Abstract
H2S signal transduction involves various physiological processes, including promoting vasodilation, regulating lipid metabolism, inducing angiogenesis, improving oxidative stress and inflammatory response, and avoiding cell apoptosis. Oxidative stress is an important mechanism that causes the pathological progression of NAFLD. However, the effect and specific mechanism of exogenous H2S on oxidative stress in NAFLD are still unclear. Here, we investigated the specific regulatory mechanism of exogenous H2S on oxidative stress and inflammation induced by LM in HepG2 cells. HepG2 cells were stimulated with LM with or without GYY4137 (200 µM) treatment for 24 h. The levels of MDA, SOD, ROS, TNF-α, IL-6, and antioxidant related proteins of cells were detected. We found exogenous H2S remarkably reduced the levels of MDA, ROS, TNF-α and IL-6 and elevated SOD contents as well as the expression of antioxidant-related proteins in LM-induced HepG2 cells. Moreover, exogenous H2S improved the expression of USP22 protein in LM-induced HepG2 cells and inhibited the ubiquitination degradation of SIRT1 through USP22. After USP22 was knocked down, the efficacy of exogenous H2S on mitigating LM-induced oxidative damage and inflammatory reaction in HepG2 cells had been weakened. In conclusion, exogenous H2S inhibited SIRT1 ubiquitination degradation through USP22, thereby alleviating LM-induced oxidative stress and inflammatory responses in HepG2 cells.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is gradually becoming the main cause of liver transplantation. The occurrence of NAFLD involves multiple factors, including genes, metabolism and environment, especially closely related to metabolic diseases such as obesity, insulin resistance and hyperlipidemia. According to relevant survey data, NAFLD has reached about 25% worldwide, of which the incidence rate of the disease among diabetes and obese people has exceeded 70%, which has caused serious negative impact on the physical and mental health and quality of life of patients1,2.
Oxidative stress is a cellular physiological process characterized by the disruption of the balance between reactive oxygen species (ROS) and antioxidants within the cell, leading to oxidative damage and affecting various biological processes. Oxidative stress is related to the pathophysiology of over 100 diseases3,4,5,6,7. In NAFLD, dysfunction such as mitochondria β-oxidation and endoplasmic reticulum stress, followed by excessive production of endogenous active substances: composed of ROS, reactive nitrogen species (RNS), and reactive sulfur substance (RSS), leading to hepatocyte damage and promoting the secretion of inflammatory cytokines (TNF-α, IL-6 and IL-10) secretion and cell death. The imbalance of inflammatory factors in the body participates in the second blow of NAFLD and promotes the process of NAFLD. The serum levels of TNF-α, IL-6, IL-10 and high-sensitivity C-reactive protein were significantly increased in patients with clinical NAFLD. NAFLD is closely related to oxidative stress. Disorders of lipid metabolism leads to lipid accumulation in the liver, which affects different ROS generators, including mitochondria and endoplasmic reticulum. In NASH mice induced by high-fat and high fructose diet and HepG2 cells exposure to palmitic acid, the decreasing of nuclear translocation of NRF2 inhibited the expression of downstream antioxidant genes and increased intracellular ROS, which lead to hepatocyte damage. Increased intracellular ROS resulted in down-regulation of mitochondrial functional genes mitochondrial power-related protein (dynamin-likeprotein1, Drp1), mitochondrial transcription factor A (TFAM), PGC-1α and NRF1 expression, which caused mitochondrial damage and exacerbated hepatocyte oxidative damage. Carbon monoxide releasing molecule A1 can regulate this process8. Catalase (CAT) has the strongest antioxidant capacity in liver cells compared with other tissue cells, indicating that CAT is the main means of clearing hydrogen peroxide in liver cells9. Hydrogen peroxide can freely diffuse within the organism, causing a decrease in ATP, glutathione, NADPH levels, and damaging mitochondrial DNA. Endogenous CAT plays a crucial role in improving lipid accumulation in hepatocytes caused by a high-fat diet10. Research on oxidative stress damage and antioxidants may provide new ideas and methods for the treatment of NAFLD. Sirtuin (SIRT1) is an important member of the Sirtuin family of class I protein deacetylases, playing a key role in metabolic regulation, oxidative stress, and physiological and pathological processes related to aging. SIRT1 also participates in the regulation of reactive oxygen species in vivo. Therefore, regulating SIRT1 levels may become a strategy for combating the generation of reactive oxygen species, as well as a new target for treating various pathological diseases11.
