Abstract
This study aimed to develop models for predicting forkhead box protein A1 (FOXA1) gene mutations in prostate cancer using clinicoradiological and MRI radiomics data. Totally 367 prostate cancer patients (109 with FOXA1 mutations and 258 without) from three centers underwent multiparametric MRI. Patients from Center 1 (n = 236) were randomly divided into training and internal validation sets (7:3). Patients from Centers 2 and 3 (n = 131) were used for external validation. The index tumor lesion’s volume of interest was delineated on MRI images to obtain 428 radiomics features for each patient. Radiomics features were selected by least absolute shrinkage and selection operator regression. Clinicoradiological features were compared between mutant and wild-type patients for feature selection. Those selected features were further chosen by binary logistic regression (LR) analysis, and used to develop prediction models for FOXA1 mutations with LR, support vector machine (SVM), and random forest (RF) classifiers. Models’ performances were assessed by area under the receiver operating characteristic curve (AUC). No clinicoradiological feature was associated with FOXA1 mutations, while three radiomics features were selected to build models. AUCs of RF model in internal and external validation sets (0.82 and 0.81) were significantly greater than LR (0.74 and 0.71) and SVM (0.60 and 0.65) models (all p < 0.05), while AUC of LR model was greater than SVM model in internal validation set (p = 0.03). Radiomics method with RF classifier could effectively predict FOXA1 mutations in prostate cancer.
Similar content being viewed by others
Introduction
Prostate cancer ranks among the most prevalent malignancies and is the leading cause of cancer death among men in a quarter of the world’s countries1. Androgen deprivation therapy (ADT) remains the primary treatment modality due to its efficacy in targeting the steroid hormone receptor androgen receptor (AR), a pivotal essential mediator of prostate cancer development and progression2. Regrettably, prolonged ADT may lead to the progression of prostate cancer into castration-resistant prostate cancer (CRPC)3 and even neuroendocrine prostate cancer (NEPC)4, which are fatal and rapidly progressing malignancies, and the transcriptional network also shifts5. Therefore, revealing the factors contributing to the progression of prostate cancer is imperative for the development of efficacious treatment strategies.
Forkhead box protein A1 (FOXA1) is a transcriptional activator of steroid hormone receptors such as AR6,7. As a regulator of differentiation in prostate luminal epithelial cells, FOXA1 has been found to play a role in promoting the transition from closed chromatin to open chromatin. This transition facilitates gene expression, enhances cellular proliferation, and ultimately contributes to prostate tumorigenesis5; moreover, FOXA1 promotes both tumor growth and metastasis in CRPC and NEPC5,8. Even for patient undergoing radical prostatectomy (RP), a recent study has proved that foxa1 gene status was an important factor to identify subtypes of prostate cancer which were closely associated with biochemical recurrence (BCR)-free survival after RP, playing an important role in the prognosis evaluation9. Another study also proved that, after receiving neoadjuvant ADT plus apalutamide and RP, the BCR of prostate patients with FOXA1 gene mutations was significantly shorter than that of patients without, which meant the FOXA1 gene status could be a factor that effect the designment of neoadjuvant treatment10. Specifically, in the Chinese population, the mutation rate of FOXA1 is greater than that in the Western population, and the mutation rate increases to 20% in CRPC patients11. Moreover, recent studies have shown that FOXA1 itself or its related pathways can serve as treatment targets12,13. Those results indicated that FOXA1 gene mutations could be potential therapeutic targets for a large portion of prostate cancer patients.
At present, genetic testing is recommended for high-risk, very-high-risk, regional or metastatic prostate cancer patients14,15. The testing of prostate cancer involves the analysis of tissue samples and circulating tumor DNA (ctDNA), and tumor tissue testing is the gold standard for the assessment of genetic mutations and alteration16,17.This approach has certain drawbacks, including high cost, technical complexity, and invasiveness, which can limit its widespread adoption and accessibility. Moreover, most genetic testing of prostate only included one malignant sample per patient11,18, but the majority of prostate cancers are multifocal19 and there is a very high degree of interfocal heterogeneity among tumors18,20. Therefore, there is an urgent necessity to develop noninvasive and cost-effective surrogate methods that can be used to comprehensively evaluate FOXA1 gene status of prostate cancer, and select lesions and patients suspected of FOXA1 mutations before genetic testing, allowing for more targeted and effective interventions.
