Abstract
This study aims to compare the sperm retrieval rate (SRR) achieved through microdissection testicular sperm extraction (micro-TESE) with the outcomes of intracytoplasmic sperm injection (ICSI) in patients with non-obstructive azoospermia (NOA) who exhibit a partial deletion of the azoospermia factor c(AZFc) region on the Y chromosome. From December 2016 to September 2023, the clinical data of patients with NOA who had AZFc deletions, which were identified through high-throughput sequencing, and who underwent micro-TESE were retrospectively analyzed. Based on the results of screening the AZFc region, the patients were categorized into three groups—the gr/gr deletion group (n = 17), the b2/b4 deletion group (n = 62), and the b2/b3 deletion group (n = 11). The SRR, embryo development, and embryo transfer outcomes were compared between the three groups. The SRR in the gr/gr deletion group was significantly lower than that in the b2/b4 deletion group (P = 0.010). Conversely, the SRR in the b2/b4 deletion group and b2/b3 deletion group were relatively high, at 64.5% (40/62) and 54.5(6/11),respectively; however, the number of micro-TESE procedures that extracted sperm with severe morphological abnormalities was 13, resulting in an available sperm rate of only 43.5% (27/62). No significant differences were observed across all groups regarding fertilized oocyte (2PN) rate, 2PN cleavage rate, D3 available embryo rate, high-quality embryo rate, blastocyst formation rate, number of transferred embryos, miscarriage rate, clinical pregnancy rate, or live birth delivery rate (P > 0.05). The micro-TESE outcomes in NOA patients with partial deletions in the AZFc region of the Y chromosome are most significantly negatively impacted by gr/gr deletion.Patients with b2/b4 deletion and b2/b3 deletion type should actively undergo surgical treatment to obtain biological descendants.
Similar content being viewed by others
Introduction
Y chromosome microdeletion is one of the major genetic factors contributing to male infertility in cases of azoospermia or severe oligospermia1. The AZF (azoospermia factor) is located on the long arm of the Y chromosome, and the absence of this region may lead to a decrease in sperm count or disordered spermatogenesis2. As recommended by the European Society of Andrology and the European Molecular Genetic Quality Control Network, the detection of Y chromosome AZF deletion is a crucial step in the diagnosis of azoospermia and severe oligospermia3. Based on different regions, AZF deficiency can be categorized into three regions—AZFa, AZFb, and AZFc4. Since the AZFc region is particularly vulnerable to non-allelic (intrachromosomal) influences, this can lead to microdeletions or duplications5,6. Clinically, we have observed that carriers of AZFc region deletion (ranging from azoospermia to normal sperm count) exhibit surprising phenotypic variations, although the reasons remain unclear.
Thanks to the development of high-throughput sequencing technology, we have been able to conduct an in-depth investigation of the AZF region and have described several different AZFc partial deletion patterns. The AZFc region can be divided into gr/gr, b2/b4, b1/b3, b1/b2, u3/g1, b1/u3, b2/b3, etc7. It is of great significance to explore whether there are significant clinical differences among different deletion patterns.
The pathogenesis of patients with idiopathic NOA remains unclear. However, deletions in the AZFc region are recognized as being significant genetic factors. Notably, the effects of various microdeletion patterns on the success rates of sperm extraction in NOA patients and the outcomes of ICSI-assisted pregnancies have not been thoroughly investigated. To address this gap, we reviewed and analyzed the clinical data of NOA patients with AZFc deletions who underwent ICSI-assisted pregnancies. According to the incidence characteristics of AZF microdeletions, there are many types of microdeletions, but the probability of their occurrence is extremely low. Therefore, based on their probability of occurrence, we specifically focused on three common deletion types, gr/gr, b2/b4, and b2/b3, to provide guidance for the development of assisted reproductive strategies for these patients.
Data and methods
Study object and group
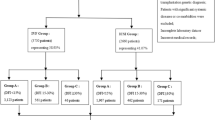
This study employed a retrospective cohort design. It analyzed patients diagnosed with NOA who exhibited either a total or partial deletion of the AZFc region on the Y chromosome. These patients were admitted to the Reproductive Center of Henan Provincial People’s Hospital between December 2016 and September 2023 and underwent micro-TESE. Subsequently, those from whom sperm was successfully extracted received treatment through ICSI to facilitate assisted pregnancy. The study received ethical approval from the Reproductive Medicine Ethics Committee of Henan Provincial People’s Hospital (SYZZ-LL-2019110401), and the guidelines outlined in the Declaration of Helsinki were followed. All participants signed a written informed consent form.
