Abstract
To develop and validate a clinical prediction model for Iliac Vein Compression Syndrome (IVCS) in outpatients with Varicose veins of the lower extremities (VVLE), to aid clinical decision-making and early identification of high-risk patients. A retrospective cohort study was conducted, including 732 outpatients diagnosed with VVLE between 2014 and 2023. Independent predictors of IVCS were identified through multivariable logistic regression, and a nomogram was developed. The model was evaluated using receiver operating characteristic (ROC) curve analysis, calibration curves, and decision curve analysis (DCA). Four independent predictors for IVCS were identified: history of deep vein thrombosis (DVT), history of vascular interventions, pain symptoms, and Clinical Etiological Anatomical Pathophysiological (CEAP) grade. The nomogram showed strong performance, with an area under the ROC curve (AUC) of 0.79 in the training set and 0.74 in the validation set. of 0.79 in the training set and 0.74 in the validation set. Calibration curves confirmed good agreement between predicted and observed outcomes. DCA demonstrated the clinical utility of the model across different risk thresholds. A simple and cost-effective nomogram for predicting IVCS in VVLE patients was developed and validated. This tool helps outpatient clinicians identify high-risk IVCS patients early, supporting personalized treatment strategies. Further validation is needed, but the model holds promise for improving early diagnosis and patient outcomes.
Similar content being viewed by others
Introduction
Varicose veins of the lower extremities (VVLE) are a prevalent peripheral vascular condition marked by the dilation, tortuosity, and reflux in the veins of the legs1,2. As individuals age, the function of the venous valves gradually deteriorates, leading to the expansion of the venous walls and the obstruction of blood flow, ultimately resulting in varicose veins and associated complications such as skin ulcers, phlebitis, and deep vein thrombosis (DVT)3. The global prevalence of varicose veins is approximately 25–30%, with a notably higher incidence among the elderly population 4,5. Beyond affecting the appearance and quality of life, varicose veins can cause long-term leg pain, edema, and restricted mobility, imposing a persistent health burden on patients6,7.
A lesser-recognized pathological condition associated with varicose veins is iliac vein compression syndrome (IVCS), also known as May-Thurner syndrome. IVCS is a condition caused by the mechanical compression of intra-abdominal vessels, primarily at the junction of the right iliac artery and the left iliac vein8,9. The compression of blood vessels in this area leads to impaired venous return, resulting in lower limb edema, pain, and venous distention. Although the diagnosis and treatment of IVCS have gained some attention in recent years, many patients with varicose veins have not been promptly diagnosed with coexisting IVCS, leading to suboptimal treatment outcomes10.
There exists a complex interplay between IVCS and VVLE. In some cases, IVCS may serve as a primary pathogenic factor for varicose veins, particularly when venous dilation is more pronounced in the left leg11. Since iliac vein compression typically presents with chronic progressive symptoms and its clinical manifestations are relatively subtle, many patients are not diagnosed until the condition worsens or complications such as deep vein thrombosis arise12. Therefore, timely recognition of varicose vein patients with concomitant IVCS is crucial for early intervention and the formulation of personalized treatment plans.
Currently, the clinical diagnosis of IVCS relies mainly on imaging techniques, such as CT venography, magnetic resonance imaging (MRI), and ultrasound. However, these methods have limitations, including high costs, low accessibility, and operational complexity, which make them difficult to apply in large-scale population screenings13. Given the limitations of expensive and complex diagnostic tests, a simple, cost-effective, and reliable clinical prediction model based on outpatient clinical characteristics would significantly enhance clinical decision-making and improve patient outcomes.
Therefore, this study aims to investigate the clinical characteristics associated with IVCS among outpatients diagnosed with VVLE and to establish a predictive model based on routinely available clinical data. Identifying relevant risk factors and constructing a practical screening tool may contribute to improved early recognition of IVCS and support individualized management approaches in outpatient settings.