Multiparametric magnetic resonance imaging (mpMRI) has emerged as an invaluable tool for the comprehensive evaluation, diagnosis, and treatment response monitoring in prostate cancer patients21. Previous studies have shown that MRI-based radiomics signatures can serve as biomarkers to predict gene expression patterns and mutation status in brain, lung, colorectal, breast, and kidney tumors22. In prostate cancer patients, the potential of MRI combined with radiomics has also been proven in the prediction of gene status23. Recently, Skingen et al.24 suggested that radiogenomics could reveal two gene expression programs activated at different hypoxia levels, and Chen et al.25 proved that models integrating pathological and radiomics features can predict the likelihood of TP53 mutations in prostate cancer patients, which preliminarily proved the ability of this technique to identify genetic mutations in prostate cancer patients.
Consequently, the aim of this study was to utilize mpMRI based radiomics analysis and clinicoradiological data for predicting the occurrence of FOXA1 mutations in prostate cancer.
Materials and methods
Patients
Approval for this retrospective investigation was obtained from the Institutional Review Boards (IRB) of Fudan University Shanghai Cancer Center (No. 050432-4-2307E), Shanghai Proton and Heavy Ion Center (No. 230614EXP-01), Suzhou Municipal Hospital, Jiangsu, P.R. China (K-2021-GSKY20210209). All methods and experimental protocols were performed in accordance with the relevant guidelines and regulations. The requirement for obtaining informed consent was waived under the approval of IRBs above, due to the retrospective nature of the study.
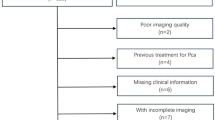
From July 2018 to January 2024, data for 696 patients with biopsy or radical prostatectomy-proven prostate cancer were retrieved from the history systems of three centers (Center 1, Fudan University Shanghai Cancer Center; Center 2, Shanghai Proton and Heavy Ion Center; Center 3, Suzhou Municipal Hospital) according to the following criteria: the patient (a) underwent gene testing and had available data on FOXA1 mutation status; (b) had undergone whole-body CT or PET exams before surgery; (c) had not received any prior treatment before surgery; (d) had prostate mpMRI images with acceptable-quality; and (e) had an interval between prostate mpMRI and biospy/surgery of less than 2 weeks (Fig. 1).
A total of 236 consecutive patients from Center 1 were included in the study; 74 had FOXA1 mutations, and 162 did not have FOXA1 mutations. The patients were allocated into two datasets at a random ratio of 7:3. The training dataset comprised 165 patients, whereas the internal validation dataset encompassed 71 patients. The predictive models for FOXA1 were constructed using the training dataset and validated with the internal validation dataset. Additionally, a set of 131 patients from Center 2 (24 with FOXA1 mutations and 66 without FOXA1 mutations) and Center 3 (11 with FOXA1 mutations and 30 without FOXA1 mutations) was used for external validation of the prediction model. Baseline clinical characteristics, including patient age, index lesion’s Gleason score, prostate-specific antigen (PSA) level, imaging T stage, pelvic lymph node and distant metastasis status were extracted from patient medical records (Table 1).
Pathological procedures and FOXA1 mutation status identification
Mutation analysis of FOXA1 was conducted using DNA extracted from formalin-fixed, paraffin-embedded tumor sections obtained from patients who underwent postoperative resection or needle biopsy. Pathologists examined the histopathology slices and utilized the modified Gleason scoring system to determine the Gleason scores for tumor lesions. The tissue involvement and Gleason scores of needle biopsy specimens were individually assessed and recorded for each core subjected to MRI segmentation. Index lesion was identified and the tissue was sampled for DNA extraction.
The DNA extraction was performed with a DNA extraction kit, and the mutation analysis was conducted through the polymerase chain reaction (PCR) method. Patients without FOXA1 mutations were referred to as wild type.
