Abstract
To explore the relationship between resting EEG features and physical exercise among university students with depressive symptoms. A total of 1221 university students were recruited to complete a basic information form, the Patient Health Questionnaire-9 (PHQ-9), and the Physical Activity Rating Scale-3 (PARS-3). Resting EEG signals were collected from a subsample of 132 students. Latent Class Analysis was used to classify depressive symptoms, and the relationship between different resting EEG features and physical exercise was analyzed. Depressive symptoms among university students were classified into three latent classes, with Class 3 exhibiting the most severe symptoms. Significant differences were observed among the three groups in delta lateralization in the central region, alpha1 and alpha2 power in the parietal region, theta and alpha1 lateralization in the lateral frontal region, and alpha1 lateralization in the posterior temporal region (all p < 0.05). Alpha1 lateralization in the dorsolateral frontal cortex (F3, F4) was positively correlated with PARS-3 scores (r = 0.183, p = 0.036) and physical exercise intensity (r = 0.225, p = 0.010). Theta and alpha1 lateralization in the lateral frontal region (F7, F8) were positively correlated with exercise frequency (r = 0.229, p = 0.008; r = 0.214, p = 0.014, respectively). EEG lateralization features in the dorsolateral prefrontal and lateral frontal regions may serve as shared neurophysiological markers reflecting both depressive symptom severity and engagement in physical exercise among university students.
Similar content being viewed by others
Introduction
According to statistics from the World Health Organization, nearly 280 million people worldwide suffer from depression, with approximately 100 million cases in China alone. The Global Burden of Disease Study in 2010 revealed that mental and substance use disorders are among the leading causes of reduced healthy life expectancy worldwide, with depression accounting for 40.5% of mortality associated with these disorders, thus constituting a major contributor to the global disease burden1. Depression-related illnesses pose a significant and growing challenge to healthcare systems worldwide2. Furthermore, the clinical manifestations of depression are highly complex and heterogeneous across individuals, complicating diagnosis and posing challenges to personalized treatment approaches3.
The detection, diagnosis, and classification of depressive symptoms have long been focal points in mental health research. Recent advancements in electrophysiological technology and statistical methodologies have facilitated breakthroughs in this ___domain. Electroencephalography (EEG), a non-invasive method for measuring neural electrical activity in the cerebral cortex, offers a valuable and objective means to assess depression due to its high temporal resolution, convenience, and efficiency. Studies have revealed characteristic EEG patterns in individuals with depression, such as abnormal power in delta and beta frequency bands, hemispheric asymmetries in theta and alpha waves4,5, and discrepancies in power between high-frequency (beta) and low-frequency (delta, theta) bands6,7. A substantial body of research indicates that depression is frequently associated with disrupted hemispheric asymmetry—specifically, reduced activity in the left dorsolateral prefrontal cortex and increased activity in the right8. These asymmetries are believed to underlie deficits in emotional regulation commonly observed in depression9. Resting-state EEG studies have consistently shown significant differences in alpha wave activity between the left and right hemispheres, which correlate with the severity of depressive symptoms10. Typically, individuals with depression exhibit decreased alpha activity in the left dorsolateral prefrontal cortex and increased activity on the right side11, a pattern that is associated with heightened negative affect and diminished motivation, thereby exacerbating depressive symptoms. These findings have been corroborated by functional magnetic resonance imaging (fMRI) studies12,13, underscoring the central role of cerebral hemispheric asymmetry in the pathophysiology of depression. In this study, we employed resting-state EEG as both a biomarker for depressive symptoms and a tool for assessing the potential modulatory effects of physical exercise. Resting-state EEG enables the capture of spontaneous neural activity in the absence of external stimuli and provides insights into underlying abnormalities in brain function among individuals with depression8. Notably, resting-state EEG allows the investigation of the default mode network (DMN), which is often abnormally activated or functionally altered in depressed individuals. These DMN alterations are closely linked to increased self-referential thought and impaired emotion regulation14,15, contributing to heightened vulnerability to depressive episodes16.
