Abstract
This study aimed to investigate the associations between the triglyceride glucose index (TyG-BRI) and cardiovascular disease (CVD) among middle-aged and elderly individuals. In the REACTION study, a baseline survey was conducted in 2011, which involved the collection of triglyceride levels, fasting blood glucose, height, waist circumference, and other relevant data, from which the TyG-BRI was subsequently calculated. A total of 8,935 participants who met the inclusion criteria were followed up until the conclusion of the study in 2021, during which pertinent data regarding mortality and CVD events were gathered. The study subjects were categorized into quartiles on the basis of TyG-BRI score. To analyze the impact of the TyG-BRI on all-cause mortality and CVD events, a multifactorial Cox proportional hazards model was employed. Additionally, receiver operating characteristic (ROC) curves were generated to assess the ability of the TyG index, BRI, and TyG-BRI to predict cardiovascular events. Among the 7532 participants who completed the follow-up, 474 experienced a CVD event during this period. After multifactor adjustment, a nonlinear correlation was observed between the TyG index, TyG-BRI, and CVD events. When the first quartile (Q1) was used as a reference, the hazard ratios (HRs) with 95% confidence intervals (CIs) for CVD events in the fourth quartile (Q4) were 1.997 (1.361–2.928) and 2.130 (1.535–2.957), respectively. Additionally, in the receiver operating characteristic (ROC) curve analysis, the areas under the curve (AUCs) for the TyG index, BRI, and TyG-BRI concerning the risk of CVD events were 0.614 (0.588, 0.640), 0.609 (0.584, 0.633), and 0.626 (0.601, 0.651), respectively. In middle-aged and elderly individuals, both the TyG index and the TyG-BRI serve as independent risk factors for cardiovascular disease. Notably, the TyG-BRI has a stronger ability to predict the risk of cardiovascular events than does the TyG index.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs) have emerged as significant contributors to mortality and disability among middle-aged and elderly people globally, imposing a substantial burden on public health1. According to statistics from the World Health Organization, CVD is responsible for approximately 17.9 million deaths annually, which represents approximately 32% of total global mortality2. The prevalence of CVD increases markedly with age, particularly among middle-aged and elderly individuals. In China, both the incidence and mortality rates of cardiovascular diseases have increased in recent years, accounting for 47% of all deaths3. Therefore, conducting in-depth research on the pathogenesis and effective predictors of CVD holds critical clinical importance for early prevention, timely diagnosis, and intervention.
In recent years, the triglyceride-glucose (TyG) index has emerged as a significant indicator of insulin resistance (IR) and has garnered increasing attention in cardiovascular disease research4,5,6. IR influences the metabolic processes of blood sugar and lipids through various mechanisms, thereby facilitating the formation and progression of atherosclerosis and substantially increasing the risk of cardiovascular disease6. Numerous studies have demonstrated strong associations between the TyG index and the onset, progression, and prognosis of cardiovascular diseases. Generally, elevated TyG index values are indicative of a heightened risk of cardiovascular disease7,8,9,10. Nevertheless, the TyG index has inherent limitations as a standalone predictor of cardiovascular disease (CVD). Firstly, it primarily reflects insulin resistance and dyslipidemia but does not account for central adiposity, which is a key driver of CVD risk through mechanisms such as chronic inflammation and adipokine dysregulation11,12,13,14. Additionally, the pathogenesis of CVD involves multifaceted pathways, such as endothelial dysfunction and oxidative stress, which are not fully captured by glucose-lipid metabolism alone15,16,17. These limitations may account for its variable predictive performance across populations with differing metabolic profiles4.
Studies have clearly demonstrated a strong relationship between abdominal obesity and cardiovascular disease11,12. Central obesity contributes to the development of cardiovascular diseases through multiple mechanisms, including the secretion of various adipokines, the induction of chronic inflammatory responses, and the impairment of vascular endothelial function13,14. The body roundness index (BRI), an obesity assessment tool, offers a more precise measurement of central obesity and effectively reflects the accumulation of abdominal fat18. Several studies have revealed a significant positive correlation between the BRI and the risk of cardiovascular disease. Compared with traditional obesity indicators such as body mass index (BMI), the BRI has exhibited greater accuracy and reliability in assessing cardiovascular disease risk19,20.
