.071; from 2005 to 2020, RR: 1.09, 95% CI: 0.72–1.64, P = 0.742) (Fig. 8 D).
There were slight differences in the RR of developing SPBC after chemotherapy among different age groups, but these differences did not reach statistical significance (from 18 to 50 years old, RR: 2, 95% CI: 1.09–3.65, P = 0.0569; from 50 to 70 years old, RR: 1.12, 95% CI: 0.9–1.39, P = 0.387; over 70 years old, RR: 1.32, 95% CI: 0.97–1.8, P = 0.13) (Fig. 8 E).
Regarding radiotherapy, the RR value of developing SPBC was the highest in the 18–50 age group (from 18 to 50 years old, RR: 3.11, 95% CI: 1.76–5.67, P = 0.00135; from 50 to 70 years old, RR: 1.16, 95% CI: 0.95–1.41, P = 0.2286; over 70 years old, RR: 1.31, 95% CI: 0.99–1.74, P = 0.1029) (Fig. 8 F).
Given that the current study results show no association between radiotherapy or chemotherapy and the risk of developing SPKC and SPUrC, there is no need for a dynamic assessment of the correlation between these factors and the incidence of SPKC and SPUrC. Additionally, since only 37 cases of patients who developed SPUaC after rectal cancer were included in this study, and after grouping according to relevant factors, the sample size in some groups was zero. Due to the small and unevenly - distributed sample size, it was not possible to dynamically evaluate the impact of radiotherapy on the relative risk of developing SPUaC.
The specific RRs, 95% CIs, and P values are presented in Table S 2 of the supplementary file.
RRs and 95% CIs of developing SPBC. (A) The RR of chemotherapy during the latency period from RC to SPBC. (B) The RR of radiotherapy during the latency period from RC to SPBC. (C) The RR of chemotherapy for patients with RC diagnosed in different years in developing SPBC. (D) The RR of radiotherapy for patients with RC diagnosed in different years in developing SPBC. (E) The RR of chemotherapy for patients with RC at different age groups in developing SPBC. (F) The RR of radiotherapy for patients with RC at different age groups in developing SPBC.
Survival outcomes of spuns
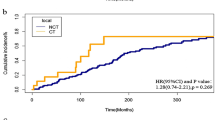
We used the Kaplan - Meier method to conduct CSS and OS analyses on patients with SPUNs in the groups of rectal cancer patients who received postoperative chemotherapy, postoperative radiotherapy, postoperative chemoradiotherapy, and surgery alone. The results showed that there were significant differences in CSS and OS among the four groups (CSS, P = 1.6 × 10⁻³; OS, P = 3.6 × 10⁻²). Overall, patients who received postoperative radiotherapy had relatively lower survival rates (Fig. 9 A - B).
Subsequently, we performed propensity score matching (PSM) at a ratio of 1:10 for patients with SPUNs in the four groups and those with OPUNs based on age, gender, race, and age at rectal cancer diagnosis (Table S 3). Then, we used the Kaplan - Meier method to draw survival curves. The results of the survival analysis showed that the overall survival rate of patients with SPUNs in the postoperative radiotherapy group was lower than that of patients with OPUNs (P = 1.1 × 10⁻²) (Fig. 10 A), and there were no significant differences in the overall survival rates between patients with SPUNs and those with OPUNs in the other three groups (Fig. 10 B - D).
Survival Outcomes of Patients with SPUNs. (A) Comparison of OS between patients with SPUNs in the postoperative radiotherapy group after rectal cancer and patients with OPUNs. (B) Comparison of OS between patients with SPUNs in the surgery - alone group after rectal cancer and patients with OPUNs. (C) Comparison of OS between patients with SPUNs in the postoperative chemoradiotherapy group after rectal cancer and patients with OPUNs. (D) Comparison of OS between patients with SPUNs in the postoperative chemotherapy group after rectal cancer and patients with OPUNs.
Discussion
This study aimed to evaluate the risk of developing SPUNs in patients during the perioperative period of RC under different treatment strategies. Through the analysis of a large amount of patient data, we obtained some meaningful results. The study utilized the multi-center SEER database, which had a large sample size and covered patient information over a long time range, making the research results highly representative. Meanwhile, various statistical methods were employed for analysis, enhancing the reliability of the results.