In parallel, latent class analysis (LCA) has emerged as a useful method for symptom classification. LCA identifies subgroups of individuals based on the probability distribution of observed behavioral or psychological indicators. It maintains the specificity of intra-group homogeneity and inter-group heterogeneity, offering objective model-fit indices to evaluate the validity of the classifications. This approach accommodates individual variability while helping identify shared symptom features across latent classes, thereby expanding the methodological toolkit for depression research.
Exercise therapy has shown promise as a non-pharmacological intervention for depression due to its low cost, high accessibility, and minimal side effects. Numerous studies have demonstrated that regular physical activity can significantly reduce the risk of depression. Mechanistically, physical exercise can enhance the expression of PGC-1α in skeletal muscle, modulate peripheral kynurenine metabolism, suppress inflammatory responses, regulate neurotrophic factors and glucocorticoids, and promote neurogenesis in the hippocampus. These effects improve central nervous system function and alleviate depressive symptoms. Additionally, resting-state EEG has been proposed as a sensitive biomarker for evaluating the effects of exercise interventions. Exercise has been shown to alter EEG patterns—such as increasing beta power17 and improving frontal alpha asymmetry18—thereby improving mood and cognitive function. These findings suggest that physical exercise promotes cortical relaxation, enhances interhemispheric coordination and synchronization, and supports neuronal survival, ultimately helping to reduce depressive symptoms.
Given this background, the present study aimed to classify university students with depressive symptoms using LCA, examine the EEG characteristics of each subgroup, and summarize the heterogeneity and neurophysiological features of depressive symptoms among university students. Furthermore, we explored the relationships among EEG indicators, depressive symptoms, and physical exercise to investigate whether exercise could alleviate depressive symptoms by modulating EEG features. Our findings are intended to inform clinical interventions and contribute to the development of tailored mental health strategies in higher education settings.
Methods
Participants
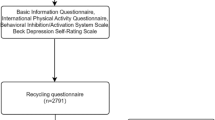
In accordance with the principle of voluntariness, university students were recruited from Songjiang University Town, Shanghai, using convenience sampling. The inclusion criteria were as follows: participants were aged 17–25 years, right-handed, and non-sports majors, with no history of mental illness, head injury, or contraindicated medical conditions such as respiratory, cardiovascular, or cerebrovascular diseases. Participants were also required to have never used barbiturates, benzodiazepines, or chloral hydrate. Prior to the test, participants were instructed to avoid intense physical exercise for 24 h, refrain from consuming caffeinated or alcoholic beverages, and avoid being either fasting or overly full during the test. This study was approved by the Ethics Committee of Shanghai Sport University (102772021RT004), and all procedures were conducted in accordance with the relevant ethical guidelines and regulations. Informed consent was obtained from all participants, who were fully aware of the study procedures and potential risks. The participant recruitment flow chart is shown in Fig. 1.
Process of measurement
From the pool of participants who completed the questionnaire survey, a subset was further selected for EEG signal acquisition, with a target sample size of 150 individuals. All testing procedures, including the questionnaire administration and EEG data collection, were conducted between 8:15 AM and 11:45 AM. 12 of the samples were excluded due to not completing all the testing processes, 6 samples were excluded due to signal quality issues, and ultimately only 132 samples were included for analysis. A flowchart of the EEG signal data collection process can be found in Fig. 2.
Questionnaires
Basic information table
Information was collected on participants’ gender, age, height, weight, registered residence (urban/rural), only-child status, single-parent family background, family relationships, parental education levels, smoking history (defined as smoking more than one cigarette per day for at least six consecutive or cumulative months), drinking habits (defined as consuming alcohol more than once per week during the past year), interpersonal relationships, history of mental illness, and presence of any exercise contraindications.