Methods
Study population
This retrospective cohort study included 732 outpatients diagnosed with VVLE who sought either initial or follow-up care at our institution between 2014 and 2023. The cohort consisted of patients with and without a history of vascular interventions. Inclusion criteria were: (1) age ≥ 18 years; (2) a diagnosis of VVLE confirmed by imaging studies (ultrasound, CT, or MRI); and (3) no history of severe infections or other significant diseases. Exclusion criteria included: (1) incomplete clinical records; (2) presence of severe infections or other significant comorbidities (e.g., acute venous inflammation, uncontrolled diabetes); and (3) postoperative complications that could interfere with IVCS diagnosis (e.g., severe bleeding, critical infections, ICU admissions, or neurological complications).
Data collection
Data were extracted from patient medical records, including demographic information, medical history, clinical symptoms, and imaging results. The following clinical variables were collected: gender, age, body mass index (BMI), smoking history, diabetes, hypertension, history of vascular interventions, history of DVT, pain symptoms, and Clinical Etiological Anatomical Pathophysiological (CEAP) grade. History of vascular interventions was defined as any prior invasive procedure targeting the lower extremity venous system, including superficial venous ablation (e.g., endovenous laser or radiofrequency therapy), foam or liquid sclerotherapy, surgical ligation or stripping, and deep venous interventions such as stenting or angioplasty. Central venous catheterization or unrelated vascular procedures were not included. Imaging data, including venous ultrasound, CT angiography, and MRI scans, were reviewed, with particular attention to the degree of iliac vein compression. All data were retrieved from the hospital’s electronic medical records and cross-checked for accuracy through dual review. Pain symptoms were documented as a binary variable (present/absent) based on the clinical encounter notes. No standardized pain scale or qualitative description was used in this dataset.
Definition of IVCS
IVCS was defined as the mechanical compression or obstruction of the iliac vein in patients with VVLE, leading to symptoms such as lower limb edema, pain, and venous distention. The diagnosis was confirmed through imaging studies (CT or MRI) showing compression of the iliac vein, in conjunction with clinical presentation14,15.
IVCS diagnostic criteria
Iliac vein compression was diagnosed when cross-sectional imaging (CT or MRI) demonstrated a luminal narrowing of ≥ 50% at the site of arterial crossover, typically between the right common iliac artery and the left common iliac vein, in conjunction with relevant clinical symptoms such as unilateral lower limb edema, pain, or varicosities. This diagnostic threshold is supported by prior studies indicating that a ≥ 50% compression is clinically significant and associated with symptomatic IVCS16,17,18.
Imaging protocol
All patients initially underwent duplex ultrasound examination of the lower extremity veins to evaluate venous reflux, valve insufficiency, and vessel dilation. For patients with clinical suspicion of proximal venous obstruction—such as unilateral lower limb edema, pain, or severe varicosities—further imaging with contrast-enhanced computed tomography venography (CTV) or magnetic resonance venography (MRV) was performed to assess for iliac vein compression. CTV was performed in the venous phase using a multi-slice CT scanner, with scanning coverage from the abdominal aorta to the femoral veins. Intravenous contrast was administered at a rate of 3–4 mL/s, and imaging was timed to capture optimal venous enhancement. MRV utilized a 3D contrast-enhanced gradient-echo sequence with similar anatomical coverage. Both imaging modalities were interpreted by two experienced radiologists blinded to clinical outcomes. Conventional venography or intravascular ultrasound was not employed in this outpatient cohort due to the non-invasive nature of the diagnostic workflow and the limited availability of these procedures in routine outpatient settings.