Inclusion Criteria: (1) Patients who underwent three semen analyses, in accordance with the WHO Laboratory Manual for the Examination and Processing of Human Semen (5th edition), with no sperm detected via microscopy following centrifugation; (2) patients with partial or complete deletions in the AZFc region of the Y chromosome, as identified through high-throughput sequencing, were categorized into three groups based on deletion type—gr/gr deletion, b2/b4 deletion, and b2/b3 deletion; (3) patients who underwent micro-TESE, with those successfully retrieving sperm proceeding to ICSI for assisted reproductive treatment; and (4) the patient was not exposed to an environment that significantly affected fertility, such as smoking, alcohol, drug use, etc.
Exclusion Criteria: (1) Patients exhibiting chromosomal karyotype abnormalities or single-gene disorders that necessitate preimplantation genetic diagnosis or screening; and (2) couples in which the female partner has severe ovarian dysfunction or endometrial diseases that may significantly impact the outcomes of ICSI-assisted pregnancies.
Research method
-
1.
Micro-TESE: Patients who were included in the study underwent surgery according to a routine surgical plan. If sperm were found during the operation, it was considered to be positive for sperm collection, and the embryologist determined whether such a collection method was available. The evaluation of sperm during the operation was based on the description of sperm morphology in the WHO Guidelines 5/6; priority was given to sperm with normal sperm morphology. Sperm with “malformations” or “deformities” were confirmed by the embryologist as unable to undergo ICSI, i.e., sperm head vacuolation, double-headed sperm deformity, point-headed sperm deformity, big-headed sperm deformity, etc.
-
2.
AZF: The sequencing library was constructed according to the instructions of the DNA extraction kit (Tiangen Biochemical Technology, Beijing, China) and the Y chromosome microdeletion detection kit (Beijing Zhongyi Health Medical Equipment Co., LTD.). High-throughput sequencing is performed using a MiSeq sequencer (Illumina, Nextseq 550 DX) according to the instructions of the general sequencing reaction kit (Beijing Zhongyi Kangwei Medical Equipment Co., LTD.). Captures were performed according to the specific probes designed, and the relative copy number of the AZF region was calculated, using the average number of autosomal housekeeper probes as a reference. Subsequently, the Z-Score method was applied to validate the preliminary findings and to determine the final variation type of the sample.
-
3.
ICSI and ET (embryo transfer): The ovulation induction, ICSI, and embryo transfer protocols were developed based on the relevant routine procedures of the department. Blood samples were collected 14 days post-transfer to measure β-hCG levels, and a vaginal ultrasound was performed 28 to 35 days after the transfer. If a gestational sac is visible, clinical pregnancy is confirmed, and the number of gestational sacs is recorded. Follow-up via telephone continues until the birth of the fetus.
Statistical methods
For data analysis, measurement data consistent with a normal distribution were expressed as mean ± standard deviation (SD). Group comparisons were conducted using either the independent samples t-test or one-way analysis of variance (ANOVA), as appropriate. Qualitative data were presented as percentages (%), and group comparisons were performed using the Chi-square test or Fisher’s exact test, depending on the data characteristics. All statistical analyses were conducted using SPSS version 23.0, with a p-value of < 0.05 being considered as statistically significant.
Results
Overview of the basic situation and comparison of SRR
According to the established inclusion and exclusion criteria, a total of 90 patients with deletions in the AZFc region associated with NOA were included in this study. In total, 50 micro-TESEs resulted in sperm, yielding an SRR of 55.6% (50/90). The number of micro-TESE procedures with sperm that had severe morphological abnormalities, which is deemed unsuitable for ICSI in assisted reproduction, was 13. Consequently, the number of micro-TESEs that resulted in available sperm was 37, translating to an available sperm retrieval rate of 41.1%.
As illustrated in Table 1, the SRR in the gr/gr deletion group was significantly lower than that in the b2/b4 deletion group, at a statistically significant level (P < 0.05). The unusable malformed sperm were derived from the b2/b4 deletion group. Additionally, the volume of the left testicle in the b2/b3 deletion group was smaller than that in the b2/b4 deletion group, and the follicle stimulating hormone (FSH) levels were significantly higher in the b2/b3 group compared to the other two groups (P < 0.05). the luteinizing hormone (LH) levels were significantly higher in the b2/b3 group compared to the other two groups (P < 0.05). No statistically significant differences were observed in age, volume of the right testicle, prolactin (PRL), testosterone (T), or estradiol (E2) levels among all groups (P > 0.05).