Patient health questionnaire-9 (PHQ-9)19
The Patient Health Questionnaire-9 (PHQ-9) is a widely used self-report scale for screening depressive symptoms. It comprises nine items, including anhedonia, depressed mood, sleep disturbances, fatigue, appetite changes, low self-esteem, difficulty concentrating, psychomotor retardation or agitation, and suicidal ideation. These items correspond directly to the diagnostic criteria for major depressive disorder outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM) by the American Psychiatric Association. Each item is scored on a 4-point Likert scale: 0 = "not at all," 1 = "several days," 2 = "more than half the days," and 3 = "nearly every day." Higher total scores indicate more severe depressive symptoms. The PHQ-9 has demonstrated good internal consistency, with a Cronbach’s alpha coefficient of 0.882.
The physical activity scale-3 (PARS-3)20
The Physical Activity Scale-3 (PAS-3), translated and revised by Liang Deqing et al., is currently recognized as one of the effective tools for assessing physical activity levels in adults. The scale quantifies physical activity using the formula: amount of exercise = intensity × duration × frequency. Both intensity and frequency are rated on a five-point scale (scored 1 to 5), while duration is rated on a five-grade scale (scored 0 to 4). The total score ranges from 0 to 100 scores. According to the scoring criteria, a total score of ≤ 19 scores indicates low physical activity, 20–42 scores indicates moderate activity, and ≥ 43 scores indicates high physical activity. The scale demonstrates good internal consistency, with a Cronbach’s alpha coefficient of 0.817.
EEG signal collection
EEG signals were recorded using the BEAM system (Model NCERP-190012) developed by NCC Electrophysiology. The system featured a preamplifier and unipolar leads, with a sampling frequency of 500 Hz. Signals were filtered using a 0.3 Hz high-pass filter, a 30 Hz low-pass filter, and a 50 Hz notch filter to eliminate artifacts and power line noise. EEG signals were analyzed across six frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), beta1 (13–20 Hz), and beta2 (20–30 Hz). All EEG data were collected in a soundproof, dark, and well-ventilated room to minimize external disturbances. Participants were not allowed to bring sensory-stimulating electronic devices (e.g., mobile phones) into the testing area. Upon arrival, participants adapted to the environment and were seated comfortably. The EEG cap was applied according to the international 10–20 electrode placement system, with electrodes positioned at Fp1, Fp2, F3, F4, F7, F8, C3, C4, P3, P4, O1, O2, T3, T4, T5, and T6. The ground electrode (GND) and reference electrodes were attached to the bilateral earlobes. All electrode impedances were maintained below 5 kΩ. Participants were instructed to remain awake, breathe steadily, relax, rest their hands naturally at their sides, close their eyes, and avoid teeth clenching or swallowing. After confirming waveform stability, a five-minute resting-state EEG recording was conducted.
The EEG data were preprocessed as follows:(1) The raw EEG signal data in edf format were imported into the EEGLAB toolbox in MATLAB and converted into multichannel time-series data.(2) Electrode channels were identified and non-informative or irrelevant channels were removed. The remaining electrodes included Fp1, Fp2, F3, F4, C3, C4, P3, P4, F7, F8, O1, O2, T3, T4, T5, T6, A1, and A2. The bilateral mastoids (A1 and A2) were used for re-referencing.(3) Independent Component Analysis (ICA) was applied to remove artifacts such as those originating from ocular movements and muscle activity.(4) The EEG signals were decomposed into standard frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), beta1 (13–20 Hz), and beta2 (20–30 Hz).(5) Power spectra were computed using the Fast Fourier Transform (FFT) method.(6) For each electrode, the mean power within each frequency band was calculated to obtain absolute power values.(7) Electrodes were grouped into anatomical regions based on the 10–20 system: orbitofrontal cortex (Fp1, Fp2), dorsolateral frontal cortex (F3, F4), lateral frontal cortex (F7, F8), central region (C3, C4), parietal region (P3, P4), occipital region (O1, O2), anterior temporal (T3, T4), posterior temporal (T5, T6). The index of EEG power values for each brain region was determined by adding P left to P right. The index of EEG lateralization for each pair of homologous electrode sites in the left and right brain was calculated by (P right—P left) / (P left + P right). Higher values indicate greater right lateralization (P indicates absolute power value and left and right refer to the symmetrical electrode sites on the left and right sides of the brain).