Through organ-specific analysis, the results showed that the cumulative incidence of SPBC and SPUaC in rectal cancer patients who received postoperative combined radiotherapy was significantly higher than that in patients who did not receive combined radiotherapy. This is consistent with the conclusions of most cutting-edge research in the relevant field, that is, ‘radiotherapy is an important risk factor for SPC’12,33,34. Cox regression analysis showed that factors such as age, gender, race, and the year of diagnosis were closely related to the occurrence of SPUNs. In particular, elderly men, white individuals, and patients diagnosed in later years had a higher risk, which is similar to the research conclusion of Jia Huixun et al.35. The increase in the incidence of SPBC and SPUaC after radiotherapy may be attributed to the damaging effect of radiation on the DNA of bladder and urethral cells, which disrupts the normal functions of cells and the stability of genetic material, potentially triggering the transformation of normal cells into cancer cells36. Many studies have shown that patients receiving radiotherapy face the risk of radiation-related SPC, and this risk persists for decades after exposure. Since the risk of most radiation-sensitive malignancies increases with the radiation dose, tissues within or near the radiotherapy treatment field usually have the highest risk37. For example, a study in South Korea pointed out that for thyroid cancer patients receiving radioactive iodide treatment, the risk of SPC is linearly positively correlated with the cumulative dose of RAI. The risk significantly increases starting from 3.7 GBq, varies in different dose ranges, and is significantly higher in the high-dose group38. Previous studies have confirmed that ionizing radiation can cause gene mutations. In 2010, Mullenders et al. found that cells exposed to ionizing radiation can lead to single-strand and double-strand DNA breaks (DSBs). Single-strand breaks can be converted into DSBs during cell replication, and DSBs can cause gene mutations, resulting in the malignant transformation of irradiated cells39. Changes in DNA repair proteins may also increase the risk of a second malignancy. For example, ataxia-telangiectasia mutated is a protein that can sense DNA damage and initiate the DNA repair cascade. Mutations in this gene can lead to increased radiosensitivity and cancer susceptibility40. It is known that T-cell subsets play a role in ionizing-radiation-induced normal tissue damage, acquiring pro-inflammatory and pro-fibrotic phenotypes in the acute and chronic stages of tissue response41. Therefore, patients with autoimmune diseases such as interstitial lung disease, inflammatory bowel disease, and connective tissue diseases are more likely to experience radiation toxicity reactions42.In addition, the study found that the risk of SPBC in rectal cancer patients who received postoperative combined chemotherapy was significantly increased, but it did not increase the incidence of SPKC, SPUrC, and SPUaC. For a long time, cytotoxic chemotherapy has been considered to increase the risk of hematological malignancies such as leukemia and is also associated with an increased risk of various other types of SPC43. The use of chemotherapy and hormone therapy in systemic anti-cancer treatment is associated with an increased risk of SPC. Treatment-related acute myeloid leukemia and myelodysplastic syndrome are the most well-defined examples. Alkylating agents, topoisomerase-II inhibitors, and antimetabolites have the greatest potential for leukemogenesis44. In addition, chemotherapy can also increase the risk of solid malignancies, which can occur more than ten years after exposure to chemotherapy. In particular, exposure to alkylating agents can increase the risk of developing lung cancer, thyroid cancer, gastrointestinal cancer, bladder cancer, and sarcoma43. The association between bladder cancer and cyclophosphamide exposure is one example of the carcinogenic potential of a specific alkylating agent for a specific site, due to the direct exposure of the bladder epithelium to cyclophosphamide metabolites. Furthermore, exposure to procarbazine can increase the risk of gastrointestinal malignancies45. Most cases occur 3 to 10 years after exposure to chemotherapy drugs such as alkylating agents, accompanied by the loss of chromosomes 5 or 7 and TP53 gene mutations. Other abnormalities, such as recurrent translocations involving T(3;21), T(8;21), T(11q23), T(15;17), and INV(16), are related to the use of topoisomerase II inhibitors46. This study was mainly carried out based on the SEER database, which has unique advantages and limitations in cancer research. On the one hand, the data of the SEER database come from a large number of individuals, covering patients’ personal information, tumor characteristics, diagnostic stages, and detailed treatment plans, providing rich materials for cancer research. In addition, the database also contains data of cancer patients who received non-surgical treatment, making up for the deficiency of some databases that are limited to surgical patients, thus providing a more accurate calculation basis for the research. However, the SEER database also has some limitations. First, it does not collect some key data before and after surgery, making it not the most ideal choice when studying the surgical outcomes of different cancers. Second, the SEER database only covers the data of some states participating in the project and cannot comprehensively reflect the overall situation of cancer incidence, treatment utilization, and treatment outcomes across the United States. In addition, although the SEER database can track the long-term survival of patients, the available research endpoints are relatively limited in long-term studies47,48. Given the above limitations, there may be a problem of sample bias in this study. Since the analysis is only based on the patient data in the SEER database, it is difficult to accurately compare the differences between different patient groups, which limits the reliability and general applicability of the research conclusions to a certain extent.In conclusion, our research results emphasize the need for a more refined approach in the postoperative treatment of rectal cancer patients to balance the benefits of adjuvant treatment with the potential risks of SPUNs. Further research is crucial for optimizing treatment strategies and improving the long-term prognosis of cancer survivors.