In the late 1990s, Abe and Kimura’s pioneering work demonstrated the ability of hydrogen sulfide (H2S) to act as a neuromodulator and vasodilator, confirming that H2S was the third physiological signaling gas after nitric oxide (NO) and carbon monoxide (CO)12. The liver is a key organ for producing and clearing H2S. The critical factors of NAFLD include lipid metabolism dysfunction, endoplasmic reticulum stress, oxidative stress, insulin resistance, and inflammation, all of which seem to be closely related to the H2S system in the liver. Exogenous hydrogen sulfide was used as a hydrogen sulfide donor for in experiments to explore its pharmacological mechanism. Our previous research showed that exogenous H2S relied on SIRT1 to alleviate liver endoplasmic reticulum stress thereby alleviating hepatocyte fat deposition13. Considering that endoplasmic reticulum stress is also a type of inflammatory response, there is mutual interference between it and oxidative stress. Although studies have shown exogenous hydrogen sulfide can alleviate oxidative stress in fatty liver, its mechanism is not clear. Therefore, based on previous research, this paper focuses on exploring the effects of exogenous H2S on oxidative stress and inflammation induced by lipid mixture in HepG2 cells and further explores its specific molecular mechanisms involved.
Results
GYY4137 reduced oxidative stress and inflammation levels in LM-induced HepG2 cells
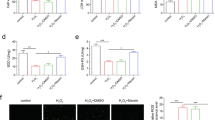
HepG2 cells were stimulated by 10%LM and treated with or without GYY4137(200 µM) for 24 h. Afterwards, the cells were collected to detect the levels of cellular oxidative stress and inflammation related indicators. We used GYY4137 which was described in detail in our previous studies as an exogenous H2S donor for intervention13. The results showed the level of MDA significantly increased in HepG2 cells exposure to LM, along with the decline of SOD. While the intervention of GYY4137 lowered the MDA level and elevated SOD content (Fig. 1A). In addition, GYY4137 reduced levels of inflammatory factors (TNF-α, IL-6) in the LM group (Fig. 1B). Analysis of ROS detection indicated ROS had a remarkable increase in the LM group, which was alleviated by GYY4137 (Fig. 1C).
GYY4137 decreased oxidative stress, inflammatory cytokine levels and ROS in LM-induced HepG2 cells. (A) The levels of MDA and SOD in each group. (B) TNF-α and IL-6 levels in each group. (C) Detection of ROS content by flow cytometry. Data were presented as mean ± SEM. **P < 0.01. LM, lipid mixture; GYY (200), GYY4137 (200 µM).
GYY4137 increased the expression of antioxidant related proteins in LM group cells
CAT, SOD and GSH can clear lipid hydroperoxides. In order to clarify the changes in antioxidant related indicators in this segment, we used western blot to detect the proteins expression of CAT, SOD and GSH. The results showed CAT, SOD, and GSH proteins were remarkably reduced in the LM group. After GYY4137 intervention, these indicators all had a significant recovery (Fig. 2A, B and C). This indicated GYY4137 not only reduced oxidative stress and inflammatory response induced by LM, but also enhanced the antioxidant capacity in HepG2 cells under LM stimulation.
GYY4137 elevated the expression of antioxidant related proteins in LM group cells. (A) Western blot analysis of CAT, with α-Tubulin used as a loading control. (B) Western blot analysis of SOD, and α-Tubulin used as a loading control. (C) Western blot analysis of GSH, with α-Tubulin used as a loading control. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01. LM, lipid mixture; GYY (200), GYY4137 (200 µM).
GYY4137 inhibited LM induced degradation of SIRT1 protein in HepG2 cells through the proteasome pathway
Our previous study indicated GYY4137 significantly increased the expression of SIRT1 protein in HepG2 cells exposure to LM13. Next, we first examined whether GYY4137 regulated SIRT1 through protein synthesis or degradation layers. We added the protein synthesis inhibitor cycloheximide (CHX, 2 µg/mL) to HepG2 cells in the LM group and LM + GYY4137 group dealt with 0, 24, 48, 72 and 96 h, respectively. Cells were collected at various time points for western blot. We found the degradation rate of SIRT1 protein in the LM + GYY4137 group was slower than that in the LM group (Fig. 3A). We further explored which pathway GYY4137 inhibited the degradation of SIRT1 in the LM group. In the above-mentioned CHX-added cells, the autophagy inhibitor chloroquine (10 µM) and the proteasome inhibitor MG132 (1 µM) were respectively added to intervene the cells for 24 h. The results showed the existence of MG132 slowed down the degradation rate of SIRT1 protein in LM and LM + GYY4137 group cells (Fig. 3B). In addition, we detected the level of SIRT1 ubiquitination in LM and LM + GYY4137 groups. The IP result showed GYY4137 reduced the ubiquitination level of SIRT1 in LM group cells, stabilized the protein, and correspondingly increased the expression of SIRT1 protein (Fig. 3C).