The TyG-BRI integrates the strengths of the TyG index and BRI, offering a more comprehensive reflection of an individual’s metabolic status and level of obesity. It also shows promise as an effective predictor of cardiovascular disease risk. Currently, research exploring the relationship between the TyG-BRI and CVD is still in its early stages, particularly long-term follow-up studies. Consequently, a ten-year follow-up study was conducted to examine the association between the TyG-BRI and cardiovascular disease in middle-aged and elderly populations. This research aims to enhance our understanding of the intrinsic relationship between these two factors and to clarify the role of the TyG-BRI in predicting cardiovascular disease risk. The findings are expected to be significant, providing a new theoretical basis and practical guidance for the clinical prevention and treatment of cardiovascular diseases.
Methods
Research subjects
The data for this study were collected from the Guizhou region as part of the “Risk Evaluation of Cancers in Chinese Diabetic Individuals: A Longitudinal (REACTION) Study”. This study received approval from the Ethics Committee of the Affiliated Hospital of Guizhou Medical University, in accordance with the Declaration of Helsinki (Approval Number: Clen Review [2011(51)], 2021 Clinical Review Number: 280). All participants provided written informed consent prior to data collection. Additional supporting data is provided in the Supplementary Information file submitted with this manuscript, which is available from the corresponding author upon reasonable request.
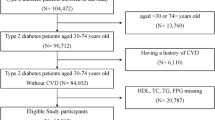
The research subjects comprised middle-aged and elderly residents of Yunyan District, Guiyang City, with specific inclusion criteria requiring participants to be permanent residents aged 40 years or older. Baseline data were collected through physical examinations, laboratory tests, and standardized questionnaires. Individuals diagnosed with cardiovascular disease at baseline, those with incomplete baseline data, and those who had regularly used antidiabetic or lipid-lowering medications in the month preceding the study were excluded. Ultimately, the study followed up with 8,935 eligible participants over a ten-year period, yielding an average follow-up duration of 9.30 ± 1.83 years and a follow-up rate of 84.30%. Analyses were conducted on 7,532 participants who completed the follow-up, among whom 474 experienced a cardiovascular disease event during the follow-up period (Fig. 1).
Data collection
Baseline data collection
Experienced medical staff conducted a standardized survey of the research subjects via a uniform questionnaire to collect basic demographic information systematically from all participants, including age, sex, marital status, and education level. Additionally, detailed inquiries were made regarding lifestyle-related factors, such as smoking and drinking habits. Concurrently, the participants’ past medical histories and treatments for chronic conditions, including hypertension, diabetes, cardiovascular disease, and malignant tumors, were documented. To minimize the potential for errors during the data entry process, the questionnaire results were double-entered and meticulously reviewed.
Demographic indicators
The physical parameters of the research subjects were measured by professionally trained medical staff via standardized measurement tools. Specific indicators included height, weight, and waist circumference. Height and weight were measured via Jianmin brand measuring instruments, with a measurement accuracy of 0.1 cm for height and 0.1 kg for weight. The waist circumference was measured at the midpoint between the lower edge of the ribs and the iliac crest, with the accuracy maintained at 0.1 cm. All the measurements were conducted twice, and the final results for each parameter were averaged from these two measurements to ensure accuracy.
Laboratory indicators
Laboratory indicators were collected from study subjects who had fasted for more than 10 h, with blood samples drawn in a quiet resting state the following morning. Venous blood was tested for glucose levels via the hexokinase method via an Abbott fully automated immunoassay analyzer (Architect cil 6200, USA). For lipid measurements, fasting venous blood collected in the early morning was centrifuged and stored at -70 °C. The samples were then sent to the Shanghai Institute of Endocrinology, where high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured via the homogeneous method on the same Abbott analyzer. Triglycerides (TGs) and total cholesterol (TC) were assessed via the glycerol phosphate oxidase method and the enzyme reagent method, respectively. Additionally, the level of serum creatinine (Cr) was determined via the kinetic alkaline picric acid method, whereas the level of glycated hemoglobin (HbA1c) was measured via ion-exchange high-pressure liquid chromatography.
