Abstract
To investigate the risk factors for postoperative hepatic dysfunction (HD) in patients undergoing acute Stanford type A aortic dissection (ATAAD) surgery and to develop an individualized prediction model. We retrospectively analyzed cardiac surgery patients with ATAAD treated at our hospital from January 2020 to March 2024, dividing them into 7:3 training and validation cohorts and grouping them into HD and non-HD categories based on postoperative liver function. Least absolute shrinkage and selection operator (LASSO) and multivariate logistic regression were used to identify independent predictive factors for postoperative HD, which formed the basis of a nomogram prediction model. We assessed model accuracy, calibration and clinical utility using C-statistics, calibration plots and decision curve analysis (DCA) curves. Internal validation with 1000 Bootstrap resamples was performed to reduce overfitting bias. LASSO and multivariate logistic regression identified key risk factors for HD in ATAAD patients, including chronic kidney disease, preoperative creatinine, international normalized ratio (INR), red blood cell (RBC) transfusion volume, peak intraoperative lactate, aortic cross-clamping time greater than 99 min, and reoperation. Based on these factors, a nomogram prediction model was successfully developed. The Hosmer–Leme show test yielded a p value of 0.952, indicating a good model fit. The area under the curve (AUC) values in the training and validation cohorts were 0.856 (95% CI 0.777–0.936) and 0.958 (95% CI 0.915–1) respectively, indicating good discriminatory power. The calibration curve shows that the bias corrected line is close to the ideal line. The DCA curve indicates that the use of the nomogram provides greater net clinical benefit. The AUC values before and after Bootstrap validation were 0.860 (95% CI 0.795–0.924) and 0.858 (95% CI 0.795–0.924), respectively, reflecting stable model performance and minimal risk of overfitting. The internally validated prognostic nomogram demonstrates excellent discriminative power, calibration, and clinical utility for predicting the risk of HD in patients who have undergone ATAAD surgery. This allows for an individualized evaluation and the optimization of clinical outcomes.
Similar content being viewed by others
Introduction
Acute Stanford type A aortic dissection (ATAAD) is a severe cardiovascular disease, with a mortality rate of up to 50% within 3 days and exceeding 70% within 1 week1,2. Currently, surgical intervention is considered the gold standard for treating this disease3. Although surgical techniques and medical care have advanced, the postoperative mortality and complication rates for ATAAD continue to be elevated due to a complex interplay of factors such as extracorporeal circulation, involvement of the dissection, ischemia–reperfusion injury, inflammatory responses, transfusions, and medications4,5,6.
Hepatic dysfunction (HD) is a common postoperative complication among patients with ATAAD, characterized by a postoperative serum transaminase (Aspartate Aminotransferase/Alanine Aminotransferase, AST/ALT) elevation exceeding 10 times the upper limit of normal7,8,9. The reported incidence of HD varies widely in the literature, ranging from 8.7 to 22.9%7,10,11. In ATAAD patients, postoperative HD increases the incidence of adverse events and mortality7,12,13. Currently, aside from pharmacotherapy, there are no specific treatments available to prevent or cure postoperative HD in ATAAD patients14. Moreover, predicting hepatic dysfunction is complicated by its multifactorial nature, often leading to challenges in accurately assessing risk using traditional methods. Therefore, early identification of patients at risk for postoperative HD and timely intervention are critical for improving outcomes in ATAAD.
Despite HD being common in ATAAD patients and closely associated with poor postoperative outcomes, studies on postoperative HD in ATAAD patients remain limited. Most existing research is constrained by small sample sizes and produces inconsistent or even contradictory findings15,16,17,18,19. Therefore, we conducted this study to address the gap by identifying independent risk factors for HD in a large cohort of ATAAD patients undergoing surgery. A predictive model for postoperative HD following ATAAD surgery was developed using retrospective analysis. The model, constructed with LASSO, logistic regression, and bootstrap verification, was represented as a nomogram for assessing patient risk. The model’s performance was evaluated using receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA), showing strong discrimination, good calibration, and clinical utility. This model helps clinicians identify at-risk patients, guide interventions, and improve prognosis while reducing mortality in ATAAD patients (Fig. 1).
Materials and methods
Patient selection
A total of 273 patients diagnosed with ATAAD and undergoing surgical treatment at the First Affiliated Hospital of China Medical University between January 2020 and March 2024 were included in a retrospective analysis of clinical data. This study was conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board of the First Affiliated Hospital of China Medical University. Prior to their inclusion in the study, informed consent was obtained from all patients.