GYY4137 inhibited LM induced degradation of SIRT1 protein in HepG2 cells through the proteasome pathway (A) The protein expression of SIRT1 after adding CHX, with α-Tubulin used as a loading control. (B) After inhibition of protein synthesis followed by inhibition of autophagy lysosomal protein degradation pathway and ubiquitin proteasome protein degradation pathway, the performance of SIRT1 protein degradation, with α-Tubulin used as a loading control. (C) The SIRT1 ubiquitination level. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01. LM, lipid mixture; GYY (200), GYY4137 (200 µM); CHX, cycloheximide; Cq, chloroquine.
GYY4137 suppressed degradation of SIRT1 protein under LM exposure through USP22
Existing researches have reported USP7 and USP22 proteins could interact with SIRT1 protein, thereby reducing its ubiquitination level and stabilizing its expression. Therefore, we tested the mRNA contents and protein levels of USP22 and USP7. The results showed there were no significant difference in the expression of USP7 between groups. Fortunately, we found the mRNA and protein expression of USP22 in LM group was decline. And the intervention of GYY4137 could increase the protein expression and levels of mRNA of USP22 in the LM group (Fig. 4A, B). Therefore, we speculated GYY4137 may stabilize the expression of SIRT1 by increasing USP22. When USP22 was knocked down, we found GYY4137 did not reduce the ubiquitination of SIRT1 in the LM group. This further supported GYY4137 to stabilize the expression of SIRT1 protein through USP22, in order to reduce the ubiquitination level and degradation of SIRT1 (Fig. 4C).
GYY4137 inhibited degradation of SIRT1 protein under LM exposure through USP22 (A) Western blot analysis of USP7 and USP22, with α-Tubulin used as a loading control. (B) Relative mRNA levels of USP22 and USP7 by qRT-PCR. (C) Ubiquitination level of SIRT1, and western blot analysis of SIRT1 and USP22, with α-Tubulin used as a loading control. Data were presented as mean ± SEM. **P < 0.01, ns: no statistical difference. LM, lipid mixture; GYY (200), GYY4137 (200µM); NC, negative control; siRNA, small interfering RNA.
GYY4137 relied on USP22 to regulate SIRT1 to alleviate LM induced oxidative damage in HepG2 cells
Knockdown of USP22 was conducted by transfecting siRNA into HepG2 cells. After knocking down USP22, ORO staining was detected, as well as the levels of oxidative stress and inflammation in each group of cells were retested. Firstly, the ORO staining results showed the effect of GYY4137 on lipid droplet deposition in the LM group was greatly weakened when USP22 was knocked down (Fig. 5A). Simultaneously, it was observed that the improvement effects of GYY4137 on oxidative stress and inflammation in LM-induced HepG2 cells were also diminished when USP22 was knocked down (Fig. 5B and C). Only difference was observed in declining MDA levels on cells exposure to LM (Fig. 5B). And yet the function of GYY4137 on reducing ROS content and inflammatory factors (TNF-α, IL-6) in LM-induced HepG2 cells was all intercepted, as well as disappearance of the effect on raising SOD level (Fig. 5B, C and D). In addition, the abilities of GYY4137 on increasing the proteins expression of CAT, SOD and GSH in LM group had also vanished (Fig. 5E). These results further indicated GYY4137 improved oxidative stress and inflammatory response in LM-induced HepG2 cells by depending on USP22 to regulate SIRT1.
