Abstract
Deep vein thrombosis (DVT) is a significant contributor to the global disease burden. This study aimed to identify metabolic biomarkers to compensate for the limitation in specificity and singular nature of D-dimer test. UHPLC–MS was used to examine γ-butylbetaine and l-carnitine levels in clinical and rat blood and urine at different stages of DVT along with analysis of their diagnostic titer and correlation analyses with DVT. γ-Butylbetaine and l-carnitine levels in plasma and urine which trends were similar in plasma and urine of humans and rats with DVT were lower in patients with DVT and rats than those in the control group (P < 0.05); in human plasma, area under the curve (AUC) of γ-butylbetaine and l-carnitine combined with D-dimer was 0.914 (P < 0.001) in the acute group and 0.895 (P < 0.001) in the subacute group, respectively. In human urine, AUC of l-carnitine combined with γ-butylbetaine in the subacute group was 0.855 (P < 0.001). γ-Butylbetaine and l-carnitine can be used to screen and diagnose DVT at different phases. Moreover, γ-butylbetaine and l-carnitine expression levels are similar in humans and rats, providing precise indicators and animal models for in-depth study of the mechanisms underlying DVT development.
Similar content being viewed by others
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are types of venous thrombosis. With the incidence increasing by 1–2 cases per 1000 people/year worldwide1,2, they have become the third leading cause of death among cardiovascular diseases3. DVT is a multifactorial disease caused by a complex interaction of external environmental and genetic risk factors and is characterized by three major factors: blood hypercoagulation, endothelial dysfunction, and hemodynamic disorders; it is most common in the lower extremities4. Currently, clinical diagnosis of DVT includes assessment of pretest probability such as the Wells score, which has the highest consensus, D-dimer, and imaging tests such as ultrasonography5. D-Dimer is a serum biomarker for the diagnosis of DVT with high sensitivity but poor specificity6. Consequently, studies are increasingly focused on the metabolomics for biomarker discovery from various organisms7,8,9.
Inhibition of thrombus formation by untargeted metabolomics findings indicates that acylcarnitines in venous thromboembolism (VTE) plasma affect factor Xa-related anticoagulant activity10. Jiang et al. similarly found an association between carnitine, triacylglycerol, phosphatidylethanolamine, and the amino acid tryptophan, and the risk of VTE and PE through metabolomic analysis of prospective studies11. A metabolomics approach incorporating 21 biomarkers was used to construct an equation to stratify the VTE population according to disease risk12. However, these biomarkers only partially explain the potential risk factors for DVT. Therefore, it is necessary to explore more reliable biomarkers for accurate early diagnosis of patients suspected of having DVT, timely and targeted prophylactic treatment, and reduction of DVT-related morbidity and mortality.
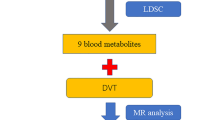
Urine has long been used to facilitate the diagnosis of various renal, oncological, hematologic, and metabolic disorders7,13; however, current research on urine analysis to improve the early diagnosis and prognosis of DVT is unclear, given that urine collection is a noninvasive procedure unlike blood testing. This study aimed to identify changes in urinary metabolites in patients with DVT using untargeted metabolomics to efficiently screen for DVT through noninvasive methods. Two biomarker combinations, γ-butylbetaine (GBB) and l-carnitine (L-CN), were identified at the molecular level and validated using ultra-performance liquid chromatography mass spectrometry (UPLC–MS) of blood and urine samples from ergonomics and animals. We aimed to validate targeted metabolomics to clarify their concentration ranges in circulating blood and urine in DVT and to assess their accuracy in DVT screening and disease stratification. The results of this study provide basic and clinical data to support further research on the prevention and treatment of DVT. (The corresponding flow chart is presented in Fig. 1.)
Results
Baseline characteristics of clinical cases
Blood samples from 171 patients (83 from the control group and 88 [51 men and 37 women] from the DVT group) and urine samples from 141 patients (76 from the control group and 65 [40 men and 25 women] from the DVT group) were analyzed separately. The demographic and clinical characteristics of patients with DVT and those in the control groups were significantly different in terms of age (P < 0.05), although no differences were observed in sex distribution, BMI, or other comorbidities (Table 1).