Conclusion
This study mainly explored the association between RC treatment modalities and the development of SPUNs. Organ-specific analyses showed that patients who received postoperative radiotherapy or chemotherapy for rectal cancer had a higher incidence of SPBC compared with those who did not receive either treatment. Patients who received postoperative radiotherapy had a higher incidence of SPUaC than those who did not receive radiotherapy. There was no association between combined chemotherapy or radiotherapy after rectal cancer and the risk of developing SPKC and SPUrC. In the univariate analysis, the relevant risk factors for different urinary system cancers in rectal cancer patients had their own characteristics. For SPBC, the risk factors were associated with advanced age, male gender, racial differences, year of diagnosis, and whether postoperative radiotherapy was administered. The risk factors for SPKC mainly involved age, gender, and year of diagnosis. SPUrC was only related to gender. The risk factors for SPUaC included gender and whether radiotherapy was combined.In the multivariate analysis, the independent prognostic factors for SPBC included age, year of diagnosis, postoperative radiotherapy, and race. The independent factors affecting SPKC were age and year of diagnosis. No significant influencing factors were found for SPUrC. Postoperative combined radiotherapy was an independent risk factor for SPUaC. Survival analysis showed that patients who received radiotherapy after surgery had a lower survival rate.
Data availability
The data supporting the findings of this study are publicly available through the Surveillance, Epidemiology, and End Results (SEER) Program, managed by the National Cancer Institute (NCI). The SEER database can be accessed via the following URL: https://seer.cancer.gov/.
Abbreviations
- ICD-O-3:
-
International Association for Cancer Coding, Classification of Oncological Diseases, Third Edition
- RC:
-
Rectal Cancer
- SPUNs:
-
Secondary Primary Urinary System Neoplasms
- SEER:
-
Surveillance, Epidemiology, and End Results database
- S:
-
Surgery Alone Group
- SR:
-
Surgery plus Radiotherapy Group
- SC:
-
Surgery plus Chemotherapy Group
- SCR:
-
Surgery plus Chemoradiotherapy Group
- CRC:
-
Colorectal Cancer
- SPC:
-
Second Primary Cancer
- OPUNs:
-
Original Primary Urological Neoplasms
- PSM:
-
Propensity Score Matching
- RR:
-
Relative Risk
- CI:
-
Confidence Interval
- OS:
-
Overall Survival
- CSS:
-
Cancer-Specific Survival
- COX :
-
Proportional Hazards Regression Model
- SPBC :
-
Secondary Primary Bladder Cancer
- SPKC :
-
Secondary Primary Kidney Cancer
- SPUaC:
-
Secondary Primary Urethral Cancer
- SPUrC:
-
Secondary Primary Ureteral Cancer
- HR:
-
Hazard Ratio
- FOLFOX :
-
Fluorouracil, Leucovorin, and Oxaliplatin
References
Eng, C. et al. A Comprehensive Framework for early-onset Colorectal cancer Research (The Lancet Oncology, 2022).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA: A Cancer. J. Clin. 74, 12–49 (2024).
DeSantis, C. E. et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer. J. Clin. 64, 252–271 (2014).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2019. CA: A Cancer. J. Clin. 69, 363–385 (2019).