GYY4137 depended on USP22 to regulate SIRT1 to alleviate LM induced oxidative damage in HepG2 cells (A) Lipid droplets were visualized by ORO staining (25 μm). (B) The levels of MDA and SOD. (C) The TNF-α and IL-6 levels. (D) Detection of ROS content by flow cytometry. (E) Western blot analysis of CAT, SOD and GSH, with α-Tubulin used as a loading control. Data were presented as mean ± SEM. #P < 0.05, **P < 0.01, ns: no statistical difference. LM, lipid mixture; GYY (200), GYY4137 (200 µM); NC, negative control; siRNA, small interfering RNA.
Discussion
This study investigated the effects of exogenous H2S on oxidative stress and inflammatory response in HepG2 cells induced by LM. Our results indicated exogenous H2S reduced SIRT1 ubiquitination levels through USP22, thereby increasing SIRT1 protein expression and inhibiting LM-induced oxidative stress and inflammatory response in HepG2 cells, which provided new ideas for the treatment of NAFLD.
The most prominent factors in the pathogenesis of hepatic steatosis are insulin resistance, low-grade chronic inflammation and atherosclerosis with abnormal blood lipid14,15,16. Increased oxidative stress is a physiological and pathological characteristic of hepatic steatosis. Actually, oxidative stress increases in many chronic diseases, such as metabolic syndrome, atherosclerosis, hypercholesterolemia, obesity, peripheral artery disease and obstructive sleep apnea syndrome. Similarly, oxidative stress plays an important role in the initiation and progression of NAFLD. ROS generation in NAFLD patients leads to lipid peroxidation, which in turn results in inflammation and activation of hepatic stellate cells, causing liver fibrosis17. ROS and inflammation induced oxidative stress may be important mechanisms leading to liver cell death and tissue damage18. In fact, it is often found NAFLD patients have elevated levels of markers of systemic oxidative stress and lipid peroxidation in many clinical studies19,20,21. Our research showed oxidative stress related indicators and inflammatory factors in HepG2 cells induced by LM were significantly increased, while the intervention of exogenous H2S could improve this malignant change. CAT is a cytoplasmic antioxidant enzyme that protects cells from the toxic effects of H2O2 by catalyzing the decomposition of H2O2 into O2 and H2O22. As one of the most important antioxidants, SOD can convert anionic superoxide (O2 •) into hydrogen peroxide (H2O2) and O223. Glutathione (GSH) is a key non-enzymatic antioxidant participating in free radical scavenging24. Multiple studies have shown that levels of antioxidants such as SOD, CAT, and GSH are reduced in NAFLD25,26,27,28,29,30. Therefore, the decrease in antioxidant levels may be the main cause of NAFLD progression and further liver damage. Antioxidants can improve the status of antioxidants, reduce fat production and oxidative stress, and have preventive and therapeutic effects on liver steatosis25. Our results suggested the expression of CAT, SOD and GSH proteins in LM group cells significantly decreased. Fortunately, after treatment with GYY4137, these antioxidant indicators all increased. This illustrated not only did GYY4137 reduce oxidative stress levels, but GYY4137 also increased antioxidant related indicators to improve cellular adverse changes under LM stimulation. However, the specific molecular mechanism by which exogenous H2S ameliorated oxidative stress in LM-induced HepG2 cells is still unclear.
Our previous research13 has shown exogenous H2S alleviated endoplasmic reticulum stress in NAFLD and improved liver steatosis by increasing the expression of SIRT1 protein. However, it is currently unclear how exogenous H2S regulates the SIRT1 protein. Firstly, our study found exogenous H2S regulated the degradation of SIRT1 protein by inhibiting its degradation through the proteasome pathway. Moreover, recent studies have reported that SIRT1 was regulated by ubiquitin specific proteases (USP), where USP7 and USP22 could interact with SIRT1 to deubiquitinate and stabilize its expression31,32. Therefore, we detected the protein expression of USP7 and USP22 in each group of cells through western blot. Fortunately, we detected exogenous H2S increased the protein expression of USP22 in LM group cells. This indicated exogenous H2S could inhibit the ubiquitination degradation of SIRT1 through USP22, thereby stabilizing the expression of SIRT1 protein. Further experiments after knocking down USP22 showed exogenous H2S relied on USP22 to regulate the SIRT1 protein. There is currently limited research on USP22 in NAFLD, and its specific roles still require further exploration. In addition, the deficiency of our study is that we only conducted in vitro experiments. And we will validate it in vivo in the future.
Overall, our research findings suggested exogenous H2S alleviated LM induced oxidative stress and inflammatory response in HepG2 cells, specifically regulated by the USP22/SIRT1 axis. Exogenous H2S inhibited the ubiquitination degradation of SIRT1 through a proteasome pathway dependent on USP22, stabilizes the expression of SIRT1, and thus exerted its biological functions.