MRI protocol
MRI scans were performed using four 3.0-T MRI systems (Siemens, Magnetom Skyra or GE SIGNA Pioneer), equipped with 16-channel body array coils.
T2-weighted imaging (T2WI), dynamic contrast enhancement (DCE) MRI, and diffusion-weighted imaging (DWI) were all utilized to image the entire prostate and seminal vesicle. Apparent diffusion coefficient (ADC) maps were generated with the DWI images (b = 0 or 50, 1000 s/mm2) after postprocessing. Sequence parameters are displayed in Table 2.
Lesion mpMRI radiological feartures assessment
The MRI images were assessed by two urogenital radiologists, one with 8 years of experience (reader 1) and another with two decades of experience (reader 2). All pathological and clinical data except the gene profiles were provided to the radiologists. For each patient involving multifocal tumors, only the index lesion was included. The index lesion was determined to be the lesion with the highest Gleason score, if there were multiple lesions with the highest Gleason score, the largest one among them would be identified as the index lesion26,27. For each patient, Reader 1 and 2 assessed the mpMRI characteristic of index lesion, including mpMRI lesion size, European Society of Urogenital Radiology Extraprostatic extension (ESUR-EPE) score, seminal vesicle invasion and the PI-RADS score together, according to a previous study28, the differences between readers were resolved by consensus, these results and ADC value of index lesion were recorded as mpMRI radiological feartures in Table 1.
Lesion segmentation
The regions of interest (ROIs) of index lesion were contoured by ITK-SNAP 4.0 (www.itksnap.org), an open-source image processing software29. First, the lesions were delineated along the tumor border on consecutive images obtained through axial T2WI, DCE, DWI (at the highest b value) and ADC images referring to the pathological results by reader 1 (Fig. 2A–C), the ROIs covered the entire lesion volume while excluding the surrounding normal tissue. To enhance the differentiation between prostate cancer and the surrounding tissues, the DCE images were acquired during the 4th phase out of a total of 18/20 phases in this study25.
Second, the reader 2 drew and conducted ROI depiction on a random selection of 55 cases (15% of the whole sample) to evaluate the interobserver correlation coefficient (Fig. 2D–F).
Radiomics feature extraction
The gray levels of each image were restricted to the range of μ ± 3σ, where μ represents the mean value of gray levels within the VOI, and σ denotes the standard deviation of gray levels. Subsequently, the resulting gray levels were normalized to a scale ranging from zero to one.
Radiomics features were extracted using PyRadiomics, a flexible open-source platform from a previous study30, with the grey levels of each image discretized into 25 levels. A total of 7 categories of radiomics features were extracted, encompassing 14 shape features, 18 first-order statistical features, 24 grey-level co-occurrence matrix (Glcm) features, 16 grey-level size zone matrix (Glszm) features, 16 grey-level run length matrix (Glrlm) features, 14 grey-level dependence matrix (Gldm) features, and 5 normalized grey-level texture distribution matrix (Ngtdm) features. All radiomics features were derived from four distinct image types, resulting in a total of 428 quantitative 3D radiomics features for each volume of interest (VOI).
Feature selection
To tackle the problem of class imbalance in the training dataset, a Synthetic Minority Over-Sampling Technique (SMOTE) was employed to upsample the data, ensuring a balanced distribution of positive and negative samples31. Furthermore, the feature matrix was normalized using zero to one normalization, ensuring that each feature vector was centered by subtracting its mean value and scaled by dividing the result by its length.
Due to the high dimensionality of the feature space, we evaluated the similarity of each feature pair. In cases where the Pearson correlation coefficient of a feature pair exceeded 0.9, indicating high similarity. One of them was eliminated in order to reduce the dimensionality of the feature space and maintain feature independence. Afterwards, we used interclass correlation coefficients to further evaluate the interobserver repeatability. Features with interclass correlation coefficients values below 0.8 were excluded from the analysis.