Definitions and standards
Cardiovascular disease
In this study, cardiovascular disease (CVD) was defined as myocardial infarction (MI), coronary heart disease (CHD), stroke, heart failure (HF), or any death resulting from cardiovascular disease21. CVD deaths encompass all CVD-related fatalities that occurred during the follow-up period. The time to onset of CVD was determined by the self-reported date of first diagnosis or the date of death due to CVD. According to the ICD-10, the classification codes for cardiovascular death, ischemic heart disease, acute myocardial infarction, and stroke are 100–199, 120–125, 121, and 160–169, respectively. Diagnoses are based on the patient’s disease certificate, electrocardiogram, coronary angiography, and auxiliary examination results, such as computed tomography (CT) and magnetic resonance imaging (MRI), obtained during hospitalization. In the follow-up data collected after 10 years, cardiovascular and cerebrovascular events were defined as the first identified cardiovascular and cerebrovascular events, along with deaths resulting from the first cardiovascular event. Throughout the follow-up process, cases of death and death certificates, along with other pertinent information, were verified by public security authorities through examination of the death certificate or death registration. The final review and confirmation were conducted by two chief physicians from the Department of Endocrinology, both of whom possess extensive clinical experience and adhere to unified clinical diagnostic standards.
Indicator calculation formula22,23.
Statistical analysis
The baseline TyG-BRI measurement results were categorized into quartiles, with participants divided into four groups on the basis of these results to compare the baseline characteristics across groups. Normally distributed measurement data are expressed as the mean ± standard deviation (x̄ ± s), and group comparisons were conducted via one-way ANOVA. For nonnormally distributed measurement data, results are presented as median (P50) with interquartile range (P25, P75), with comparisons between two groups performed via the rank sum test. Categorical measurement data are reported as the number of cases (percentages) n (%), with the χ2 test employed for group comparisons.
The Cox proportional hazards model was utilized to assess the impact of the TyG-BRI index on the risk of cardiovascular disease, incorporating the TyG-BRI index as the primary independent variable while accounting for potential confounding factors such as age, sex, smoking, drinking, hypertension, diabetes, and marital status. This approach allows for an accurate estimation of the relationship between the TyG-BRI and the incidence of cardiovascular disease. Independent associations between risk factors, along with hazard ratios (HRs) and 95% confidence intervals (CIs), were calculated. Furthermore, restricted cubic spline (RCS) regression models were employed to investigate potential linear or nonlinear associations between the TyG index, TyG-BRI, and cardiovascular disease events. To evaluate the stability of the relationship between the TyG-BRI score and cardiovascular disease across different populations, we analyzed this relationship according to sex, age (40–60 years vs. ≥ 60 years), hypertension, diabetes, smoking status, drinking status, and marital status (married or cohabiting vs. living alone). Interaction and subgroup analyses were performed. The ROC curve was used for diagnostic value analysis, with the area under the curve (AUC) calculated to quantify the predictive ability of the TyG, BRI, and TyG-BRI for cardiovascular disease.
All analyses were performed via Free Statistics software (version 2.0) and SPSS statistical software (version 25.0). A two-sided P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the study subjects
This study included 7,532 participants for analysis, with a mean age at baseline of 57.87 ± 7.82 years. Among the participants, 2,014 (26.74%) were male. The participants were categorized into four quartiles on the basis of the baseline TyG-BRI test results, and the differences in baseline characteristics among the four groups were assessed. The results indicated that, compared with those in the low-level group, participants in the high-level TyG-BRI group presented higher age, BMI, BRI, FBG, 2hPG, Cr, LDL-C, TC, HbA1c, and TyG. Conversely, HDL-C showed a downward trend, with the differences being statistically significant (p < 0.05) (Table 1 for details).
Associations between the tyg, TyG-BRI, and CVD risk
Figure 2 shows the RCS regression model examining the relationships between TyG, TyG-BRI, and CVD risk after adjusting for confounding factors. The findings indicate a nonlinear relationship between the TyG index and CVD risk, with a nonlinear p value of < 0.05. Subsequently, segmented linear regression analysis was conducted on the TyG and TyG-BRI. The results revealed that prior to a TyG value of 8.825, each unit increase in TyG was associated with a 38.7% increase in the risk of cardiovascular events; however, this effect was not statistically significant (p = 0.339). Beyond this threshold, each unit increase in TyG corresponded to a 61.0% increase in the risk of cardiovascular events, which was statistically significant (p = 0.014). With respect to the TyG-BRI, before reaching a value of 35.57, each unit increase in the TyG-BRI score was associated with a 6.2% increase in cardiovascular event risk, and this effect was significant (p = 0.001). Conversely, after surpassing 35.57, each unit increase in the TyG-BRI resulted in only a 0.3% increase in cardiovascular event risk, which was not statistically significant (p = 0.600) (Table 2).