Materials and methods
Materials
Lipid mixture (LM) which contained non-animal derived fatty acids (2 µg/mL arachidonic acid, with a concentration of 10 µg/mL of linoleic acid, linolenic acid, myristic acid, oleic acid, palmitic acid, and stearic acid, 0.22 mg/ml cholesterol from New Zealand wool, 2.2 mg/mL Tween 80, 70 µg/mL tocopherol acetate and 100 mg/mL Pluronic F-68, was purchased from Sigma-Aldrich (St. Louis, MO). Oil Red O staining kit and ROS detection kit were purchased from Beyotime Institute Biotechnology (Shanghai, China). Rabbit anti-α-Tubulin primary antibody (Cat#11224-1-AP, RRID: AB_2210206), rabbit anti-SIRT1 primary antibody (Cat#13161-1-AP, RRID: AB_10646436), rabbit anti-CAT primary antibody (Cat# 21260-1-AP, RRID: AB_10733099), rabbit anti-SOD primary antibody (Cat# 10269-1-AP, RRID: AB_2193750) and rabbit anti-GSH primary antibody (Cat#15712-1-AP, RRID: AB_2878171) were purchased from Proteintech (Wuhan, China). Rabbit anti-Ub Antibody (Cat#YT5498, RRID: AB_2864643) was purchased from Immunoway (Suzhou, China). Mouse anti-USP22 primary antibody (Cat# 3813-1, RRID: AB_10896543) mouse anti-USP7 primary antibody (Cat# 3693-1, RRID: AB_10933054), GYY4137 and Protein A/G PLUS Agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The MDA (malonaldehyde) and SOD (superoxide dismutase) detection kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). ELISA kits of TNF-α and IL-6 were purchased from Jingmei Biotechnology (Jiangsu, China). Other reagents and consumables were purchased from local reagent vendors.
Cell culture and treatments
HepG2 cell was purchased from the cell collection of Chinese Academy of Sciences (RRID: CVCL_C5RP). The culture medium system was DMEM containing 10% fetal bovine serum, 100 U/mL penicillin, 1 mM sodium pyruvate, and 100 µg/mL streptomycin. The cells’ culture conditions were at 37 °C under 5%CO2. As mentioned earlier13NAFLD model in vitro was established using 10% lipid mixture (LM) for 24 h. Cells needed to starve for 4 h before being added with LM or/and GYY4137(200 µM).
MDA and SOD detection
The cell supernatants were collected at the completion time of cell processing. The MDA and SOD levels were tested according to the instructions of the respective reagent kits. Finally, microplate reader was used to measure the absorbance value of MDA at 532 nm and the absorbance value of SOD at 550 nm, respectively.
Detection of ROS by flow cytometry
ROS detection was used by ROS Assay Kit in accordance with instructions. The cells were collected for detection using a flow cytometer. The fluorescence intensity of ROS was analyzed by FlowJo software.
ELISA
The cells were collected after digestion and wash them 3 times with pre cooled PBS. An appropriate amount of pre cooled PBS containing protease inhibitors was used to resuspend cells. Subsequently, the cell samples were repeatedly freeze-thawed at -20 ℃ or -80 ℃ and room temperature, repeating the freeze-thaw process several times until the cells reached a state of complete lysis. Centrifuge at 1500 rpm for 10 ~ 20 min under 4 ℃ conditions, and collect the supernatant for testing according to the instructions of the ELISA kits. Finally, microplate reader at 450 nm was used to measure the absorbance on which the standard curves in excel were based.
RNA extraction and quantitative real-time PCR (qRT-PCR)
The qRT PCR steps were as described in the previous article13. The primers used in this study are shown in Table 1.
Western blot
The protein lysis solution was used to extract cell protein samples. Proteins were separated using 10% SDS-PAGE and then transferred onto the membrane. The membrane was blocked in 5% skim milk for 1 h, then cut the membrane according to the range of target molecular weight. The corresponding primary antibodies were added to the trimmed membranes overnight at 4 ℃. The next day, the membranes were washed 3 times by TBST, followed by adding to the corresponding species of secondary antibody on the membranes for 1 h at room temperature. Thereafter, the membranes were washed 3 times with TBST again, followed by chemiluminescence. The results were analyzed by Image J software.