Statistical analysis
Statistical analyses were performed using SPSS 26.0 and R 4.2.2. Patients were randomly assigned to training (512) and validation (220) groups in a 7:3 ratio using the createDataPartition() function from the caret package in R (version 4.2.2), with set.seed(123) applied to ensure reproducibility. Continuous variables were presented as mean ± SD, and categorical variables as frequency (percentage). Univariate analysis, including chi-square and t-tests, identified potential risk factors. Variables with P < 0.05 were entered into multivariable logistic regression to identify independent IVCS predictors. A nomogram for IVCS risk was developed using the ‘rms’ package in R. Model performance was assessed using the concordance index (C-index), receiver operating characteristic (ROC) curves, and calibration curves. The area under the ROC curve (AUC) was calculated to quantify model discrimination. Decision curve analysis (DCA) evaluated the model’s clinical utility.
Ethics statement
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Jiujiang University (Approval No. [20240108]). The research was conducted in accordance with the ethical standards of the Declaration of Helsinki. Given the retrospective nature of this study, the requirement for informed consent was formally waived by the Medical Ethics Committee of the Affiliated Hospital of Jiujiang University.
Results
Clinicopathologic characteristics
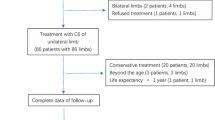
Between 2014 and 2023, a total of 943 outpatients diagnosed with VVLE were initially identified from the electronic medical records at our center. After applying the predefined inclusion and exclusion criteria, 732 patients were ultimately included in the final analysis. The patient selection process is illustrated in Fig. 1. As shown in Table 1, these patients were randomly assigned to the training group (512 cases) and validation group (220 cases) using R software, with no significant differences observed between the two groups regarding gender, age, BMI, smoking history, and other clinical characteristics (P > 0.05). Among the 732 patients, 42 were diagnosed with IVCS, and 690 did not meet the criteria for IVCS. Significant differences were found between the IVCS and non-IVCS groups in terms of history of DVT, history of vascular interventions, left side affected, pain symptoms, intermittent claudication and CEAP grade. Specifically, the IVCS group had a higher prevalence of DVT (P = 0.001), a higher rate of vascular interventions (P < 0.001), and more severe CEAP grading (≥ 4) compared to the non-IVCS group (P < 0.001). Pain symptoms were also significantly more common in the IVCS group (P = 0.003). However, no significant differences were found between the two groups regarding age, BMI, and other factors.
Independent predictive factors for IVCS
Multivariable logistic regression analysis identified four independent predictive factors for IVCS: a history of DVT (OR = 3.66, 95% CI: 1.69–7.94, P = 0.001), history of vascular interventions (OR = 4.63, 95% CI: 2.80–7.65, P < 0.001), pain symptoms (OR = 1.61, 95% CI: 1.01–2.67, P = 0.048), and CEAP classification ≥ 4 (OR = 3.38, 95% CI: 1.80–6.35, P < 0.001) (Table 2). These factors were significantly associated with the occurrence of IVCS and were used to develop the clinical prediction model.
Development and validation of the nomogram
A nomogram was developed based on four key predictive factors for IVCS: history of DVT, history of vascular interventions, pain symptoms, and CEAP grade. As shown in Fig. 2, each variable was assigned specific scores: history of DVT (No: 0 points, Yes: 66 points), history of vascular interventions (No: 0 points, Yes: 100 points), pain symptoms (No: 0 points, Yes: 42.5 points), and CEAP classification (< 4: 0 points, ≥ 4: 90 points) (Table 3). The total score was calculated by summing these individual scores, providing a tool to predict IVCS risk and assist in clinical decision-making.
The nomogram’s performance was evaluated using the C-index, which was 0.79 for the training set and 0.74 for the validation set. ROC curve analysis showed an AUC of 0.79 (95% CI: 0.69–0.88) for the training set and 0.74 (95% CI: 0.63–0.85) for the validation set (Fig. 3A and B). Calibration curves confirmed strong agreement between predicted and observed outcomes (Fig. 3C and D). DCA demonstrated the model’s clinical usefulness, with threshold probabilities ranging from 7 to 75% in the training set and 5–47% in the validation set (Fig. 3E and F).