Prior to model construction, we screened the radiomics features using the least absolute shrinkage and selection operator (LASSO) regression, a technique that adds the absolute values of the weights to the regression objective function as a penalty to prevent overfitting. This allowed us to identify the most relevant features that exhibited a high correlation with the target variable. We also compared the clinical and mpMRI radiological features between FOXA1-mutant and FOXA1-wild-type patients, and clinical features with significant differences would be included. The selected radiomics features and clinical features were subjected to binary logistic regression (LR) analysis, and features significantly related to FOXA1 mutation were used to construct radiomics models to predict FOXA1 mutant and wild-type patients.
Machine learning model
Three machine learning models, LR, support vector machine (SVM) and random forest (RF) classifiers were utilized for predicting the FOXA1 mutation status of patients. The optimal hyperparameters for the model were determined using a tenfold cross-validation (CV) technique on the training dataset. The hyperparameter selection was based on the model’s performance on the validation datasets, and an L2 regulation technique was applied to penalize large coefficients. This comprehensive approach ensured outstanding performance and capacity to effectively generalize for unseen data, and the tenfold CV and L2 regulation techniques were further used to avoid overfitting in the models.
Statistical analysis
These analyses were conducted using STATA 17 software, and a p value below 0.05 was considered statistically significant. The Mann‒Whitney U test was utilized to compare the differences in various clinicoradiological features between FOXA1-mutant and FOXA1-wild-type patients. Continuous variables that follow a normal distribution are reported as the mean ± standard deviation (SD), while those with skewed distribution are reported as the median and range. The clinicoradiological features that were significantly different between FOXA1-mutant and FOXA1-wild-type patients would be included. The selected clinicoradiological features and radiomics features were selected using LR analysis, and only the features significantly associated with mutations (P < 0.05) were included.
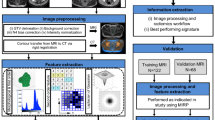
The performance of models was evaluated and their diagnostic accuracy compared using Receiver Operating Characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was computed to provide a quantitative assessment. Additionally, 95% confidence intervals were calculated for each of these parameters to evaluate their robustness. Furthermore, the accuracy, sensitivity, and specificity were calculated at the optimal cut-off value that maximized the Youden index. Figure 3 illustrates an overview of the workflow utilized in this study.
Workflow of radiomics analysis. DCE, dynamic contrast-enhanced imaging; T2WI, T2-weighted imaging; DWI, diffusion weighted imaging; ADC, apparent diffusion coefficient; VOI, volume of interest; LASSO, shrinkage and selection shrinkage and selection operator; LR, logistic regression; SVM, support vector machine (SVM); RF, random forest; ROC, receiver operating characteristic.
Results
Clinical and mpMRI radiological characteristics
No statistically significant differences were observed in the distribution of the FOXA1 mutation rate among the training, internal validation, and external validation datasets (32.1%, 29.6%, and 26.7%, respectively, all p > 0.05). Additionally, there was no statistically significant difference in any clinical or mpMRI radiological feature between patients with and without FOXA1 mutations (p = 0.39–0.86) (Table 1), therefore, no clinical or mpMRI radiological feature was included in further research.
Radiomics analysis of the RF, SVM, and LR models for the training, internal and external validation cohorts
For each patient, the T2WI, DCE, DWI images, and ADC maps yielded a total of 428 radiomics features. After conducting Pearson correlation analysis and interclass correlation coefficients assessment, the initial number of radiomics features was reduced to 40. Then, LASSO analysis was performed, resulting in the selection of 18 features. Since no clinical or radiological features were included, the following the binary logistic regression analysis only on selected radiomics features, it was determined that two radiomics features from DWI and one from T2WI exhibited significant differences between the wild-type and mutated groups (Fig. 4) (Table 3).
Plots (A–C) presented the Violin boxplots of the radiomics features firstorder_Minimum_DWI (A), Neighbourhood gray-tone difference matrix (Ngtdm)_Strength_DWI (B) and Ngtdm_Strength_ T2WI (C) with significant difference between the FOXA1 mutated and wild-Type prostate cancer in the training datasets, respectively. Among the above three features, one features (A) was positively correlated with Foxal mutations, and two feature (B, C) were negatively correlated with FOXAl mutations.