Immunoprecipitation
Immunoprecipitation was used to detect the ubiquitination level of SIRT1. An appropriate amount of SIRT1 specific antibody was added to the total protein of cells and mixed continuously at 4 ℃ for 1 h. Subsequently, Protein A/G PLUS Agarose was added to the above mixture overnight at 4 ℃. The next day, the precipitate was collected through centrifugation and washing. The obtained precipitate was resuspended using electrophoresis buffer. After boiling for 2 ~ 3 min, the sample can be used for protein blotting as described above. Incubate the transferred membrane with ubiquitinated antibody.
Cell transfection assay
The small interfering RNA (siRNA) to USP22 designed by Genechem (Shanghai, China) was used to knock down the mRNA and protein expression levels of USP22. Cell transfection assay was executed in conformity to the manuals of Lipo8000™ transfection reagent. The sequences of siRNA against USP22 have been listed in Table 2.
Oil red O (ORO) staining
After cell intervention, the ORO staining was performed according to the instructions of the ORO staining kit.
Statistics
Statistical analysis and plotting were conducted by SPSS 25.0 and Graph Prism 8.0 software, respectively. Data were expressed as mean ± SEM. ANOVA method was used to compare the differences between multiple groups of data. P < 0.05 was considered significant. All experiments should be repeated at least 3 times or more.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the restrictions of the laboratory confidentiality agreement, but are available from the corresponding author on reasonable request.
References
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. https://doi.org/10.1002/hep.28431 (2016).
Teng, M. L. et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 29 (Suppl), S32–S42. https://doi.org/10.3350/cmh.2022.0365 (2023). Epub 2022 Dec 14. PMID: 36517002; PMCID: PMC10029957.
Rosado-Pérez, J. et al. The biological significance of oxidative stress, effects of fruits as natural edible antioxidants. Curr. Pharm. Design. 24 (40), 4807–4824. https://doi.org/10.2174/1381612824666190114164758 (2018).
Liguori, I. et al. Oxidative stress, aging, and diseases. Clin. Interv Aging. 13, 757–772. https://doi.org/10.2147/CIA.S158513 (2018).
Yu, H. F. et al. HB-EGF Ameliorates Oxidative Stress-Mediated Uterine Decidualization Damage. Oxid Med Cell Longev. ; 2019:6170936. (2019). https://doi.org/10.1155/2019/6170936
Colagar, A. H. & Marzony, E. T. Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. J. Clin. Biochem. Nutr. 45 (2), 144–149. https://doi.org/10.3164/jcbn.08-251 (2009).
Yen, C. Y. et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 15 (1), 94. https://doi.org/10.1186/s12906-015-0621-8 (2015).
Upadhyay, K. K. et al. Carbon monoxide releasing molecule-A1 improves nonalcoholic steatohepatitis via Nrf2 activation mediated improvement in oxidative stress and mitochondrial function[J]. Redox Biol. 28, 101314. https://doi.org/10.1016/j.redox.2019.101314 (2020).
Shin, D. K. Catalase and nonalcoholic fatty liver disease[J]. Pfluegers Archiv: Eur. J. Physiol. 470 (12). https://doi.org/10.1007/s00424-018-2195-z (2018).
Shin, S. K. et al. Ablation of catalase promotes nonalcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflügers Archiv-European J. Physiol. 471 (6), 829–843. https://doi.org/10.1007/s00424-018-02250-3 (2019).
Ubaid, S. et al. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation 43 (5), 1589–1598. https://doi.org/10.1007/s10753-020-01242-9 (2020).
Abe, K. & Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16 (3), 1066–1071. https://doi.org/10.1523/JNEUROSCI.16-03-01066.1996 (1996).
Cui, X. M. et al. Exogenous hydrogen sulfide alleviates hepatic Endoplasmic reticulum stress via SIRT1/FoxO1/PCSK9 pathway in NAFLD. FASEB J. 37, e23027. https://doi.org/10.1096/fj.202201705RR (2023).
Sookoian, S. et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 209 (2), 585–591. https://doi.org/10.1016/j.atherosclerosis (2010).
Nseir, W. et al. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig. Dis. Sci. 56 (12), 3439–3449. https://doi.org/10.1007/s10620-011-1767-y (2011).
Mangge, H. et al. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. (6), 16. https://doi.org/10.4330/wjc.v6.i6.462 (2014).