Sebag-Montefiore, D. et al. Preoperative Radiotherapy Versus Selective Postoperative Chemoradiotherapy in Patients with Rectal cancer (MRC CR07 and NCIC-CTG C016): a Multicentre, Randomised Trial (The Lancet, 2009).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median Follow-Up of 11 years. J. Clin. Oncol. 30, 1926–1933 (2012).
Garcia-Aguilar, J. et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J. Clin. Oncol. 40, 2546–2556 (2022).
Sauer, R. et al. Preoperative Versus Postoperative Chemoradiotherapy for Rectal cancer (The New England Journal of Medicine, 2004).
Akgun, E. et al. The Effects of Chemoradiotherapy on Recurrence and Survival in Locally Advanced Rectal Cancers with Curative Total Mesorectal Excision: a Prospective, Nonrandomized Study (World Journal of Surgical Oncology, 2017).
Martling, A. et al. Risk of second primary cancer in patients treated with radiotherapy for rectal cancer. Br. J. Surg., (2016).
Wang, T-H. et al. Second primary malignancy risk after radiotherapy in rectal cancer survivors. World J. Gastroenterol. 24, 4586–4595 (2018).
Rombouts, A. J. M. et al. Incidence of Second Tumors after Treatment with or Without Radiation for Rectal cancer (Annals of Oncology, 2017).
McPartland, C. et al. Risk of secondary malignancies after pelvic radiation: A Population-based analysis. Eur. Urol. Open. Sci. 63, 52–61 (2024).
Okajima, K. et al. Multiple primary malignancies in patients with prostate cancer: increased risk of secondary malignancies after radiotherapy. Int. J. Clin. Oncol., (2012).
Wang, Y. et al. Incidence and Associated Factors of Developing Second Pelvic Malignant Neoplasms among Prostate cancer Patients Treated with Radiotherapy (Frontiers in Oncology, 2023).
Adjei Boakye, E. et al. Risk of Second Primary Cancers among Survivors of Gynecological Cancers (Gynecologic Oncology, 2020).
Mukand, N. H. et al. Abstract C020: Racial Differences in the Risks of Second Primary Gynecologic Cancers Following Chemotherapy for Malignant Ovarian Tumors: Asian Subgroups in the United States, 2000–2016 (Cancer Epidemiology Biomarkers & Prevention, 2020).
Reddy, V. K. & Vapiwala, N. Secondary malignancy after radiotherapy: not always a secondary concern. Nat. Reviews Urol. 18, 513–514 (2021).
Shiota, M. et al. Secondary bladder cancer after anticancer therapy for prostate cancer: reduced comorbidity after androgen-deprivation therapy. J. Clin. Oncol., (2015).
de la Taille, A. & Zerbib, M. Complications urologiques de La radiothérapie. Ann. Urol. (Paris). 37, 345–357 (2003).
Chorbińska, J., Krajewski, W. & Zdrojowy, R. Urological complications after radiation therapy—nothing ventured, nothing gained: a narrative review. Translational Cancer Res. 10, 1096–1118 (2021).
Liang, F. et al. Risk of second primary cancers in cancer patients treated with cisplatin: a systematic review and meta-analysis of randomized studies. BMC Cancer 17, (2017).
Allodji, R. S. et al. Role of radiotherapy and chemotherapy in the risk of leukemia after childhood cancer: an international pooled analysis. Int. J. Cancer. 148, 2079–2089 (2020).
Wei, J-L., Jiang, Y-Z. & Shao, Z-M. Survival and chemotherapy-related risk of second primary malignancy in breast cancer patients: a SEER-based study. Int. J. Clin. Oncol. 24, 934–940 (2019).
Yuan, Y. et al. Risk of second primary thyroid cancer in cancer survivors. Sci. Rep. 14, (2024).
Gao, T. et al. Association between chemotherapy for surgically treated rectal cancer and second primary endometrial cancer. Sci. Rep. 14, (2024).
GOREN, M. A. R. S. H. A. L. L. P., MD, R. E. B. A. K., WRIGHT, P. H. D. & HOROWITZ, M. A. R. C. E. M.D. AND CHARLES B. PRATT (M.D.: Cancer Chemotherapy-Induced Tubular Nephrotoxicity Evaluated by Immunochemical Determination of Urinary Adenosine Deaminase Binding Protein, 1986).