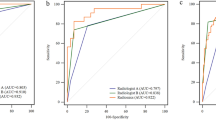
The ROC curves for predicting FOXA1 mutations in the radiomics models are shown in Fig. 5 and Table 4. In the internal validation set, the RF model achieved the highest AUC (0.82) compared with the other two models (0.74 for LR and 0.60 for SVM) (p = 0.03 and 0.01), and the AUC of the LR model was also significantly greater than that of the SVM model (p = 0.03). In the external validation sets, the AUC of the RF model (0.81) was found to be significantly higher than the LR model (0.71) and SVM model (0.65) (p = 0.04 and 0.01). However, the AUC did not show a statistically significant difference between the SVM and LR models (p = 0.41).
Receiver operating characteristic (ROC) curves of the MRI-based radiomics predictive models for forkhead box protein A1 (FOXA1) mutation discrimination in training (A), internal validation (B), and external validation (C) datasets. The values of area under the ROC curve (AUC) were listed as mean (95% confidence interval). In internal (B) and external validation (C), the random forest (RF) model exhibited significantly higher AUCs than those of logistic regression (LR) and support vector machine (SVM) model.
Discussion
The presence of FOXA1 mutations in prostate cancer has the potential to provide valuable insights into predicting resistance to tumor treatment, prognosis, and identifying therapeutic targets9,10,13,32. In this study, we investigated the potential of mpMRI utilizing radiomics features and clinical characteristics to detect FOXA1 mutations in patients with prostate cancer.
Clinical, conventional mpMRI radiological and radiomics features were included in the study, but interestingly, there was no significant difference for the clinical or mpMRI features between the mutation and non-mutation groups.
There is controversy regarding whether foxal mutations are associated with clinical features. A previous study has suggested that FOXA1 expression levels were significantly associated with Gleason score and T stage in primary prostate cancer in Germany population33, but in another study, FOXA1 expression was positively correlated with tumor size, extraprostatic extension, angiolymphatic invasion, AR and lymph node metastases at diagnosis but did not correlate with age, T stage, Gleason score, presence of prostatic intraepithelial neoplasia or multifocality, seminal vesicle or perineural invasion, or surgical excision margin status8. A recent study indicated no association between FOXA1 mutation and metastasis in the patients of the African race34, while in a study in China with a larger sample size, the mutation rate of locoregional prostate cancer (11%) was lower than metastatic CRPC (mCRPC) (18%), but was higher than metastatic castration-sensitive prostate cancer (mCSPC)11. In our study, no associations between FOXA1 mutation and tumor stage, Gleason score or metastasis status were detected. The controversy in those studies may be explained by the differences in race among cohorts. The FOXA1 mutations could be classified into class 1 to 3. In Western prostate cancer patients, Class-2, which promotes metastasis mutates at a higher prevalence, thus the FOXA1 mutations were prove to be associated with aggressiveness. While among Asian patients, the class 1 is more common, it can strongly transactivate a luminal AR program of prostate oncogenesis and play a important role in CRPC, thus is more closely associated with CRPC rather than aggressiveness at diagnosis11,35. Due to the variety of the classes, the association between clinical characteristics and FOXA1 mutation could be more uncertain.
Also, there might be two reasons why the mpMRI radiological features were not significantly related to the FOXA1 mutations. First, the microstructural changes caused by FOXA1 mutations might be too subtle to detect for radiological features assessed by naked eyes. Second, in terms of variability and subjectivity of mpMRI features, previous study has proved low agreement among readers in some mpMRI features, especially highly subjective features: shape, signal intensity level, and type of lesion margins, which could further reduce the interreader and intrareader reproducibility of the PI-RADS lexicon36. As a result, the variability and subjectivity in assessment of mpMRI features would also weaken the their ability for the detecting of FOXA1 mutations. Therefore, although the mpMRI radiological and clinical features are available and commonly used in the clinical diagnosis of prostate cancer, it is hard to depend on them to detect of FOXA1 mutations with traditional statistic method or deep learning modelling approach.