Comparison of embryo development and embryo transfer outcomes assisted by ICSI
A total of 37 couples with available sperm for ICSI-assisted pregnancy were included in the study. As presented in Table 2, the overall characteristics of both partners in each group exhibited no statistically significant differences in any indicators (P > 0.05).
The development of embryos in ICSI cycles across various AZFc deletion groups was compared. The number of micro-TESEs that resulted in available sperm was 37, and 52 oocyte retrieval cycles were performed. As illustrated in Table 3, there were no significant differences among the groups in terms of the total number of oocytes collected, the number of MII oocytes, the fertilized oocyte (2PN) rate, the 2PN cleavage rate, the D3 available embryo rate, the high-quality embryo rate, and the blastocyst formation rate (P > 0.05).
In our comparative analysis of embryo transfer outcomes within ICSI cycles across distinct AZFc deletion subgroups, we examined a total of 57 transfer cycles distributed among the three groups. Our findings, as detailed in Table 3, indicate that there were no significant variations in the number of transferred embryos, live birth delivery rate, clinical pregnancy rate, or miscarriage rate (P > 0.05). However, the implantation rate for the gr/gr deletion group was notably higher than that observed in the other two groups; this is a difference that was statistically significant (P < 0.05).
Discussion
The AZFc region of the Y chromosome spans approximately 4.5 megabases (Mb) and harbors critical genes, including DAZ and CDY1, which are closely associated with male spermatogenesis and sperm morphology. Notably, the presence of multiple gene copies within this region means that partial deletions of AZFc may not necessarily result in the complete loss of these genes8,9,10. Advances in high-throughput sequencing technology have enabled the classification of AZFc into distinct subtypes, such as gr/gr, b2/b4, b1/b3, b1/b2, u3/g1, b1/u3, and b2/b3. Among these, the b2/b4 deletion is considered equivalent to the complete deletion of the AZFc region. Research in murine models has demonstrated that deletions in the long arm of the Y chromosome (MSYq) lead to varying degrees of spermatogenic impairment, ranging from abnormal sperm morphology to complete infertility11. Furthermore, evidence suggests that the DAZ gene family within the AZFc region plays a pivotal role in the regulation of sperm morphology, with its dysfunction being linked to morphological abnormalities12.
The gr/gr deletion subtype is characterized by a partial deletion that primarily reduces the copy number of the DAZ, CDY1, and BPY2 genes. Patients with this type of deletion exhibit significant phenotypic variation, ranging from azoospermia to normal sperm parameters. Currently, the clinical significance of the gr/gr deletion remains a topic of debate. Some studies suggest that the functional differences in AZFc gene copies among various populations may contribute to the inconsistent relationship between gr/gr deletion and spermatogenic dysfunction13. Conversely, other studies have supported the notion that gr/gr deletion is a risk factor for spermatogenic dysfunction3.
The b2/b3 deletion, which also represents a partial deletion, primarily results in the deletion of DAZ1 and DAZ2, distinguishing it from the gr/gr deletion14. The differentiation between these deletions may influence the regulatory roles of other non-coding regions, leading to varying clinical implications. However, the effects of different AZFc region subtypes on SRR and outcomes of ICSI have yet to be reported.
This study is the first to compare the SRR of patients with NOA who have different deletions in the AZFc region and who underwent micro-TESE. The overall SRR for patients with AZFc region deletions in this study was 55.6% (50/90), which aligns closely with findings reported by Mao Jiaming et al.15. According to our classification of deletion types, we found that patients with a complete deletion of the b2/b4 region exhibited the highest SRR at 64.5% (40/62). However, patients with partial deletions demonstrated severe sperm malformation [n = 13, accounting for 32.5% (13/40)] and the inability to provide sperm suitable for ICSI. The SRR for the gr/gr deletion group was 23.5% (4/17). This rate is significantly lower than that of the b2/b4 deletion group. The SRR for the b2/b4 deletion group was similar to that of the b2/b3 deletion group, which had an SRR of 54.5% (6/11). There was no statistically significant difference in the right testicle volume of patients in each group, but the left testicle volume of the b2/b3 deletion group was smaller than that of the other groups, that is, the total testicle volume of the b2/b3 deletion group showed a downward trend compared with other groups. In our study, the FSH levels and the LH levels in the b2/b3 deletion group were significantly higher than those in the other groups, which may provide clues to the mechanism of impaired spermatogenesis function caused by this type of deletion, and we can further explore the causes of this correlation in subsequent studies.