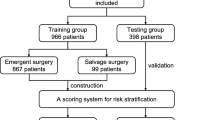
Inclusion criteria: (1) No gender restrictions (age ≥ 18 years); (2) Patients diagnosed with ATAAD through echocardiography and/or computed tomography angiography, who underwent surgical treatment; (3) Time from onset to surgery not exceeding 1 week; (4) Complete preoperative clinical data, including age, gender, underlying diseases, and biochemical indicators. The exclusion criteria and patient selection process are shown in Fig. 2.
Clinical data collection and definition
A retrospective analysis was conducted to gather and summarize the clinical information of the selected patients, utilizing the database and Hospital Information System. The data encompasses (1) Basic characteristics: age, sex, body mass index (BMI), body surface area (BSA), history of smoking, and alcohol consumption. (2) Medical history. (3) Preoperative assessments: blood tests (e.g., hemoglobin, leukocytes), liver function tests (e.g., ALT: alanine aminotransferase, ALP: alkaline phosphatase, INR: international normalized ratio, GGT: gamma-glutamyl transferase), and coagulation tests (e.g., D-dimer, INR). (4) Surgical-related details: selection of surgical method, cardiopulmonary bypass (CPB) duration, aortic cross-clamping time, and deep hypothermic circulatory arrest time, among others. (5) Postoperative Complications and 30-day mortality rate.
HD is defined as a postoperative elevation of serum transaminases (AST/ALT) exceeding 10 times the upper limit of normal.
Statistical analysis
Following the collection of clinical data, statistical analysis and visualisation were conducted utilising R version 4.3.3. The analysis process utilised various R packages, including readxl, dplyr, glmnet, rms, caret, pROC, rmda, and regplot. Summary statistics, including counts, percentages, ratios, and interquartile range (IQR), were utilized based on the data type in question. The continuous variables with a normal distribution are presented as mean ± standard deviation (SD), while those with a non-normal distribution are expressed as median and IQR. Categorical variables are summarized as frequencies and percentages. For continuous variables, either an independent sample t-test or a Mann–Whitney U test was applied. Categorical variables were expressed as frequencies and percentages, and group comparisons were conducted using chi-square tests or Fisher’s exact tests. The patients were randomly assigned to the training and validation groups in a 7:3 ratio and subsequently classified into two groups based on the incidence of postoperative hepatic dysfunction: a group with HD and a group without hepatic dysfunction (non-HD). Least absolute shrinkage and selection operator (LASSO) regression and multivariate logistic regression were employed to ascertain the independent predictors of postoperative HD in Stanford type A aortic dissection patients, and collinearity was evaluated using the variance inflation factor (VIF). A personalized HD nomogram prediction model was constructed and its predictive capability, calibration, and clinical utility were evaluated using c-statistics, calibration plots, and decision curve analysis (DCA) curves. To mitigate the potential for overfitting, internal validation was performed using 1000 bootstrap resamples.
Results
General information
In the cohort of 273 patients, the mean age was 51.11 ± 12.35 years (range 19–84), and the median BMI was 26.20 (IQR 23.40–29.40) kg/m2, with 49.5% reporting a smoking history. Hypertension was present in 78.4% of patients, 5.5% had diabetes, and 11.0% had chronic kidney disease. It is worth noting that 33.3% of patients had dissections involving the celiac trunk (see in Table 1).
For the preoperative clinical indicators, the median left ventricular ejection fraction (LVEF) was recorded at 60.00 (IQR 58.00–63.00)%, while the median left ventricular end-diastolic diameter (LVEDD) was 48.00 (IQR 46.00–52.00) mm. The leukocyte count measured 12.51 (IQR 10.02–15.37) × 109/L, while the preoperative creatinine level was 81.00 (IQR 63.00–110.00) μmol/L. Notably, preoperative creatinine levels in HD patients were noted at 104.00 (IQR 83.50–175.50) μmol/L, which was significantly higher compared to 78.00 (IQR 59.00–102.50) μmol/L in non-HD patients (see in Table 2).