In terms of the involved radiomics features, the Neighbourhood gray-tone difference matrix (ntgdm)_Strength of DWI and T2WI were negatively correlated with FOXA1 mutations, and the minimum of DWI was positively correlated with FOXA1 mutations, and high coefficients of the correlations demonstrated their potential as noninvasive biomarkers for identifying FOXA1 mutations. Ntgdm_Strength evaluates the signal intensity and spatial correlation between neighboring voxels across adjacent image planes, characterizing the dynamic range of intensities at a local level and providing a more precise quantification of heterogeneity within the tumor. Since FOXA1 mutation represses androgen signaling and increases tumor growth33, lower Ntgdm_Strength of DWI and T2WI might suggest a greater percentage of cancer epithelial cells. A higher minimum (firstorder_Minimum) of DWI indicates a denser structure and less normal tissue mixed in lesions.
Numerous studies have demonstrated the ability of radiomics features to predict gene status in prostate cancer patients. Sun et al.37 showed that 16 T2WI texture features exhibited weak but significant correlations with hypoxia-related gene expression, and another study of Ogbonnaya et al.38 based on quantitative MRI indicated that three T2WI features and one ADC textural feature were correlated with three hypoxia-related genes. In the study by Chen et al.25, three DCE features and two DWI features were significantly correlated with TP53 mutation. In our study, features from T2WI and DWI were involved. In these studies, we observed that different genes could be assessed according to different radiomics biomarkers, and features from T2W and DWI appeared more commonly used in radiogenomics studies than ADC and DCE-related features. Since DCE imaging did not yield good results in this study, we might be able to predict FOXA1 mutation status without contrast media; thus, this technique could be applied several times without injury and at high cost. Therefore, DWI and T2WI could serve as stable sources of texture features for the assessment of FOXA1 gene status in cancer patients.
Our study further indicated that the radiomics features with RF classifier was effective in detecting FOXA1 mutations among prostate cancer patients. The performance of the developed prediction model was validated in both internal and external datasets, with an AUC of 0.83 and 0.80 for the internal and validation cohorts, respectively, indicating the reliability and reproducibility of the model. The findings indicate that radiomics features extracted from DWI and T2WI have the potential to serve as noninvasive tools for the detection of FOXA1 mutations. This may aid in identifying suitable candidate lesions and patients for genetic testing, facilitating patient prognosis assessment and help to adjust the therapy strategy, or even offering potential biomarkers for further therapy. For example, as the prognosis of FOXA1 mutated patients with neoadjuvant ADT and RP is unsatisfied10, patient who was suspected of FOXA1 mutation by radiomics model would be acquired to undergo genetic tests to confirm FOXA1 mutations and search for other therapy targets. The confirmed FOXA1 mutations would help to adjust the neoadjuvant ADT, such as adding neoadjuvant chemotherapy to improve the effect39. Meanwhile, the application of genetic testing is now mainly recommended for high-risk, very-high-risk, regional or metastatic prostate cancer patients14,15, since MRI is a routine and noninvasive exam for prostate cancer, low-/intermediate-risk patients suspected of foxa1 mutation will also benefit from this radiogenomics analysis on MRI. And in low- and middle-income countries, those result could also provide reference for the treatment of patients who could not afford expensive genetic tests. Meanwhile, some clinical risks should also be vigilant against. For example, false positive result of these models, such as a patient or lesion was misdiagnosed as FOXA1 mutation, may cause a unnecessarily genetic test to confirm it, or cause more frequent following up than routine and overtreatment, result in higher cost and more anxiety. The false negative result might cause underestimations of the risk of progression of prostate cancer and shortage of attentive treatment. However, since the genetic test is not commonly applied, FOXA1 mutation is infrequently detected accordingly, the false negative result would not affect the treatment of most patients, its influence might be relatively smaller.