Based on the above data, we conclude that microdeletions in different regions of the AZFc region have varying impacts on spermatogenesis in patients with NOA. The deletion of the gr/gr gene has a significant impact on the SRR of micro-TESE, which is comparable to that observed in cases of idiopathic NOA16,17,18. Therefore, we boldly hypothesize that gr/gr deletion may not be the cause of NOA in such patients. Some researchers have suggested that microdeletions in the AZFc region may not occur in isolation; rather, they may coincide with the activation of other genes and influence spermatogenesis through mechanisms such as gene duplication or dosage compensation. This interaction could mitigate the effects of microdeletion on spermatogenesis, leading to variations in clinical phenotype11. Therefore, while the precise mechanisms underlying these differences remain unclear, the findings of this study carry significant implications for developing clinical strategies for affected patients.
The impact of the AZFc microdeletion on assisted reproductive outcomes, particularly in the context of ICSI, has garnered significant attention in recent studies. Research has primarily focused on the sources of sperm, specifically comparing ejaculated sperm and sperm obtained through testicular biopsy (testicular sperm extraction—TESE). Previous investigations have indicated that AZFc microdeletion negatively affects ICSI outcomes. Specifically, when compared to a control group without Y chromosome deletions, patients with AZFc microdeletions exhibit reduced clinical cumulative pregnancy rates, cumulative live birth delivery rates, embryo transfer suitability rates, fertilization rates, and implantation rates19. A recent meta-analysis encompassing 12 studies further substantiated these findings, revealing a significant decrease in fertilization rates among patients with AZFc microdeletions. However, other parameters, including embryo quality, clinical pregnancy rates, miscarriage rates, and live birth delivery rates, were found to be comparable between these patients and those without genetic abnormalities, regardless of whether sperm was obtained via ejaculation or TESE20. For patients with NOA due to AZFc deletions, micro-TESE extraction combined with ICSI presents a viable option for achieving pregnancy and producing biological offspring, particularly when spermatogenic function is preserved. This study is the first to report on the differences in ICSI outcomes among NOA patients with distinct AZFc deletion subtypes. Our findings reveal that sperm successfully retrieved from NOA patients with AZFc deletions exhibited comparable embryo development and transfer outcomes across all groups, with no significant differences observed. However, the embryo implantation rate was notably higher in the gr/gr deletion group compared to the other two groups. Despite this, AZFc deletions—whether in patients with ejaculated sperm, testicular sperm, or NOA testicular sperm—did not significantly influence the final pregnancy outcomes. Furthermore, the specific region of AZFc deletion had no substantial impact on embryo development or pregnancy outcomes during ICSI-assisted reproduction. These preliminary results suggest that AZFc deletions, irrespective of their subtype, do not adversely affect the overall success of ICSI in achieving pregnancy.
In summary, this study represents the first comparative analysis of the SRR in NOA patients with deletions in various segments of the AZFc region, as well as an examination of the ICSI outcomes for sperm obtained from these patients. Our findings indicate that the gr/gr deletion has the most significant impact on micro-TESE among patients with AZFc deletions. In patients with b2/b4 deletions, although the high incidence of sperm malformation is found, SRR is high.Although there is no statistical difference in SRR between the b2/b3 deletion group and the gr/gr deletion group, it can also be seen that the SRR is increased.Embryo development or pregnancy outcomes following ICSI treatment are not significantly affected by all types of deletions. Therefore, patients with b2/b4 deletion and b2/b3 deletion type should actively undergo surgical treatment to obtain biological descendants.It is important to note that the prevalence of these small deletions is quite low, particularly within the NOA patient population. Only a limited number of cases were included in this study; therefore, future research should consider multi-center collaborations to enhance the sample sizes in order for more comprehensive comparative analyses to be conductedocc.
Data availability
The datasets generated and analysed during the current study are available in the NGDC repository (HRA010038).
References
Gunes, S. & Esteves, S. C. Role of genetics and epigenetics in male infertility. Andrologia 53(1), e13586. https://doi.org/10.1111/and.13586 (2021).
Sercan Ergun, S. et al. In silico analysis of MicroRNA genes in azoospermia factor Y-chromosome microdeletions. Int. Urol. Nephrol. 54(4), 773–780. https://doi.org/10.1007/s11255-022-03133-4 (2022).
Csilla Krausz, P. et al. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state of the Art 2023. Andrology 12(3), 487–504. https://doi.org/10.1111/andr.13514 (2024).
Witherspoon, L., Dergham, A. & Flannigan, R. Y-microdeletions: A review of the genetic basis for this common cause of male infertility. Transl Androl. Urol. 10(3), 1383–1390. https://doi.org/10.21037/tau-19-599 (2021).