In terms of surgical-related data, the median duration of cardiopulmonary bypass was recorded at 143.00 (IQR 125.00–172.00) minutes, with an aortic cross-clamp time of 101.00 (IQR 87.00–123.00) minutes, and the peak intraoperative lactate level reaching 9.30 (IQR 6.10–12.80) mmol/L. The median amount of red blood cell transfusion across all patients was 3.50 (IQR 2.00–7.00) units, with HD patients receiving significantly more than non-HD patients (p < 0.001) (see in Table 3).
In our study cohort, a total of 46 patients were diagnosed with HD. Among them, the all-cause mortality rate associated with HD was 41.3% (19 of 46 patients). HD patients had a significantly increased incidence of acute kidney injury (78.3% vs. 33.9%, p < 0.001) and postoperative respiratory failure (84.8% vs. 34.4%, p < 0.001) with prolonged mechanical ventilation time (96.95 h vs. 42.00 h, p < 0.001). The need for postoperative dialysis (54.3% vs. 14.5%, p < 0.001) and the incidence of neurological dysfunction (80.4% vs. 30.8%, p < 0.001) were more frequent in the HD group. The DAOH30 was significantly shorter for HD patients (0.00 days vs. 8.00 days, p < 0.001), while the ICU-free days were also notably reduced in the HD group (6.50 days vs. 14.00 days, p = 0.001). Mortality within 30 days was significantly higher in the HD group than in the non-HD group (41.3% vs. 10.1%, p < 0.001) (see in Table 4).
LASSO regression for screening risk factors
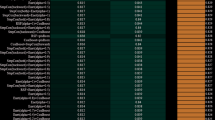
Due to the inclusion of numerous variables, correlations between them, and limited sample sizes for positive outcomes in this study, LASSO regression was used to filter the included variables. The VIF was used to assess collinearity between variables, with those with high VIFs removed to select optimal risk factors for postoperative HD. Ten-fold cross-validation was performed to determine the optimal value of Lambda = 0.076353 as the solution for the variable selection model, counting the number of variables with non-zero regression coefficients at this stage (Fig. 3). The LASSO regression results showed that the following seven variables were selected and included in the model: history of chronic kidney disease, preoperative creatinine, INR, red blood cell (RBC) transfusion volume, highest intraoperative lactate level, aortic cross-clamp time and reoperation.
Logistic regression
The risk factors identified by LASSO regression were used as independent variables, with the occurrence of HD as the dependent variable in a multivariate logistic regression analysis. The results showed that RBC transfusion volume, peak intraoperative lactate level, aortic cross-clamp time and reoperation were independent risk factors for postoperative HD (p < 0.05) (Fig. 4). Figure 5 shows the ROC curves for various predictive factors in assessing the risk of occurrence of HD.
Nomogram and performance of the model
Based on the risk factors selected from the multivariate logistic regression analysis as predictive factors, and taking into account the dual aspects of statistical significance levels and the clinical utility of LASSO regression, which can help to remove redundant variables and thus improve the generalizability of the model, we included chronic kidney disease (CKD), INR and preoperative creatinine as predictive factors in the model, in line with comparable research20. We developed a nomogram to predict the risk of HD in patients following ATAAD surgery, shown in Fig. 6. The total score is obtained by adding the individual scores derived from each predictive variable marked vertically on the horizontal axis, which is then used to estimate the likelihood of HD occurring.
Model validation was conducted through the construction of ROC, calibration, and DCA curves to assess the model’s discriminatory ability, calibration accuracy, and clinical utility. The ROC curve indicates that the area under the curve (AUC) values were 0.856 (95% CI 0.777–0.936) for the training group and 0.958 (95% CI 0.915–1) for the validation group, both of which demonstrate the model’s strong discriminative ability (see in Fig. 7). The calibration curve comprises three distinct lines (see in Fig. 8): the dotted line denotes the model’s performance on the training dataset (Apparent), the red line represents the ideal scenario where predicted probabilities align perfectly with actual occurrences (Ideal), and the blue line displays the calibration results post-resampling (Bias-corrected). The Fig. 8 demonstrates that the model’s estimated probabilities are in close alignment with the actual outcomes in both the training and validation groups, indicating a high degree of consistency. As illustrated by the DCA curve (see in Fig. 9), when the predicted probabilities of the nomogram model fall within the range of 0–0.85, the model’s clinical net benefit exceeds that of both the “full intervention” and “no intervention” scenarios, thereby confirming the nomogram’s favorable clinical utility.