Our study had several limitations. Firstly, due to its retrospective nature, potential bias in patient selection may have been present. For example, the proportion of patients with FOXA1 mutation was greater than that in previous literature11 because patients at high risk are likely to undergo complete genetic testing and MRI. Second, there may be potential discrepancies between MRI ROIs and pathology results, especially considering that some patients’ pathology was evaluated based on biopsy. For instance, the ROIs on MR images may not fully encompass the actual tumour area, resulting in the loss of information on certain regions within the tumor. Third, the FOXA1 gene works on the structure and metabolism of prostate cancer, but the MRI radiomics analysis mainly focuses on the structure change of prostate. Recent study have prove the ability of PET imaging combined with Machine Learning in assessing the status of metabolism-related genes40, thus, further researches combining MRI radiomics and PET image features are need to assess the structure and metabolism together, and provided a comprehensive evaluation of FOXA1 gene status.
Conclusion
Our study demonstrated that mpMRI (T2WI and DWI) based radiomics models have potential as noninvasive and effective tools for predicting FOXA1 status in prostate cancer patients and will facilitate the assessment of prognosis and guide subsequent treatment for prostate cancer patients.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. If someone wants to request the data, he can contact corresponding author by email, upon reasonable request.
References
Bergengren, O. et al. 2022 update on prostate cancer epidemiology and risk factors: A systematic review. Eur. Urol. 84, 191–206 (2023).
Taplin, M. E. Drug insight: Role of the androgen receptor in the development and progression of prostate cancer. Nat. Clin. Pract. Oncol. 4, 236–244 (2007).
Vellky, J. E. & Ricke, W. A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 22, 566–575 (2020).
Lee, C. F. et al. The central role of sphingosine kinase 1 in the development of neuroendocrine prostate cancer (NEPC): A new targeted therapy of NEPC. Clin. Transl. Med. 12, e695 (2022).
Celada, S. I. et al. Lysosome-dependent FOXA1 ubiquitination contributes to luminal lineage of advanced prostate cancer. Mol. Oncol. 17, 2126–2146 (2023).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Adams, E. J. et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019).
Jain, R. K., Mehta, R. J., Nakshatri, H., Idrees, M. T. & Badve, S. S. High-level expression of forkhead-box protein A1 in metastatic prostate cancer. Histopathology 58, 766–772 (2011).
Feng, D. et al. Senescence-associated secretory phenotype constructed detrimental and beneficial subtypes and prognostic index for prostate cancer patients undergoing radical prostatectomy. Discov. Oncol. 14, 155 (2023).
Tao, H. et al. Efficacy and predictive factors analysis of androgen deprivation plus novel hormone therapy as neoadjuvant treatment for high-risk prostate cancer. Prostate 85, 198–206 (2025).
Wei, Y. et al. Prospective clinical sequencing of 1016 Chinese prostate cancer patients: Uncovering genomic characterization and race disparity. Mol. Oncol. 17, 2183–2199 (2023).
Xiao, L. et al. Targeting SWI/SNF ATPases in enhancer-addicted prostate cancer. Nature 601, 434–439 (2022).
Hwang, K. W., Yun, J. W. & Kim, H. S. Unveiling the molecular landscape of FOXA1 mutant prostate cancer: Insights and prospects for targeted therapeutic strategies. Int. J. Mol. Sci. 24 (2023).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Prostate cancer (version 2.2019) (2019). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Giri, V. N. et al. Role of genetic testing for inherited prostate cancer risk: Philadelphia prostate cancer consensus conference 2017. J. Clin. Oncol. 36, 414–424 (2018).
Wyatt, A. W. et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J. Natl. Cancer Inst. 109 (2017).
Chi, K. N. et al. Detection of BRCA1, BRCA2, and ATM alterations in matched tumor tissue and circulating tumor DNA in patients with prostate cancer screened in PROfound. Clin. Cancer Res. 29, 81–91 (2023).
Løvf, M. et al. Multifocal primary prostate cancer exhibits high degree of genomic heterogeneity. Eur. Urol. 75, 498–505 (2019).
Andreoiu, M. & Cheng, L. Multifocal prostate cancer: Biologic, prognostic, and therapeutic implications. Hum. Pathol. 41, 781–793 (2010).
Haffner, M. C. et al. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 18, 79–92 (2021).