The association between the TyG index and the TyG-BRI index with the risk of cardiovascular disease is illustrated. Solid and dashed lines represent the predicted values and their corresponding 95% confidence intervals (CIs). The restricted cubic spline model was adjusted for sex, age, hypertension, diabetes, smoking status, drinking status, marital status, education level, 2hPG, Cr, HDL-C, LDL-C, TC, HbA1c.
After adjusting for age, sex, marital status, education level, 2hPG, Cr, HDL-C, LDL-C, TC, and HbA1c, both high TyG and TyG-BRI indices were associated with an increased incidence of cardiovascular disease (CVD). The corresponding hazard ratios (HRs) were 1.740 (1.344–2.253) and 1.017 (1.009–1.025), respectively. This association remained significant when the TyG and TyG-BRI indices were categorized into quartiles. After adjusting for the same confounding factors, individuals in the high-level group (Q4) of both the TyG index and TyG-BRI index presented a greater risk of CVD than did those in the low-level group (Q1), with HRs of 1.997 (1.361–2.928) and 2.130 (1.535–2.957), respectively. These findings indicate that both the TyG index and the TyG-BRI index are independent risk factors for the occurrence of cardiovascular disease; specifically, higher index levels correlate with an increased relative risk of CVD among middle-aged and elderly individuals. These indices hold significant predictive value for assessing cardiovascular disease risk and can serve as a reference for the early clinical identification of high-risk groups (Table 3).
The ability of the TyG index, BRI, and TyG-BRI to predict the risk of CVD
Figure 3 shows the ROC curves for the TyG index, BRI, and TyG-BRI in relation to CVD event risk. The results indicate that the area under the curve (AUC) values for the TyG index, BRI, and TyG-BRI for predicting CVD event risk were 0.614 (0.588, 0.640), 0.609 (0.584, 0.633), and 0.626 (0.601, 0.651), respectively. These findings suggest that all three indices possess a certain predictive capability for CVD event risk, with the TyG-BRI demonstrating superior performance compared with both the TyG index and the BRI.
Subgroup analysis
Table 4 presents the results of the subgroup analysis based on various characteristics, including age, sex, hypertension status, diabetes status, smoking status, drinking status, and marital status. The findings indicate that the association between the TyG-BRI index and CVD incidence remained consistent across all subgroups, with no evidence of interaction.
Discussion
In this study, we aimed to combine the TyG index with the BRI to investigate the relationship between the TyG-BRI and CVD. These findings indicate that higher TyG-BRI scores are associated with increased rates of CVD events. Notably, the TyG-BRI proved to be more effective in estimating CVD risk than either the TyG or the BRI individually, and this association remained consistent across various subgroups.
The relationship between the TyG-BRI index and cardiovascular disease may involve biological mechanisms, including insulin resistance, abnormal lipid metabolism, inflammatory responses, and vascular endothelial dysfunction. These factors interact synergistically to promote the onset and progression of cardiovascular diseases.
Compared with the TyG index alone, which primarily reflects insulin resistance and lipid metabolism through fasting glucose and triglyceride levels, the TyG-BRI index integrates the body roundness index (BRI), a marker of central obesity. This integration provides a more comprehensive assessment of cardiometabolic risk. Insulin resistance causes cells to become less sensitive to insulin, thereby reducing glucose uptake and utilization, which results in elevated blood sugar levels23. Concurrently, insulin resistance can also trigger abnormal lipid metabolism in adipocytes, leading to an increase in triglyceride synthesis and excessive accumulation in tissues such as the liver. This process ultimately results in elevated serum triglyceride levels and an increase in the TyG index24. In related animal experiments, a mouse model of insulin resistance induced by a high-fat diet demonstrated that the insulin signaling pathway in the liver and adipose tissue was disrupted. This disruption was accompanied by a significant increase in blood glucose and triglyceride levels, with trends that were consistent with the TyG index25,26,27. However, these models often overlook the role of central obesity, which is directly captured by the BRI. When combined with BRI, the TyG-BRI index can simultaneously reflect both metabolic dysregulation and adipose tissue dysfunction, capturing a broader spectrum of cardiovascular risk factors.