Discussion
This study successfully developed and validated a clinical prediction model for IVCS in outpatients with VVLE, aimed at helping clinicians identify high-risk IVCS patients in routine clinical practice. IVCS, a less-recognized complication, often coexists with VVLE but remains underdiagnosed due to its subtle symptoms and reliance on expensive, complex imaging for diagnosis. Through retrospective analysis of clinical data from 732 VVLE patients, we identified four independent predictors associated with IVCS and constructed a practical, cost-effective nomogram model. This model enables outpatient clinicians to make quick and reliable clinical decisions without complex diagnostic tools, offering a valuable screening tool for clinical practice.
IVCS is a condition caused by mechanical compression leading to impaired venous return, and it is often associated with VVLE11. Previous studies have demonstrated a complex interplay between IVCS and VVLE, particularly in patients with more pronounced left-sided venous dilation19,20. Our findings align with this, showing that a history of DVT, prior vascular interventions, pain symptoms, and severe CEAP grading (≥ 4) were significantly associated with the presence of IVCS. In our cohort, the incidence of DVT and vascular interventions was notably higher in the IVCS group, highlighting the important role of these factors in the development of IVCS. Moreover, CEAP grade ≥ 4 was a strong indicator of IVCS, suggesting that more severe venous disease is closely linked to the occurrence of IVCS17,21. Although CEAP classification does not directly reflect the site or mechanism of venous obstruction, we selected a threshold of ≥ 4 to capture clinically significant chronic venous changes, such as skin damage and ulceration, which may prompt consideration of proximal venous outflow compromise in outpatient settings. This threshold, while not specific, enhances the model’s practical utility by flagging patients likely to benefit from further evaluation for IVCS.
A key discovery in our study was that IVCS may serve as a primary pathogenic factor for VVLE, particularly when left-sided venous dilation is more pronounced. Previous research also supports the notion that iliac vein compression can contribute to worsening venous insufficiency, especially in patients with prominent left-sided varices22,23. Thus, clinicians should be particularly vigilant in identifying IVCS in patients with severe left-sided VVLE, particularly those with a history of DVT or prior vascular interventions.
In this study, we used multivariable logistic regression analysis to identify four independent predictors for IVCS: history of DVT, history of vascular interventions, pain symptoms, and CEAP classification. Based on these factors, we developed a simple and effective clinical prediction model, represented as a nomogram. While the predictors incorporated into the nomogram are part of routine clinical assessment, the model’s value lies in quantifying their combined contribution to IVCS risk, thereby offering a structured and objective framework to guide further diagnostic evaluation, especially in outpatient settings where imaging resources may be limited. The model demonstrated strong predictive performance, with AUC values of 0.79 for the training set and 0.74 for the validation set, indicating that the model can effectively distinguish between patients with and without IVCS. The calibration curves confirmed a high degree of agreement between predicted probabilities and observed outcomes, further validating the model’s robustness. DCA showed that the model provides significant clinical utility across various risk thresholds, particularly in high-risk patient groups, supporting its role in clinical decision-making in outpatient settings.
The clinical significance of this study lies in the development of a simple, reliable, and cost-effective tool to assess the risk of IVCS in VVLE patients, particularly in outpatient settings. Traditionally, IVCS diagnosis relies on expensive imaging techniques such as CT venography, MRI, and ultrasound, which are not always accessible or practical for routine use in outpatient care. Our model, however, only requires basic clinical variables—such as history of DVT, history of vascular interventions, pain symptoms, and CEAP classification—enabling rapid risk screening for IVCS without the need for complex imaging tests. This provides a quick and cost-effective method for outpatient clinicians to identify high-risk patients and determine whether further imaging or interventions are necessary, thereby improving patient outcomes.