Statistic analyses
The data distribution were observed through frequency histogram, and the measurement data that conformed to normal distribution or approximate normal distribution were described by mean ± standard deviation [X ± SD], and the one-way ANOVA was used for group comparison Post hoc multiple comparisons for one-way ANOVA were performed using the Bonferroni method with a corrected p-value of 0.05/3 = 0.017. The measurement data of obvious skewed distribution were described by median (interquartile interval) [M (P25, P75)], and Mann–Whitney U nonparametric test was used for inter-group comparison. N (%) was used to describe the counting data, and χ2 test was used to compare between groups.
Taking the entries of PHQ-9 as explicit indicators, 1 ~ 6 categories were set for latent class model analyses. The fitting index was selected from Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), Entropy, Lo-Mendel-Rubin Likelihood Ratio Test (LMR) and Bootstrap Likelihood Ratio Test (BLRT). The smaller the AIC and BIC were, the higher the Entropy and the better the adaptability of the model. Thus P values of LMR and BLRT would be statistically significant, indicating that the k-class model was better than k-1 class. But the number of samples contained in each class and the reason of different classifications should also be considered when selecting the model, and it is not appropriate to divide too many classes.
Pearson correlation analysis were used to explore the relationship among physical activity level, depressive symptoms and EEG indicators. The statistical inference of all parameters adopted two-tailed test, and the test level α was set to 0.05, and p < 0.05 indicated significant statistical significance.
The data calculation was completed by SPSS Statistics 22.0 and R 4.0.3, and the results were accurate to 3 decimal places (percentages were accurate to 2 decimal places).
Results
Classes of depressive symptoms
The fitting information of latent class analysis model of university students’ depressive symptoms is shown in Table 1. With increasing number of classes, the values of AIC and BIC decreases gradually, and the Entropy value increases gradually, indicating that the classification results tend to be accurate. However, in the Class 4 and Class 5 models, the proportion of people in the smallest category is only 4.26%. Considering model indicators and simplicity, the Class 3 model is selected.
Table 2 and Fig. 3 present the self-reported scores across different PHQ-9 dimensions among the three latent classes of university students. Suicidal ideation was reported exclusively in Class 3, indicating that this group exhibited the most severe depressive symptoms. Class 2 showed moderate levels of depressive symptoms, ranking between Class 1 and Class 3, but without indications of suicidal ideation. Class 1 demonstrated the mildest symptoms and the lowest overall depression scores. Demographic characteristics of the various classes as presented in Table 3.
Demographic features of various classes
Differences of resting EEG among various depressive symptoms
Second stage sampling A total of 132 participants were recruited, with class 1 comprising 61, class 2 comprising 52, and class 3 comprising 19.
Comparing the differences in resting EEG across university students with various depressive symptom profiles, Table 4 presents the EEG metrics that show significant between-group differences. A significant difference was found in alpha1 lateralization in the prefrontal cortex (Fp1, Fp2) between Class 1 and Class 3, with Class 1 showing higher values than Class 3 (p = 0.017). Additionally, significant differences in both theta and alpha1 lateralization were observed in the lateral frontal cortex (F7, F8) between Class 1 and Class 2, with Class 1 exhibiting higher values than Class 2 (p = 0.017) (p = 0.008; p = 0.009). Furthermore, Class 2 showed significantly higher alpha1 power in the parietal region (P3, P4) compared to Class 3 (p = 0.007).
Correlation between resting EEG and physical exercise
Our research has identified EEG indicators that can differentiate between types of depressive symptoms among university students. To further address the research objectives outlined in the introduction, we will now analyze the relationship between these EEG indicators and physical exercise.
As shown in Table 5, significant differences in physical activity were observed across the three groups. Class 1 demonstrated higher exercise intensity compared to Class 2 (p = 0.017), while Class 1 also reported a higher exercise frequency than Class 3 (p = 0.017).
As shown in Fig. 4, alpha1 lateralization in the dorsolateral frontal cortex (F3, F4) was positively correlated with PARS-3 scores (r = 0.183, p = 0.036) and physical exercise intensity (r = 0.225, p = 0.010). Additionally, theta and alpha1 lateralization in the lateral frontal cortex (F7, F8) were positively correlated with physical exercise frequency (r = 0.229, p = 0.008; r = 0.214, p = 0.014).