Navarro-Costa, P., Gonçalves, J. & Plancha, C. E. The AZFc region of the Y chromosome: At the crossroads between genetic diversity and male infertility. Hum. Reprod. Update 16(5), 525–542. https://doi.org/10.1093/humupd/dmq005 (2010).
Krausz, C. et al. The Y chromosome-linked copy number variations and male fertility. J. Endocrinol. Invest. 34(5), 376–382. https://doi.org/10.1007/BF03347463 (2011).
Liu, X. et al. Y chromosome structural variation in infertile men detected by targeted next-generation sequencing. J. Assist. Reprod. Genet. 38(4), 941–948. https://doi.org/10.1007/s10815-020-02031-x (2021).
Sen, S. et al. Susceptibility of Gr/gr rearrangements to azoospermia or oligozoospermia is dependent on DAZ and CDY1 gene copy deletions. J. Assist. Reprod. Genet. 32(9), 1333–1341. https://doi.org/10.1007/s10815-015-0520-4 (2015).
Rogers, M. J. Y chromosome copy number variation and its effects on fertility and other health factors: A review. Transl Androl. Urol. 10(3), 1373–1382. https://doi.org/10.21037/tau.2020.04.06(2021).
Lardone, M. C. et al. Partial-AZFc deletions in Chilean men with primary spermatogenic impairment: Gene dosage and Y-chromosome haplogroups. J. Assist. Reprod. Genet. 37(12), 3109–3119. https://doi.org/10.1007/s10815-020-01957-6 (2020).
Li, Z. & Haines, C. J. Yibing Han.Micro-deletions of the human Y chromosome and their relationship with male infertility. J. Genet. Genomics. 35(4), 193–199. https://doi.org/10.1016/S1673-8527(08)60027-2 (2008).
Nogueira, P. N. C. P. et al. Incorrect DNA methylation of the DAZL promoter CpG Island associates with defective human sperm. Hum. Reprod. 25(10), 2647–2654. https://doi.org/10.1093/humrep/deq200 (2010).
Lepretre, A. C. et al. No partial DAZ deletions but frequent gene conversion events on the Y chromosome of fertile men. J. Assist. Reprod. Genet. 22(4), 141–148. https://doi.org/10.1007/s10815-005-4910-x (2005).
Wang, Y. et al. Association of the deleted DAZ gene copy related to Gr/gr and b2/b3 deletions with spermatogenic impairment. Zhonghua Nan Ke Xue 22(1), 17–21 (2016).
Jiaming Mao, C. et al. Micro-TESE strategy in patients with NOA caused by AZFc deletion: Synchronous or asynchronous? Zygote 31(1), 25–30 https://doi.org/10.1017/S0967199422000466 (2023).
Kaijuan Wang, D. et al. Micro-TESE surgery combined with ICSI regimen in the treatment of non-obstructive azoospermia patients and its effect analysis. Zygote 31(1), 55–61. https://doi.org/10.1017/S096719942200051X (2023).
Yang, J. et al. Results of micro-TESE and outcomes of ICSI in patients with different etiological types of non-obstructive azoospermia. Zhonghua Nan Ke Xue. 24(10), 887–892 (2018).
Mao, J. M. et al. [Effect of testicular puncture biopsy on the success rate of microdissection testicular sperm extraction for idiopathic non-obstructive azoospermia. Beijing Da Xue Xue Bao Yi Xue Ban 50(4), 613–616 (2018).
Zhang, L. et al. Poor intracytoplasmic sperm injection outcome in infertile males with azoospermia factor C microdeletions. Fertil. Steril. 116(1), 96–104. https://doi.org/10.1016/j.fertnstert.2021.01.025 (2021).
Li, X. et al. Effect of Y chromosome microdeletions on the pregnancy outcome of assisted reproduction technology: a meta-analysis. Reprod. Sci. 28(9), 2413–2421. https://doi.org/10.1007/s43032-020-00387-0 (2021).
Acknowledgements
We thank the participants described in this study for their consent and support to publish this manuscript.
Funding
This work was supported by Henan Province Medical Science and Technology Research Program Joint Construction Project(LHGJ20230046).
Author information
Authors and Affiliations
Contributions
All authors have made a great contribution to this work. Yanqing Xia and Haibin Guo conceived the study; Ke Feng, Xiaowei Qu and Feng Wan extracted and analyzed the data; This study was supervised by Cuilian Zhang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, Y., Feng, K., Qu, X. et al. Impact of AZFc deletion subtypes on sperm retrieval rates via micro-TESE and ICSI outcomes in non-obstructive azoospermia patients. Sci Rep 15, 22148 (2025). https://doi.org/10.1038/s41598-025-03312-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03312-0