Bootstrap verification
To prevent overfitting of the nomogram, this study conducted internal validation through bootstrap resampling 1000 times (see in Fig. 10). Prior to validation, the findings indicated an AUC of 0.856 (95% CI 0.777–0.936). Following Bootstrap validation, the AUC was 0.854 (95% CI 0.792–0.919). The proximity of the AUC values before and after internal validation corroborates the stability of the model’s performance and a reduced risk of overfitting. The narrow confidence interval range observed before and after internal validation also substantiates the model’s robust reliability.
Discussion
HD represents a significant postoperative risk for patients with ATAAD, strongly linked to the elevated incidence of other complications and increased postoperative mortality rates21. This liver injury is frequently the result of a combination of factors, including systemic hypoperfusion, ischemia–reperfusion injury, and metabolic disturbances22,23. In accordance with the findings of previous studies with a high mortality rate, our study also revealed a postoperative incidence of HD of 16.8% and an all-cause mortality rate of 15.4% among ATAAD patients. These results highlight the critical need for precise, individualized risk assessments and preventive strategies. The traditional liver assessment scores, including the Child–Turcotte–Pugh (Child–Pugh), model for end-stage liver disease (MELD), and acute-on-chronic liver failure scores, lack the requisite specificity for accurately predicting postoperative HD in ATAAD patients24,25. Accordingly, the creation of bespoke predictive models is vital to enable the refinement of outcome-driven clinical interventions for this high-risk population.
The objective of this study was to enhance the prediction of HD by developing a nomogram that incorporates demographic, intraoperative, and laboratory data from ATAAD patients. Although previous research has identified a number of factors, including gender, diabetes, lower CPB temperature, valvular disease, surgery duration, and celiac trunk involvement, as potential contributors to liver function impairment12,23,26,27, our model did not find any significant associations between these factors and the development of postoperative HD in ATAAD patients. Instead, through the use of LASSO regression, seven independent risk factors for HD were identified: CKD, preoperative creatinine levels, INR, RBC transfusion volume, intraoperative lactate levels, aortic cross-clamp time, and reoperation. Of these, peak intraoperative lactate levels, RBC transfusion volume, aortic cross-clamp time, and reoperation were identified as the primary drivers of HD risk.
As posited by Deeb and colleagues, aortic dissection may precipitate stenosis in the celiac and mesenteric arteries, thereby reducing liver blood flow and potentially leading to hepatic impairment or infarction17,18,28. However, as evidenced by our findings, some studies indicate that the extent and ___location of the dissection do not reliably predict postoperative HD19. It is notable that celiac artery involvement itself may not emerge as a significant risk factor. Instead, intraoperative aortic cross-clamping time was identified as an independent predictor of HD, which aligns with existing literature29. This finding suggests that anatomical involvement alone may not fully reflect the risk of HD. Instead, hepatic ischemia–reperfusion injury caused by intraoperative aortic clamping may be a key mechanism. Ischemic hepatic tissue is prone to oxidative stress, inflammatory mediator release, and microcirculatory dysfunction, leading to hepatocellular impairment or necrosis30. This underscores the need for further research on the role of hepatic blood supply status and ischemia duration before and during surgery in impacting liver function in ATAAD patients.
Building on previous findings indicating a close relationship between liver and kidney function31,32,33, our study provides further evidence that renal impairment can significantly impact hepatic outcomes. In ATAAD, dissection involvement of the abdominal aorta and renal arteries may reduce renal blood flow, often leading to acute kidney injury (AKI) and renal failure34,35,36. Our analysis of ATAAD patients revealed that a history of CKD and elevated preoperative creatinine levels were both risk factors for postoperative HD, consistent with existing research. The underlying pathophysiology may involve systemic inflammation and immune activation. Renal dysfunction can trigger the excessive release of pro-inflammatory cytokines and chemokines, such as TNF-α and IL-6, which may directly impair hepatic microcirculation and promote hepatocellular injury37,38. Conversely, the HD—especially in acute settings—may induce HRS-like AKI through similar inflammatory pathways, resulting in renal tubular injury, acute tubular necrosis, and systemic vasodilatation39. This emphasizes the necessity of monitoring renal function as part of a comprehensive approach to managing hepatic risk in these high-risk patients.