As a significant indicator of central obesity, the body roundness index (BRI) is closely associated with the accumulation of abdominal fat28. Abdominal adipose tissue is highly metabolically active and can secrete a variety of adipokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which are linked to inflammation, as well as free fatty acids (FFAs)29,30. An increase in the concentration of FFAs in the bloodstream interferes with the balance of liver lipid synthesis and metabolism, promoting the production of triglycerides while inhibiting the production of high-density lipoprotein (HDL) and increasing its decomposition31. This alteration results in decreased HDL levels and a relative increase in low-density lipoprotein (LDL) levels, contributing to a lipid profile that facilitates the progression of atherosclerosis31. There is a significant association between abnormalities in lipid metabolism and an elevated risk of cardiovascular disease32, further confirming that the BRI plays a role in the development of cardiovascular disease by influencing lipid metabolism. By combining TyG and BRI, the TyG-BRI index synergizes the predictive power of both markers, reflecting the complex interplay between adiposity, insulin resistance, and lipid metabolism.
Both insulin resistance and abdominal obesity can induce a state of chronic low-grade inflammation in the body33,34. Inflammatory factors secreted by adipose tissue, such as TNF-α and IL-6, can activate associated inflammatory signaling pathways, promoting the migration and aggregation of monocytes into the subintima of blood vessels, where they eventually differentiate into macrophages34. These macrophages phagocytose oxidatively modified low-density lipoprotein (LDL) and form foam cells, thereby initiating the inflammatory process of atherosclerosis35. Furthermore, the inflammatory response can stimulate the proliferation and migration of vascular smooth muscle cells, which enhances the synthesis of the extracellular matrix and contributes to the thickening of the blood vessel wall. This process ultimately leads to a reduction in blood vessel elasticity, significantly increasing the risk of cardiovascular disease34.
Vascular endothelial dysfunction is recognized as a critical early event in the onset and progression of cardiovascular disease15. Insulin resistance and inflammatory responses contribute to damage to vascular endothelial cells through various mechanisms. These mechanisms include the induction of oxidative stress in endothelial cells, the increased production of reactive oxygen species (ROS), and the inhibition of nitric oxide (NO) synthesis and release16. These changes compromise the normal diastolic function of the vascular endothelium while enhancing blood vessel contractility, thereby promoting platelet aggregation and thrombus formation, which create favorable conditions for the development of atherosclerosis17. Furthermore, dyslipidemia can directly impact the normal function of vascular endothelial cells36. For example, the oxidative modification products of low-density lipoprotein (LDL-C) are taken up by endothelial cells, initiating a cascade of inflammatory reactions and cytotoxic effects that exacerbate endothelial damage37. Studies utilizing animal models have shown that in metabolic disorder models induced by high-sugar and high-fat diets, the activity of NO synthase in vascular endothelial cells is reduced, whereas ROS generation is increased, leading to significant impairment of endothelium-dependent diastolic function38.
In summary, the TyG-BRI plays a crucial role in the occurrence and progression of cardiovascular diseases by comprehensively reflecting the synergistic effects of multiple pathophysiological processes, including insulin resistance, abnormal lipid metabolism, inflammatory responses, and vascular endothelial dysfunction. By integrating multiple pathophysiological pathways into a single metric, the TyG-BRI index provides a more holistic representation of an individual’s cardiometabolic risk profile. The findings of this study provide a significant theoretical foundation for clinical practitioners to conduct early screening of high-risk groups for cardiovascular disease effectively among middle-aged and elderly individuals. Given the substantial positive correlation between the TyG-BRI index and cardiovascular disease risk and its capacity to comprehensively represent an individual’s metabolic status and degree of obesity, incorporating the TyG-BRI index into routine physical examination protocols for middle-aged and elderly individuals is recommended. This addition would facilitate a more comprehensive and accurate assessment of cardiovascular disease risk. For individuals with a high TyG-BRI index, a more detailed cardiovascular risk assessment is advised to identify potential risk factors for cardiovascular disease at an early stage.