The results suggest that alpha1 lateralization in the dorsolateral frontal cortex (F3, F4) and theta and alpha1 lateralization in the lateral frontal cortex (F7, F8) serve as mutual EEG indicators of depressive symptoms and physical exercise. The former is associated with more severe depressive symptoms, while the latter two are linked to milder symptoms.
Discussion
In this study, the depressive symptoms of university students were divided into three classes: class 3(55%), class 2(14%) and class 1(31%). The most serious depression is the class 2 which showed suicidal ideation. So it suggests that we should focus on students who have had suicidal ideation or attempted suicide to prevent their suicide. The second serious one was class 1, and the results showed that attention loss and lack of energy are common cognitive symptoms of depressed people. Class 3 accounted for 55% of the total, and its dimension scores were significantly lower than the other two classes, indicating that the class 3 (no depressive symptoms group) performed well. The conclusion of this paper is basically consistent with the previous results. Liu Ailou26 analyzed the characteristics of university students’ depression through LCA analysis, and finally got three types of models. Among them, the group with the most serious symptoms (14.6%) showed depression, crying, strong hopelessness and low self-evaluation, and felt that life was meaningless and even suicidal ideation, corresponding to the suicidal ideation group found in this study; and the main characteristics of mild depression group (38.3%) were psychomotor inhibition, sleep rhythm disorder, slow response, indecision and difficulty in making decisions, and obvious emotion differences between day and night, which corresponded to class 2 found in this study. Previous researches show that only about half of the university students have no depressive symptoms, and may only have slight lack of energy and loss of pleasure. The rest of the university students have mild or severe symptoms and have different performances. It is suggested that it is difficult to distinguish symptoms only by the critical value of the scale, thus reasonable classification is an effective way to explore the best diagnostic demarcation.
The results of this study indicate significant differences in the lateralization of delta in the central area (C3, C4), the power values of alpha1 and alpha2 in the parietal area (P3, P4), the lateralization of theta and alpha1 in the lateral frontal cortex (F7, F8), and the lateralization of alpha1 in the posterior temporal area (T5, T6) when students with different types of depressive symptoms were awake with their eyes closed. After multiple comparisons, it was found that there were significant differences in delta waves in the central area between Class 1 and Class 2, respectively, indicating that university students with depressive symptoms in Class 1 and Class 2 may have insufficient EEG rhythm adjustment ability and sleep rhythm disorder. Delta frequency is related to deep sleep and behavioral inhibition21,22. Duncan et al. found that the delta wave rhythm of depressed patients is lower than that of normal people4. Harmony et al. believe that the increase of delta wave rhythm in brain tasks is related to the functional afferent of cortex or the inhibition of sensory afferent which interferes with internal concentration23. Delta wave activity is an inactive symbol of intermediate neuron and thalamus cortex input, which mostly appears in temporal lobe and occipital lobe during sleep, so it is related to sleep quality. The whole cortex would also present delta wave in normal adults under extreme fatigue and deep sleep21,24. The amplitude of delta in depressed people during sleep is low, and the incidence of delta in the first non-rapid eye movement period decreases25, which attributes to the increase of sleep latency and lack of deep sleep, so the brain activity slows down in awake state, and the increase of delta wave reflects the fatigue and drowsiness of the body26. Theta wave is related to emotional processing27. The rhythm synchronization of theta wave in EEG is enhanced in anger and weakened in happiness. In a comparison of patients with different degrees of depression, it is found that patients with mild depression have higher theta rhythm synchronization in facing happy faces28; On the contrary, when perceiving angry faces, the rhythm synchronization of theta wave in severe depression group is higher. Therefore, it can be concluded that individuals with severe depression exhibit heightened sensitivity to negative emotions and reduced ability to evoke positive emotions when processing internal emotional stimuli. Li et al. found that the synchronization of theta rhythm in the visual cognition of depressed patients increases, which is likely related to their attention deficits. Differences in alpha waves were observed in the parietal region, lateral frontal cortex, and posterior temporal region29. The alpha frequency band is considered a key rhythm of the brain’s physiological state, predominantly appearing in the parietal lobe and its posterior part, and is closely linked to cognitive processes and memory. An increase in alpha power in these brain regions may be associated with excessive vigilance and a decrease in cognitive function. Studies have shown that alpha wave activity in the resting EEG of patients with depression is enhanced30, with increased absolute power of alpha waves31. Schaffer et al. found that alpha wave activation in the left and right hemispheres of the brain was altered, with depressed patients exhibiting hyperactivity in the right frontal cortex and insufficient activity in the left frontal cortex32. Similarly, Golland et al. reported that the asymmetry of alpha waves in patients with severe depression was significantly higher than in healthy controls33.