Despite its promising results, this study has several limitations. Given the relatively small number of IVCS events (n = 42), there is an inherent risk of overfitting in logistic regression modeling. To mitigate this, we intentionally limited the number of final predictors to four, based on both statistical significance in univariate analysis and clinical relevance. While this approach balances parsimony and interpretability, future studies with larger sample sizes could consider using penalized regression techniques such as LASSO to improve model stability. As a single-center retrospective analysis, it may be subject to selection bias, and external validation in larger, multicenter cohorts is needed to confirm its generalizability. Additionally, the model is based solely on routine clinical variables; incorporating biomarkers such as hematological, genomic, or imaging data may further enhance its predictive performance. While the nomogram offers practical value for IVCS risk assessment, it remains a screening tool and should not replace imaging for definitive diagnosis. The inclusion of pain as a predictor also requires cautious interpretation due to its subjective nature and potential variability. In this study, pain was recorded as a binary variable (present/absent) based on outpatient clinical records, without quantifying severity or specifying type. This limited granularity may reduce its discriminative power. Future studies should adopt standardized assessment tools (e.g., VAS scores or pain subtyping) to improve the robustness and reproducibility of this predictor across clinical settings. Further research is warranted to explore whether combining this model with imaging techniques can improve diagnostic accuracy and guide early intervention. Evaluating its impact on long-term outcomes—such as symptom recurrence, quality of life, and thrombotic complications—will help optimize IVCS management and support evidence-based clinical decisions.
Conclusion
This study successfully developed and validated a clinical prediction model for IVCS in VVLE patients, identifying key factors such as history of DVT, history of vascular interventions, pain symptoms, and CEAP classification as strong predictors of IVCS risk. The model provides outpatient clinicians with a simple, cost-effective, and reliable tool for early identification of high-risk IVCS patients, supporting personalized treatment strategies. While further validation and refinement are necessary, this model holds promise for improving early diagnosis and intervention in IVCS, ultimately enhancing patient outcomes and quality of life.
Data availability
The main data can be obtained from the corresponding authors while adhering to privacy and ethical guidelines.
References
Youn, Y. J. & Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 34, 269–283. https://doi.org/10.3904/kjim.2018.230 (2019).
Alsaigh, T. & Fukaya, E. Varicose veins and chronic venous disease. Cardiol. Clin. 39, 567–581. https://doi.org/10.1016/j.ccl.2021.06.009 (2021).
Raetz, J., Wilson, M. & Collins, K. Varicose veins: diagnosis and treatment. Am. Fam Physician. 99, 682–688 (2019).
Aslam, M. R. et al. Global impact and contributing factors in varicose vein disease development. SAGE Open. Med. 10, 20503121221118992. https://doi.org/10.1177/20503121221118992 (2022).
Robertson, L., Evans, C. & Fowkes, F. G. Epidemiology of chronic venous disease. Phlebology 23, 103–111. https://doi.org/10.1258/phleb.2007.007061 (2008).
Raffetto, J. D. Pathophysiology of chronic venous disease and venous ulcers. Surg. Clin. North. Am. 98, 337–347. https://doi.org/10.1016/j.suc.2017.11.002 (2018).
Atkins, E., Mughal, N. A., Place, F. & Coughlin, P. A. Varicose veins in primary care. Bmj 370, m2509. https://doi.org/10.1136/bmj.m2509 (2020).
Brinegar, K. N., Sheth, R. A., Khademhosseini, A., Bautista, J. & Oklu, R. Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J. Radiol. 7, 375–381. https://doi.org/10.4329/wjr.v7.i11.375 (2015).
Poyyamoli, S. et al. May-Thurner syndrome. Cardiovasc. Diagn. Ther. 11, 1104–1111. https://doi.org/10.21037/cdt.2020.03.07 (2021).
Radaideh, Q., Patel, N. M. & Shammas, N. W. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag. 15, 115–122. https://doi.org/10.2147/vhrm.S203349 (2019).
Lugo-Fagundo, C., Nance, J. W., Johnson, P. T. & Fishman, E. K. May-Thurner syndrome: MDCT findings and clinical correlates. Abdom. Radiol. (NY). 41, 2026–2030. https://doi.org/10.1007/s00261-016-0793-9 (2016).