Lactic acid, a byproduct of glycolysis, is frequently elevated following cardiac surgery and has been demonstrated to be a significant predictor of adverse postoperative outcomes40,41,42. Hyperlactatemia, a common occurrence in patients who have undergone cardiac surgery, can be attributed to a number of factors, including the flow rate, duration, and temperature of extracorporeal circulation, as well as fluctuations in blood pressure40,43. These conditions contribute to tissue hypoxia and metabolic stress, thereby rendering elevated lactate levels a reliable clinical marker for assessing disease severity and risk of complications. In accordance with the results of our study, we found a significant correlation between elevated intraoperative lactate levels and the occurrence of postoperative HD in ATAAD patients. This highlights the importance of monitoring lactate as a predictive factor for hepatic risk. Moreover, our findings suggest that maintaining stable perfusion and minimizing circulatory disruptions during surgery may be crucial for reducing the risk of liver-related complications in this patient group.
Red blood cells (RBCs) are essential for systemic oxygen delivery, particularly to metabolically active organs such as the liver, which is highly susceptible to hypoxia-induced injury44,45,46. Patients undergoing ATAAD often necessitating significant RBC transfusions to maintain adequate oxygenation19,47. As intraoperative blood loss is challenging to quantify with precision, the volume of RBC transfusion offers a more reliable indicator of the patient’s oxygenation status during surgery. Our findings substantiated that intraoperative RBC transfusion volume is an independent risk factor for postoperative HD. Beyond indicating the severity of bleeding and impaired oxygen delivery, transfusion of stored RBCs may aggravate liver injury through several mechanisms. During storage, depletion of antioxidants such as NADH and glutathione increases hemoglobin susceptibility to oxidative degradation, releasing free iron and heme that induce oxidative stress. Membrane damage leads to microparticle formation, which reduces nitric oxide availability and disrupts hepatic microcirculation48. In addition, aged RBCs and their breakdown products activate hepatic macrophages, promoting inflammatory cytokine release. Transfusion also raises systemic iron levels, resulting in hepatic accumulation of non-transferrin-bound iron and iron-mediated cytotoxicity, further impairing liver function48,49. This underscores the significance of transfusion management as a constituent of liver protection strategies in ATAAD patients, assisting in the mitigation of hypoxic damage and the enhancement of postoperative outcomes.
Based on the above findings and confidence in our results, we developed a nomogram that demonstrated superior predictive performance, achieving an AUC above 0.8 in both the training and validation cohorts. This model outperformed traditional liver assessment tools, offering a more precise, individualized risk assessment for ATAAD patients. By identifying key risk factors and leveraging this predictive nomogram, our study supports a more proactive approach to mitigating the impact of HD and improving patient outcomes in ATAAD.
Limitation
This research introduces a nomogram model for predicting the risk of postoperative HD in ATAAD patients based on multivariable analysis for the first time, demonstrating notable clinical value for postoperative management. Nevertheless, this study is not without limitations, including a single-center design, a relatively limited sample size, an internal validation of the predictive model, a markedly smaller number of HD patients compared to non-HD patients (approximately 17% vs. 83%) without the application of data balancing techniques during analysis, and constraints inherent to retrospective analysis, which may affect the external validity of the predictive model. Furthermore, the intricacy of interventions, including reoperation, extracorporeal membrane oxygenation, and intra-aortic balloon pump, makes it challenging to assess their impact on the risk of hepatic dysfunction in this study independently. This highlights the necessity for further investigation. Notwithstanding these limitations, the nomogram model demonstrates robust predictive performance, exhibiting good calibration and clinical utility, and offers enhanced capabilities for individualized risk assessment.
Conclusion
In conclusion, we developed a nomogram prediction model for forecasting HD following surgery for ATAAD based on a retrospective analysis of clinical data. We then performed internal validation, confirming the model’s significant clinical utility. This predictive model serves as a tool for individualized forecasting, facilitating early intervention for postoperative HD in ATAAD patients and improving treatment strategies.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Wang, W. et al. Clinical features of acute aortic dissection from the registry of aortic dissection in China. J. Thorac. Cardiovasc. Surg. 148(6), 2995–3000 (2014).
Harris, K. M. et al. Early mortality in type A acute aortic dissection: Insights from the international registry of acute aortic dissection. JAMA Cardiol. 7(10), 1009 (2022).
Erbel, R. et al. ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (ESC). Eur. Heart J. 35(41), 2873–2926 (2014).
Castrovinci, S. et al. Surgical management of aortic root in type a acute aortic dissection: A propensity-score analysis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 50(2), 223–229 (2016).