Class 2 (suicidal ideation) represents one of the most severe symptoms of depression. From a structural and functional brain perspective, suicidal ideation is linked to functional decline in the prefrontal cortex, particularly the anterior cingulate cortex34. The anterior cingulate cortex plays a crucial role in emotion regulation, pain cognition, and self-related thinking, making its dysfunction closely associated with the onset of suicidal ideation. In this study, we observed a correlation between physical exercise and alpha1 lateralization (F7, F8) in the in the lateral frontal cortex. The prefrontal cortex plays a key role in emotional regulation, decision-making, and behavior control, which may explain why exercise frequency is also correlated with alpha1 lateralization. Specifically, alpha waves are EEG activities related to relaxation and the waking state. In patients with depression and suicidal ideation, increased alpha wave activity is thought to help enhance attention and improve their waking state35. Previous studies have shown that physical exercise can increase alpha activity in the brain, thereby promoting emotional regulation and alleviating depressive symptoms. Biologically, alpha1 wave activity is typically linked to relaxation, calmness, and reduced cortical excitability36. In depressed patients, alpha1 activity may be suppressed in the left dorsolateral prefrontal cortex, a region associated with common depressive symptoms such as negative emotions, emotional regulation difficulties, and increased suicidal ideation37. Regular physical exercise has been found to increase alpha1 activity, likely due to the physiological and psychological relaxation effects of exercise, as well as increased neurotransmitter activity (e.g., gamma-aminobutyric acid, GABA)38. An “optimal” balance between exercise frequency and antidepressant effects may enhance alpha1 activity and alleviate depressive symptoms, including suicidal thoughts39. Excessive exercise can lead to physical fatigue and other related problems, while insufficient exercise may not be enough to induce beneficial EEG changes. By enhancing alpha1 activity, physical exercise helps to adjust and balance the neural networks associated with emotion and emotional regulation in the brain. This balance and adjustment may be particularly crucial for reducing suicidal ideation in depressed patients. Additionally, we found a correlation between exercise intensity and alpha2 power in the parietal lobe (P3 + P4). Activities in the alpha2 frequency band are associated with advanced cognitive functions such as attention, memory, and emotional regulation. Depressed patients often exhibit abnormal activity in the alpha2 band, which may be linked to their cognitive and emotional disorders35. The parietal lobe plays a central role in self-awareness and body sensation, with its activity being related to an individual’s emotional state, self-awareness, and social interactions. Enhancing these functions may help depressed patients perceive their emotional and bodily states from different perspectives, thereby alleviating suicidal thoughts. Based on previous studies, we propose that changes in exercise intensity can regulate emotional responses and bodily perceptions related to suicidal ideation. Increased exercise intensity may promote neural activity in the parietal lobe, improving the emotional and social functions of depressed patients. From the mechanism, moderate to high intensity physical exercise is considered as an effective treatment for depression, which can significantly increase the α2 power value, which may be related to the changes in neuroplasticity caused by exercise. For example, physical exercise can promote the production of brain-derived neurotrophic factor (BDNF), which is helpful for the growth and repair of neurons and enhance neuroplasticity40,41; secondly, endorphins are natural compounds that can improve mood. Previous studies have proved that moderate and high intensity physical exercise may promote the secretion of this neurohormone42,43,44. Moreover, there is a study indicating that high-intensity interval training can effectively enhance the self-efficacy of teenagers45. Therefore, we think that the increase of α2 power value may help to balance and adjust the brain network related to emotion and emotional regulation. This neuromodulation may play an essential role in reducing suicidal ideation in patients with depression.