This study employed a ten-year follow-up cohort design, enabling us to dynamically observe changes in the TyG-BRI index and the incidence and progression of cardiovascular diseases. This design more accurately illustrates the temporal relationships and causal connections between the two, thereby addressing the limitations associated with unclear causal directions in cross-sectional studies. Compared with other studies, our sample size is larger, allowing for a more precise evaluation of the strength of the association between the TyG-BRI index and cardiovascular disease while minimizing the impact of sampling error, thus enhancing the stability and reliability of the study results. However, despite various measures taken to ensure the integrity of the study data, some limitations remain. First, during the ten-year follow-up, some respondents were lost to follow-up. The missing information regarding the characteristics of these individuals and their health outcomes may affect the accuracy and representativeness of the final results. Furthermore, as a composite index, the specific mechanisms of action among the components of the TyG-BRI (such as triglycerides, fasting glucose, and body roundness index) have yet to be fully elucidated. Although we have discussed the relationship between the TyG-BRI index and cardiovascular disease, further investigations are needed to obtain a more comprehensive understanding.
Conclusion
In middle-aged and elderly populations, both the TyG index and the TyG-BRI serve as independent risk factors for cardiovascular disease. Among the various subgroups, the TyG-BRI index demonstrated notable stability. Furthermore, the TyG-BRI has shown superior predictive ability for cardiovascular event risk compared with the TyG index. Consequently, maintaining an optimal TyG index, implementing effective weight management strategies, and maintaining a reasonable waist circumference are crucial measures for the prevention of cardiovascular diseases.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Mensah, G. A., Fuster, V. & Roth, G. A. A heart-healthy and stroke-free world: Using data to inform global action. J. Am. Coll. Cardiol. 82(25), 2343–2349 (2023).
Mensah, G. A. et al. Global burden of cardiovascular diseases and risks, 1990–2022. J. Am. Coll. Cardiol. 82(25), 2350–2473 (2023).
Center For Cardiovascular Diseases The Writing Committee of the Report on Cardiovascular Health And Diseases. China N. Report on cardiovascular health and diseases in china 2023: An updated summary. Biomed. Environ. Sci.: BES 37(9), 949–992 (2024).
Tao, L. C. et al. ni, ting,. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 21(1), 68 (2022).
Cui, C. et al. Triglyceride-glucose index, renal function and cardiovascular disease: A national cohort study. Cardiovasc. Diabetol. 22, 325 (2023).
Liu, X. et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc. Diabetol. 21(1), 124 (2022).
Miao, M. et al. Triglyceride-glucose index and common carotid artery intima-media thickness in patients with ischemic stroke. Cardiovasc. Diabetol. 21, 43 (2022).
Liu, H. et al. Triglyceride-glucose index correlates with the occurrence and prognosis of acute myocardial infarction complicated by cardiogenic shock: Data from two large cohorts. Cardiovasc. Diabetol. 23, 337 (2024).
Li, J. et al. The triglyceride-glucose index is associated with atherosclerosis in patients with symptomatic coronary artery disease, regardless of diabetes mellitus and hyperlipidaemia. Cardiovasc. Diabetol. 22 (1), 224 (2023).
Li, Z. et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: A RCSCD-TCM study in China. Cardiovasc. Diabetol. 21, 38 (2022).
Fan, H. et al. Abdominal obesity is strongly associated with cardiovascular disease and its risk factors in elderly and very elderly community-dwelling Chinese. Sci. Rep. 6, 21521 (2016).
Kim, H. Y. et al. Association between abdominal obesity and cardiovascular risk factors in adults with normal body mass index: Based on the sixth korea national health and nutrition examination survey. J. Obes. Metabolic Syndrome 28 (4), 262–270 (2019).
Zarzour, A., Kim, H. W. & Weintraub, N. L. Understanding obesity-related cardiovascular disease. Circulation 138(1), 64–66 (2018).
Zhao, Y. et al. Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets. Signal. Transduct. Target. Therapy. 8, 431 (2023).
Calila, H. et al. Endothelial dysfunction as a key link between cardiovascular disease and frailty: A systematic review. J. Clin. Med. 13(9), 2686 (2024).
Scioli, M. G. et al. Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: Potential diagnostic biomarkers and therapeutic targets. J. Clin. Med. 9(6), 1995. (2020).