Englberger, L. et al. Deep hypothermic circulatory arrest is not a risk factor for acute kidney injury in thoracic aortic surgery. J. Thorac. Cardiovasc. Surg. 141(2), 552–558 (2011).
Isselbacher, E. M. et al. Recurrent aortic dissection. Circulation 134(14), 1013–1024 (2016).
Henrion, J. Hypoxic hepatitis. Liver Int. 32(7), 1039–1052 (2012).
Van Den Broecke, A. et al. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann. Intensive Care 8(1), 15 (2018).
Waseem, N. & Chen, P. H. Hypoxic hepatitis: A review and clinical update. J. Clin. Transl. Hepatol. 4(3), 263–268 (2016).
Yan, T. D. et al. Standardizing clinical end points in aortic arch surgery: A consensus statement from the international aortic arch surgery study group. Circulation 129(15), 1610–1616 (2014).
Zhao, R. et al. Current surgical management of acute type A aortic dissection in China. JACC Asia 2(7), 869–878 (2022).
Soltesz, E. G., Parks, R. J., Jortberg, E. M. & Blackstone, E. H. Machine learning-derived multivariable predictors of post-cardiotomy cardiogenic shock in high-risk cardiac surgery patients. JTCVS Open 22, 272–285 (2024).
Veldtman, G. R. et al. Fontan circulation and systemic disease—A retrospective cohort analysis over 35 years of follow-up: Fontan systemic disease at 35 years. Am. Heart J. 279, 40–43 (2025).
Merath, K. et al. Postoperative liver failure: Definitions, risk factors, prediction models and prevention strategies. J. Gastrointest. Surg. 27(11), 2640–2649 (2023).
Sheng, W., Qiao, H., Wang, Z., Niu, Z. & Lv, X. Analysis of prognosis and risk factors for postoperative hepatic dysfunction in patients with acute type a aortic dissection. Anatol. J. Cardiol. 27(4), 197–204 (2023).
Xu, Y. et al. Risk factors for postoperative hepatic dysfunction in overweight patients with acute type a aortic dissection. BMC Surg. 24(1), 305 (2024).
Deeb, G. M., Patel, H. J. & Williams, D. M. Treatment for malperfusion syndrome in acute type a and B aortic dissection: A long-term analysis. J. Thorac. Cardiovasc. Surg. 140(6), S98-100 (2010).
Tien, M. & Cheung, A. T. Anesthetic management of acute aortic dissection. In Aortic Dissection and Acute Aortic Syndromes (eds Sellke, F. W. et al.) 553–576 (Springer, 2021). https://doi.org/10.1007/978-3-030-66668-2_38.
Apaydin, A. Z. et al. Perioperative risk factors for mortality in patients with acute type a aortic dissection. Ann. Thorac. Surg. 74(6), 2034–2039 (2002).
Wang, H. et al. Predicting medication nonadherence risk in a Chinese inflammatory rheumatic disease population: Development and assessment of a new predictive nomogram. Patient Prefer. Adherence 12, 1757–1765 (2018).
Lopez-Delgado, J. C., Putzu, A. & Landoni, G. The importance of liver function assessment before cardiac surgery: A narrative review. Front. Surg. 9, 1053019 (2022).
Raman, J. S., Kochi, K., Morimatsu, H., Buxton, B. & Bellomo, R. Severe ischemic early liver injury after cardiac surgery. Ann. Thorac. Surg. 74(5), 1601–1606 (2002).
Kirov, H. et al. Association of liver dysfunction with outcomes after cardiac surgery—A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 35(6), ivac280 (2022).
Zheng, Y. X., Zhong, X., Li, Y. J. & Fan, X. G. Performance of scoring systems to predict mortality of patients with acute-on-chronic liver failure: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 32(10), 1668–1678 (2017).
Peng, Y. et al. Child-pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev. Gastroenterol. Hepatol. 10(8), 971–980 (2016).
Aneman, A. et al. Advances in critical care management of patients undergoing cardiac surgery. Intensive Care Med. 44(6), 799–810 (2018).
Yu, W. et al. Risk factors of hyperbilirubinemia after acute type a aortic dissection. Heart Surg. Forum 27(5), E459–E464 (2024).
Leslie, B. R. Ischemic hepatitis from aortic dissection. Ann. Intern. Med. 110(6), 495 (1989).
Liu, X. et al. Hybrid total arch replacement via ministernotomy for Stanford type a aortic dissection. Front. Cardiovasc. Med. 18(10), 1231905 (2023).