Guo, C., Gao, S., Hu, L., Shang, D. & Li, Y. Predictive factors for Iliac vein compression syndrome in patients with varicose veins. Vascular https://doi.org/10.1177/17085381241275269 (2024).
Dwivedi, A., Singh, S. N., Sharma, A., Sharma, R. & Mishra, T. A. Systematic review of radiological diagnosis and management of May-Thurner syndrome. J. Pharm. Bioallied Sci. 16, S1012–s1016. https://doi.org/10.4103/jpbs.jpbs_1135_23 (2024).
Toh, M. R., Tang, T. Y., Lim, H., Venkatanarasimha, N. & Damodharan, K. Review of imaging and endovascular intervention of Iliocaval venous compression syndrome. World J. Radiol. 12, 18–28. https://doi.org/10.4329/wjr.v12.i3.18 (2020).
Wolpert, L. M., Rahmani, O., Stein, B., Gallagher, J. J. & Drezner, A. D. Magnetic resonance venography in the diagnosis and management of May-Thurner syndrome. Vasc Endovascular Surg. 36, 51–57. https://doi.org/10.1177/153857440203600109 (2002).
Cheng, L., Zhao, H. & Zhang, F. X. Iliac vein compression syndrome in an asymptomatic patient population: A prospective study. Chin. Med. J. (Engl). 130, 1269–1275. https://doi.org/10.4103/0366-6999.206341 (2017).
Hu, H., Hu, L., Deng, Z. & Jiang, Q. A prognostic nomogram for recurrence survival in post-surgical patients with varicose veins of the lower extremities. Sci. Rep. 14, 5486. https://doi.org/10.1038/s41598-024-55812-0 (2024).
Brazeau, N. F. et al. May-Thurner syndrome: diagnosis and management. Vasa 42, 96–105. https://doi.org/10.1024/0301-1526/a000252 (2013).
Shi, W. Y., Gu, J. P., Liu, C. J., Lou, W. S. & He, X. Dual compression is not an uncommon type of Iliac vein compression syndrome. Int. J. Cardiovasc. Imaging. 33, 1277–1285. https://doi.org/10.1007/s10554-017-1112-4 (2017).
Shebel, N. D. & Whalen, C. C. Diagnosis and management of Iliac vein compression syndrome. J. Vasc Nurs. 23, 10–17. https://doi.org/10.1016/j.jvn.2004.12.001 (2005). quiz 18–19.
Eklöf, B. et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J. Vasc Surg. 40, 1248–1252. https://doi.org/10.1016/j.jvs.2004.09.027 (2004).
Esposito, A., Charisis, N., Kantarovsky, A., Uhl, J. F. & Labropoulos, N. A comprehensive review of the pathophysiology and clinical importance of Iliac vein obstruction. Eur. J. Vasc. Endovasc. Surg. 60, 118–125. https://doi.org/10.1016/j.ejvs.2020.03.020 (2020).
Assi, I. Z. et al. A comparative study of altered hemodynamics in Iliac vein compression syndrome. Front. Bioeng. Biotechnol. 12, 1302063. https://doi.org/10.3389/fbioe.2024.1302063 (2024).
Acknowledgements
This work was supported by the Science and Technology Program of Jiangxi Provincial Health Commission (Grant No. 202510725).
Author information
Authors and Affiliations
Contributions
H.H.Z. and Z.L. conceived the study. L.H. and Z.L. collected and analyzed the data. H.H.Z. drafted the manuscript. H.H. revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, HH., Hu, L., Li, Z. et al. Development and validation of a clinical prediction model for Iliac vein compression syndrome in outpatients with varicose veins of the lower extremities. Sci Rep 15, 19250 (2025). https://doi.org/10.1038/s41598-025-04175-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04175-1