Mohri, J. et al. Predisposing factors for chemotherapy-induced nephrotoxicity in patients with advanced esophageal cancer who received combination chemotherapy with docetaxel, cisplatin, and 5-fluorouracil. J. Translational Intern. Med. 6, 32–37 (2018).
Ries, F. & Klastersky, J. Nephrotoxicity induced by Cancer chemotherapy with special emphasis on cisplatin toxicity. Am. J. Kidney Dis. 8, 368–379 (1986).
Charles, G., Moertel, M. B. D., Archie, H. & Baggenstoss.: MULTIPLE PRIMARY MALIGNANT NEOPLASMS II. Tumors of different tissues or organs. CANCER 14, March - April 1961.
Shrestha, B. & Dunn, L. The declaration of Helsinki on medical research involving human subjects: A review of seventh revision. J. Nepal Health Res. Counc. 17, 548–552 (2020).
Wen, B. et al. The 2024 Revision of the Declaration of Helsinki: a Modern Ethical Framework for Medical Research (Postgraduate Medical Journal, 2024).
Rombouts, A. J. M. et al. Does pelvic radiation increase rectal cancer incidence? – A systematic review and meta-analysis. Cancer Treat. Rev. 68, 136–144 (2018).
Berrington de Gonzalez, A. et al. Risk of second cancers according to radiation therapy technique and modality in prostate Cancer survivors. Int. J. Radiation Oncology*Biology*Physics. 91, 295–302 (2015).
Jia, H. et al. Second primary malignancies in patients with colorectal cancer: A Population-Based analysis. Oncologist 25, e651–e658 (2020).
Little, J. B. Radiation carcinogenesis. Carcinogenesis vol.21 (2000).
de Berrington, A. et al. Second solid cancers after radiation therapy: A systematic review of the epidemiologic studies of the radiation Dose-Response relationship. Int. J. Radiation Oncology*Biology*Physics. 86, 224–233 (2013).
Kim, K. J. et al. Linear association between radioactive iodine dose and second primary malignancy risk in thyroid cancer. JNCI: J. Natl. Cancer Inst. 115, 695–702 (2023).
Mullenders, L. et al. Assessing cancer risks of low-dose radiation. Nat. Rev. Cancer. 9, 596–604 (2009).
Taylor, A. M. R. Molecular pathology of ataxia telangiectasia. J. Clin. Pathol. 58, 1009–1015 (2005).
Citrin, D. E. & Mitchell, J. B. Mechanisms of normal tissue injury from irradiation. Semin. Radiat. Oncol. 27, 316–324 (2017).
Daly, L. E. et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer. 116, 310–317 (2017).
Lindsay, M. et al. MD, MSCE: The Rising Incidence of Second Cancers: Patterns of Occurrence and Identification of Risk Factors for Children and Adults. American Society of Clinical Oncology Educational Book Volume 34, Number 1, (2014).
Vardiman, J. W. The World Health Organization (WHO) Classification of Tumors of the Hematopoietic and Lymphoid Tissues: an Overview with Emphasis on the Myeloid Neoplasms (Chemico-Biological Interactions, 2009).
Dracham, C. B., Shankar, A. & Madan, R. Radiation induced secondary malignancies: a review Article. Radiation Oncol. J., (2018).
Ellis, N. A. et al. MDM2 SNP309 and TP53 Arg72Pro interact to alter therapy-related acute myeloid leukemia susceptibility. Blood, (2008).
Kuo, T-M. & Mobley, L. R. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control. 27, 1117–1126 (2016).
Martin, A. et al. A guide to large data sets for population-based cancer research: strengths, limitations, and pitfalls. Cancer 130, 3802–3814 (2024).
Acknowledgements
The authors appreciate technical help by staff in the Department of General surgery at the General Hospital of Northern Theater Command.
Funding
This study was financially supported by Liaoning province livelihood science and technology joint plan (2021 JH2/10300106).
Author information
Authors and Affiliations
Contributions
NL, XNW, XYW and CZ contributed to the conception and design of this study.NL and XNW performed the statistical analysis. This first draft of the manuscript was written by NL, and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, N., Wang, X., Duan, F. et al. Association of different treatment modalities for rectal cancer and second primary urinary system neoplasms, findings from SEER. Sci Rep 15, 18782 (2025). https://doi.org/10.1038/s41598-025-02676-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02676-7