Limitations
-
(1)
Due to limitations in the research conditions, EEG data were recorded using only 16 electrodes.
-
(2)
This study employed a cross-sectional design, which limits the ability to draw conclusions about causality.
-
(3)
Self-report questionnaires were used to assess depressive symptoms and physical activity, which may introduce subjective bias. For instance, the severity of depression with suicidal ideation may be influenced by factors such as medication use or substance consumption. Similarly, the subdimensions of physical exercise assessed may have inherent limitations.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Whiteford, H. A. et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 382(9904), 1575–1586 (2013).
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859), 2163–2196 (2012).
Steffens, D. C. et al. Prevalence of depression among older Americans: The Aging, Demographics and Memory Study. Int. Psychogeriatr. 21(5), 879–888 (2009).
Duncan, W. C. et al. Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J. Affect. Disord. 145(1), 115–119 (2013).
Clark, D. L. et al. Intrinsic local beta oscillations in the Subgenual cingulate relate to depressive symptoms in treatment-resistant depression. Biol. Psychiat. 80(11), e93–e94 (2016).
Knott, V. et al. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. 106(2), 123–140 (2001).
Fingelkurts, A. A. & Fingelkurts, A. A. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol. Psychiat. 77(12), 1050–1060 (2015).
Kaiser, R. H. et al. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiat. 72(6), 603–611 (2015).
Davidson, R. J. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biol. Psychol. 67(1–2), 219–233 (2004).
Thibodeau, R., Jorgensen, R. S. & Kim, S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. J. Abnorm. Psychol. 115(4), 715–729 (2006).
Jesulola, E. et al. Frontal alpha asymmetry as a pathway to behavioural withdrawal in depression: Research findings and issues. Behav. Brain Res. 292, 56–67 (2015).
Chen, J. H. et al. Aberrant global and regional topological organization of the fractional anisotropy-weighted brain structural networks in major depressive disorder. Chin. Med. J. 129(6), 679–689 (2016).
Dutta, A., Mckie, S. & Deakin, J. F. Resting state networks in major depressive disorder. Psychiatry Res. 224(3), 139–151 (2014).
Hamilton, J. P. et al. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol. Psychiat. 70(4), 327–333 (2011).
Whitfield-Gabrieli, S. & Ford, J. M. Default mode network activity and connectivity in psychopathology. Ann. Rev. Clin. Psychol. 8, 49–76 (2012).
Knyazev, G. G. et al. Task-positive and task-negative networks in major depressive disorder: A combined fMRI and EEG study. J. Affect. Disord. 235, 211–219 (2018).
Moraes, H. et al. The effect of acute effort on EEG in healthy young and elderly subjects. Eur. J. Appl. Physiol. 111(1), 67–75 (2011).
Woo, M. et al. The influence of exercise intensity on frontal electroencephalographic asymmetry and self-reported affect. Res. Q. Exerc. Sport 81(3), 349–359 (2010).
Xiao, R. & Zheng, Z. The relationship between physical activity and depression in college students: The impact of sex differences. Chin. J. Sports Med. 36(09), 812–816 (2017) (in Chinese).
Liang, D. The stress level of college students and its relationship with physical exercise. Chin. J. Mental Health 01, 5–6 (1994) (in Chinese).
Baldini, S. et al. Localizing non-epileptiform abnormal brain function in children using high density EEG: Electric Source Imaging of focal slowing. Epilepsy Res. 159, 106245 (2020).
Putman, P. Resting state EEG delta-beta coherence in relation to anxiety, behavioral inhibition, and selective attentional processing of threatening stimuli. Int. J. Psychophysiol. Off. J. Int. Organiz. Psychophysiol. 80(1), 63–68 (2011).
Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integrat. Neurosci. 7, 83 (2013).
Choi, J. W. et al. Abnormal sleep delta rhythm and interregional phase synchrony in patients with restless legs syndrome and their reversal by dopamine agonist treatment. J. Clin. Neurol. (Seoul, Korea) 13(4), 340–350 (2017).
Armitage, R. et al. Delta sleep EEG in depressed adolescent females and healthy controls. J. Affect. Disord. 63(1–3), 139–148 (2001).
Wichniak, A., Wierzbicka, A. & Jernajczyk, W. Sleep as a biomarker for depression. Int. Rev. Psychiatry (Abingdon, England) 25(5), 632–645 (2013).
Tesler, N. et al. Increased frontal sleep slow wave activity in adolescents with major depression. NeuroImage Clin. 10, 250–256 (2016).
Spironelli, C. et al. Evidence of language-related left hypofrontality in Major Depression: An EEG Beta band study. Sci. Rep. 10(1), 8166 (2020).
Li, Y. et al. Depression-related brain connectivity analyzed by EEG event-related phase synchrony measure. Front. Hum. Neurosci. 10, 477 (2016).
Berger, A. M. & Davelaar, E. J. Frontal alpha oscillations and attentional control: A virtual reality neurofeedback study. Neuroscience 378, 189–197 (2018).
Begić, D. et al. Quantitative electroencephalography in schizophrenia and depression. Psychiatr. Danub. 23(4), 355–362 (2011).
Schaffer, C. E., Davidson, R. J. & Saron, C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biol. Psychiat. 18(7), 753–762 (1983).
Gollan, J. K. et al. Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biol. Psychol. 99, 198–208 (2014).
Davidson, R. J. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology 35(5), 607–614 (1998).
Crabbe, J. B. & Dishman, R. K. Brain electrocortical activity during and after exercise: A quantitative synthesis. Psychophysiology 41(4), 563–574 (2004).
Babyak, M. et al. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosom. Med. 62(5), 633–638 (2000).
Deslandes, A. et al. Exercise and mental health: Many reasons to move. Neuropsychobiology 59(4), 191–198 (2009).
Schacter, D. L., Addis, D. R. & Buckner, R. L. Remembering the past to imagine the future: The prospective brain. Nat. Rev. Neurosci. 8(9), 657–661 (2007).
Schneider, S. et al. The influence of exercise on prefrontal cortex activity and cognitive performance during a simulated space flight to Mars (MARS500). Behav. Brain Res. 236(1), 1–7 (2013).
Kruk, J., Kotarska, K. & Aboul-Enein, B. H. Physical exercise and catecholamines response: Benefits and health risk: possible mechanisms. Free Radical Res. 54(2–3), 105–125 (2020).
Stroehle, A. et al. Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology 35(3), 364–368 (2010).
Goldfarb, A. H. et al. Plasma beta-endorphin concentration: Response to intensity and duration of exercise. Med. Sci. Sports Exerc. 22(2), 241–244 (1990).
Harber, V. J. & Sutton, J. R. Endorphins and exercise. Sports medicine (Auckland, NZ) 1(2), 154–171 (1984).
Pahlavani, H. A. Possible role of exercise therapy on depression: Effector neurotransmitters as key players. Behav. Brain Res. 459, 114791 (2024).
Costigan, S. A. et al. High-intensity interval training for cognitive and mental health in adolescents. Med. Sci. Sports Exerc. 48(10), 1985–1993 (2016).
Author information
Authors and Affiliations
Contributions
Mrs. Qun Zhao: data collect and manuscript editing Mrs. Yanran Si: data collect Mrs. Lijuan Mao, manuscript editing Mr. Xing Wang: review and editing Mr. Peng Wang: data collect and analysis Mrs. Shufan Li: data collect Mr. Zhaohui Guo: manuscript editing Mr. Jianghua Zhu: review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Q., Si, Y., Mao, L. et al. The relationship between resting EEG features and physical exercise among university students with depressive symptoms. Sci Rep 15, 19839 (2025). https://doi.org/10.1038/s41598-025-04430-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04430-5