Povero, D. & Feldstein, A. E. Novel molecular mechanisms in the development of Non-Alcoholic steatohepatitis. Diabetes Metabolism J. 40 (1), 1–11. https://doi.org/10.4093/dmj.2016.40.1.1 (2016).
Farzanegi, P. et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur. J. Sport Sci. 19 (7), 994–1003. https://doi.org/10.1080/17461391.2019.1571114 (2019).
Pirgon, O. et al. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J. Clin. Res. Pediatr. Endocrinol. 5 (1), 33–39. https://doi.org/10.4274/Jcrpe.825 (2013).
Gaens, K. H. et al. Endogenous formation of N-epsilon-(carboxymethyl) lysineis increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J. Hepatol. 56 (3), 647–655. https://doi.org/10.1016/j.jhep.2011.07.028 (2012).
Del Ben, M. et al. Serum cytokeratin-18 is associated with NOX2-Generated oxidative stress in patients with nonalcoholic fatty liver. Int. J. Hepatol. 784–985. https://doi.org/10.1155/2014/784985 (2014).
Ankita, N. Role of catalase in oxidative Stress- and Age-Associated degenerative diseases. Oxidative Med. Cell. Longev. 9613090. https://doi.org/10.1155/2019/9613090 (2019).
Fukai, T. & Ushio-Fukai, M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox. Signal. 15 (6), 1583. https://doi.org/10.1089/ars.2011.3999 (2011).
Robaczewska, J. et al. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J. Physiol. Pharmacol. 67 (3), 331–337 (2016).
Hajighasem, A., Farzanegi, P. & Mazaheri, Z. Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 125 (2), 142–149. https://doi.org/10.1080/13813455.2018.1441872 (2018).
Irie, M. et al. Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease. Euroasian J. Hepatogastroenterology. 6 (1), 13–18. https://doi.org/10.5005/jp-journals-10018-1159 (2016).
Kumar, A. et al. Patients with nonalcoholic fatty liver disease (NAFLD) have higher oxidative stress in comparison to chronic viral hepatitis. J. Clin. Experimental Hepatol. 3 (1), 12–18. https://doi.org/10.1016/j.jceh.2012.10.009 (2013).
Ding, C. et al. New insights into Salvianolic acid A action: regulation of the TXNIP/NLRP3 and txnip/chrebp pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 6, 28734. https://doi.org/10.1038/srep28734 (2016).
Krautbauer, S. et al. Manganese superoxide dismutase is reduced in the liver of male but not female humans and rodents with non-alcoholic fatty liver disease. Experimental Mol. Pathol. 95 (3), 330–335. https://doi.org/10.1016/j.yexmp.2013.10.003 (2013).
El-Din, S. H. S. et al. Pharmacological and antioxidant actions of Garlic and. Or onion in non-alcoholic fatty liver disease (NAFLD) in rats. J. Egypt. Soc. Parasitol. 44 (2), 295–308. https://doi.org/10.12816/0006468 (2014).
Song, N. et al. USP7 deubiquitinates and stabilizes SIRT1. Anat. Rec (Hoboken). 303 (5), 1337–1345. https://doi.org/10.1002/ar.24252 (2020).
Lin, Z. et al. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol. Cell. 46 (4), 484–494. https://doi.org/10.1016/j.molcel.2012.03.024 (2012).
Acknowledgements
We appreciate the experimental platform provided by the Translational Medicine Center of the First Affiliated Hospital of Xi’an Jiaotong University. We also thank the members of the Gastroenterology Research Group at the First Affiliated Hospital of Xi’an Jiaotong University for their efforts.
Funding
This work was supported by the Key Research and Development Program of Shaanxi (No. 2021ZDLSF02-06), the National Natural Science Foundation of China (No.82303169).
Author information
Authors and Affiliations
Contributions
Xiaomeng Cui and Shuixiang He conceived and designed the study. Chengjun Li assisted the cell experiments. Xiaomeng Cui and Yarui Li performed the experiments and analyzed data. Yarui Li and Shuixiang He revised the manuscript written by Xiaomeng Cui. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cui, X., Li, C., He, S. et al. Exogenous H2S reduces oxidative stress induced by lipid mixture in HepG2 cells through USP22/SIRT1 axis. Sci Rep 15, 23129 (2025). https://doi.org/10.1038/s41598-025-04924-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04924-2