Sherafatmandjoo, H., Safaei, A. A., Ghaderi, F. & Allameh, F. Prostate cancer diagnosis based on multi-parametric MRI, clinical and pathological factors using deep learning. Sci. Rep. 14, 14951 (2024).
Qi, Y., Zhao, T. & Han, M. The application of radiomics in predicting gene mutations in cancer. Eur. Radiol. 32, 4014–4024 (2022).
Thenault, R., Gasmi, A., Khene, Z. E., Bensalah, K. & Mathieu, R. Radiogenomics in prostate cancer evaluation. Curr. Opin. Urol. 31, 424–429 (2021).
Skingen, V. E. et al. Prostate cancer radiogenomics reveals proliferative gene expression programs associated with distinct MRI-based hypoxia levels. Radiother. Oncol. 188, 109875 (2023).
Chen, R. et al. Association of pathological features and multiparametric MRI-based radiomics with TP53-mutated prostate cancer. J. Magn. Reson. Imaging 60, 1134–1145 (2024).
Karavitakis, M. et al. Histological characteristics of the index lesion in whole-mount radical prostatectomy specimens: Implications for focal therapy. Prostate Cancer Prostatic Dis. 14, 46–52 (2011).
Ghafoor, S. et al. Index lesion contouring on prostate MRI for targeted MRI/US fusion biopsy: Evaluation of mismatch between radiologists and urologists. Eur. J. Radiol. 162, 110763 (2023).
Luzzago, S. et al. Multiparametric MRI-based 5-year risk prediction model for biochemical recurrence of prostate cancer after radical prostatectomy. Radiology 309, e223349 (2023).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
van Griethuysen, J. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77, e104–e107 (2017).
Chawla, N. V., Bowyer, K. W., Hall, L. O. & Kegelmeyer, W. P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 321–357 (2002)
Dong, H. Y. et al. FOXA1 in prostate cancer. Asian J. Androl. (2022).
Gerhardt, J. et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am. J. Pathol. 180, 848–861 (2012).
Faisal, F. A. et al. CDKN1B Deletions are associated with metastasis in African American men with clinically localized, surgically treated prostate cancer. Clin. Cancer Res. 26, 2595–2602 (2020).
Parolia, A. et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 571, 413–418 (2019).
Jozwiak, R., Sobecki, P. & Lorenc, T. Intraobserver and interobserver agreement between six radiologists describing mpMRI features of prostate cancer using a PI-RADS 2.1 structured reporting scheme. Life (Basel) 13 (2023).
Sun, Y. et al. Association analysis between quantitative MRI features and hypoxia-related genetic profiles in prostate cancer: A pilot study. Br. J. Radiol. 92, 20190373 (2019).
Ogbonnaya, C. N. et al. Radiogenomics reveals correlation between quantitative texture radiomics features of biparametric MRI and hypoxia-related gene expression in men with localised prostate cancer. J. Clin Med. 12 (2023).
Ge, Q. et al. Neoadjuvant chemohormonal therapy in prostate cancer before radical prostatectomy: A systematic review and meta-analysis. Front. Oncol. 12, 906370 (2022).
Bauckneht, M. et al. Gene’s expression underpinning the divergent predictive value of [18F]F-fluorodeoxyglucose and prostate-specific membrane antigen positron emission tomography in primary prostate cancer: A bioinformatic and experimental study. J. Transl. Med. 21, 3 (2023).
Acknowledgements
Our study was supported by National Cancer Center of China, No. NCC201909B03
Author information
Authors and Affiliations
Contributions
X.L. and K.L. conceived and designed the study. L.Z., R.C. and K.L. collected the data. H.G. performed the pathological analyses. D.L. and G.H. performed the radiomics analyses of images. B.Z. performed the statistical analyses. R.C. and L.D. wrote the manuscript text, L.Z. and X.L made amendments to the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, L., Chen, R., Zhou, B. et al. The value of MRI-based radiomics and clinicoradiological data for the detection of forkhead box protein A1 gene mutated prostate cancer. Sci Rep 15, 22929 (2025). https://doi.org/10.1038/s41598-025-04562-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04562-8