Medina-Leyte, D. J. et al. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 22(8), 3850 (2021).
Li, Y. et al. Body roundness index and waist-hip ratio result in better cardiovascular disease risk stratification: Results from a large chinese cross-sectional study. Front. Nutr. 9, 801582 (2022).
Zhang, X. et al. Body roundness index and all-cause mortality among US adults. JAMA Netw. Open. 7(6), e2415051 (2024).
Yang, M. et al. Body roundness index trajectories and the incidence of cardiovascular disease: Evidence from the China health and retirement longitudinal study. J. Am. Heart Assoc. 13 (19), e034768 (2024).
Tong, Y. et al. Changes in fasting blood glucose status and incidence of cardiovascular disease: The China-PAR project. J. Diabetes. 15(2), 110–120 (2023).
Gounden, V., Devaraj, S. & Jialal, I. The role of the triglyceride-glucose index as a biomarker of cardio-metabolic syndromes. Lipids Health Dis. 23(1), 416 (2024).
Zhao, X. et al. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 14, 1149239 (2023).
Yang, Y. et al. The interaction between triglyceride-glucose index and visceral adiposity in cardiovascular disease risk: Findings from a nationwide chinese cohort. Cardiovasc. Diabetol. 23(1), 427 (2024).
Budi, Y. P. et al. The role of autophagy in high-fat diet-induced insulin resistance of adipose tissues in mice. PeerJ 10, e13867 (2022).
Elkanawati, R. Y., Sumiwi, S. A. & Levita, J. Impact of lipids on insulin resistance: Insights from human and animal studies. Drug Des. Dev. Therapy 18, 3337–3360 (2024).
Simonsson, C. et al. A multi-scale in silico mouse model for diet-induced insulin resistance. Biochem. Eng. J. 191, 108798 (2023).
Rico-Martín, S. et al. Effectiveness of body roundness index in predicting metabolic syndrome: A systematic review and meta-analysis. Obes. Rev.: Off. J. Int. Assoc. Study Obes. 21(7), e13023 (2020).
Ellulu, M. S. et al. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci.: AMS. 13(4), 851–863 (2017).
Makki, K., Froguel, P. & Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 139239 (2013).
Heeren, J. & Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 50, 101238 (2021).
Wazir, M. et al. Lipid disorders and cardiovascular risk: A comprehensive analysis of current perspectives. Cureus 15(12), e51395.
Gasmi, A. et al. Obesity and insulin resistance: Associations with chronic inflammation, genetic and epigenetic factors. Curr. Med. Chem. 28(4), 800–826 (2021).
Alfaddagh, A. et al. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 4, 100130 (2020).
Poznyak, A. V. et al. Macrophages and foam cells: brief overview of their role, linkage, and targeting potential in atherosclerosis. Biomedicines 9(9), 1221 (2021).
Yang, D. R. et al. Endothelial dysfunction in vascular complications of diabetes: A comprehensive review of mechanisms and implications. Front. Endocrinol., 15. (2024).
Hong, C. G. et al. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9, 1023651 (2023).
Man, A. W. C., Li, H. & Xia, N. Impact of lifestyles (diet and exercise) on vascular health: Oxidative stress and endothelial function. Oxid. Med. Cellul. Longev. 2020, 1496462 (2020).
Acknowledgements
The authors thank all the funding support and the study participants in the REACTION research.
Funding
Funding for the provincial key medical discipline construction project of the Guizhou Provincial Health Commission from 2023 to 2024. Cultivate project 2022 for the National Natural Science Foundation of China, Affiliated Hospital of Guizhou Medical University (22NSFCP44).
Author information
Authors and Affiliations
Contributions
Conception and design: M.Z. and F.Z.; Administrative support: Q.Z., L.S.; Provision of study materials or participants: L.S., N.P., Y.H.; Collection and assembly of data: N.P., Y.H.; Data analysis and interpretation: M.Z. and F.Z.; Manuscript writing: F.Z., M.Z.; Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was waived by the local Ethics Committee of Guizhou Medical University in view of the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, F., Peng, N., Shi, L. et al. Triglyceride-glucose index combined with body roundness index predicts cardiovascular risk in middle-aged and elderly individuals: a 10-year cohort study. Sci Rep 15, 22062 (2025). https://doi.org/10.1038/s41598-025-05114-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05114-w