Choi, E. K. & Lim, D. G. Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: A narrative review. J. Yeungnam Med. Sci. 40(2), 115–122 (2022).
Simonetto, D. A., Gines, P. & Kamath, P. S. Hepatorenal syndrome: Pathophysiology, diagnosis, and management. BMJ (Clin. Res. Ed). 14(370), m2687 (2020).
Bucsics, T. & Krones, E. Renal dysfunction in cirrhosis: Acute kidney injury and the hepatorenal syndrome. Gastroenterol. Rep. 5(2), 127–137 (2017).
Cullaro, G., Verna, E. C. & Lai, J. C. Association between renal function pattern and mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 17(11), 2364–2370 (2019).
Zhu, J. C. et al. Acute renal injury after thoracic endovascular aortic repair of Stanford type B aortic dissection: Incidence, risk factors, and prognosis. J. Formosan Med. Asso. Taiwan Yi Zhi. 113(9), 612–619 (2014).
Goda, M. et al. Risk analysis for hospital mortality in patients with acute type a aortic dissection. Ann. Thorac. Surg. 90(4), 1246–1250 (2010).
Lingzhi, C. et al. Outcome predictors in patients presenting with acute aortic dissection. J. Cardiothorac. Vasc. Anesth. 30(5), 1272–1277 (2016).
Shang, Y. et al. Kidney ischemia-reperfusion elicits acute liver injury and inflammatory response. Front. Med. (Lausanne) 7, 201 (2020).
Park, S. W. et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab. Investig. J. Tech. Methods Pathol. 91(1), 63–84 (2011).
Adebayo, D. & Wong, F. Pathophysiology of hepatorenal syndrome—Acute kidney injury. Clin. Gastroenterol. Hepatol. 21(10), S1-10 (2023).
Klee, P., Rimensberger, P. C. & Karam, O. Association between lactates, blood glucose, and systemic oxygen delivery in children after cardiopulmonary bypass. Front. Pediatr. 8, 332 (2020).
Draben, L. Hyperlactatemia and patient outcomes after pediatric cardiac surgery. Crit. Care Nurse 38(5), e1-6 (2018).
Mak, N. T. J. J., Iqbal, S., de Varennes, B. & Khwaja, K. Outcomes of post-cardiac surgery patients with persistent hyperlactatemia in the intensive care unit: A matched cohort study. J. Cardiothorac. Surg. 24(11), 33 (2016).
Matteucci, M. et al. Hyperlactatemia during cardiopulmonary bypass: Risk factors and impact on surgical results with a focus on the long-term outcome. Perfusion 35(8), 756–762 (2020).
Sadasivam, N., Kim, Y. J., Radhakrishnan, K. & Kim, D. K. Oxidative stress, genomic integrity, and liver diseases. Molecules (Basel, Switzerland) 27(10), 3159 (2022).
Quesnelle, K. M., Bystrom, P. V. & Toledo-Pereyra, L. H. Molecular responses to ischemia and reperfusion in the liver. Arch. Toxicol. 89(5), 651–657 (2015).
Cha, H. J. Erythropoiesis: Insights from a genomic perspective. Exp. Mol. Med. 56(10), 2099–2104 (2024).
Magouliotis, D. E. et al. Hemostatic properties of aortic root preservation versus root replacement for acute type a aortic dissection: A pooled analysis. Life 14(10), 1255 (2024).
Orlov, D. & Karkouti, K. The pathophysiology and consequences of red blood cell storage. Anaesthesia 1(70), 29-e12 (2014).
Baek, J. & Kim, S. Effects of transfusion of stored red blood cells on renal ischemia-reperfusion–induced hepatic injury in rats. Transplant. Proc. 55(3), 629–636 (2023).
Acknowledgements
Graphic abstract was created with Biorender.com.
Funding
National Natural Science Foundation of China (Grant Number 81870963).
Author information
Authors and Affiliations
Contributions
Xiaotian Han, Wei Wang, Tianxiang Gu and Enyi Shi wrote the main manuscript text. Xiao tian Han and Wei Wang prepared the figures and tables. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University, and informed consent was obtained from all participants.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, X., Wang, W., Gu, T. et al. Development and validation of a predictive model for postoperative hepatic dysfunction in Stanford type A aortic dissection. Sci Rep 15, 22126 (2025). https://doi.org/10.1038/s41598-025-06024-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06024-7