Screening of differential metabolites in urine samples of DVT and control groups
Untargeted metabolomics results were obtained by UHPLC–QTOF/MS, and all extracted samples were randomly uploaded for sequencing assays. Quality control (QC) samples were added separately to assess the reliability of the experimental technique, and urine samples from the DVT and control groups were analyzed in both positive (ESI+) and negative (ESI−) ion modes (supplementary materials 1 and 2).The selected metabolites with statistical differences were subjected to matchstick analysis (Fig. 2a, b), and then combined with unit variables and multivariate statistical analysis; VIP > 1 and P < 0.05 were used to identify seven main biomarkers in POS (Table 2). According to the metabolic map, differential metabolites screened were mainly amino acids, fatty acids, nucleosides, and their derivatives, including organic heterocyclic compounds, alkaloids, and sugars (Fig. 2c, d). Further analysis revealed that GBB, belonging to amino acids, and L-CN, belonging to amino acid derivatives, were on the lysine degradation pathway enriched by the Kyoto Encyclopedia of Genes and Genomes. As per the relevant literature, GBB and L-CN are closely associated with vascular function.
Untargeted metabolomics combined with bioinformatics to analyze differential metabolites. (a, b) Differential metabolites screened in positive and negative ion modes. (c, d) Classification maps of differential metabolites in Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis screened in positive and negative ion modes.
Concentrations of L-CN and GBB in human plasma and urine
Extracted ion flow chromatograms of L-CN and GBB in different samples are shown in Fig. 3 and Supplementary Materials 3. The levels of L-CN (P < 0.05 and P < 0.05, respectively) and GBB (P < 0.01 and P < 0.05, respectively) in the serum and urine of patients with DVT were lower (Fig. 4a–d, respectively) than those of the control group.
Concentration levels of γ-butylbetaine (GBB) and l-carnitine (L-CN) in the plasma and urine of patients in the control and deep vein thrombosis (DVT) groups. (a, b) Plasma levels of GBB and L-CN in the control and DVT groups. (c, d) Levels of plasma GBB and L-CN in the urine of the control and DVT groups. ***P < 0.001, **P < 0.01, *P < 0.05.
Correlations between L-CN and GBB in human blood, blood routine parameters, and coagulation function parameters
We calculated the Pearson correlation values for L-CN, GBB, coagulation function parameters, and blood routine indicators in the control and DVT groups (Fig. 5). We found that DVT was negatively correlated with L-CN (P < 0.05, r = − 0.17) and GBB (P < 0.01, r = − 0.22), and L-CN was closely related to GBB (P < 0.01, r = 0.48). L-CN had positive correlations with the percentage of monocytes (P < 0.01, r = 0.23), the absolute value of monocytes (P < 0.05, r = 0.17), and the absolute value of lymphocytes (P < 0.05, r = 0.18). Conversely, L-CN was negatively correlated with the percentage of neutrophils (P < 0.05, r = − 0.17). Although L-CN was weakly correlated with D-dimer (P < 0.05, r = − 0.17), it was statistically significant. In the correlation analysis of GBB with coagulation function and blood routine parameters, GBB was found to be negatively correlated with activated partial thromboplastin time (APTT; P < 0.01, r = − 0.20) and had a weak but statistically significant positive correlation with hemoglobin (P < 0.05, r = − 0.16) and thrombin time (TT; P < 0.05, r = − 0.15).
Correlations of γ-butylbetaine (GBB) and l-carnitine (L-CN) with hematological and coagulation parameters in patients with deep vein thrombosis (DVT). WBC, white blood cell count; NEUT#, neutrophil count; NEUT%, neutrophil percentage; LYMPH#, lymphocyte count; LYMPH%, lymphocyte percentage; MONO#, monocyte count; MONO%, monocyte percentage; RBC, red blood cell count; PLT, platelet count; HGB, hemoglobin; PT, prothrombin time; FDP, fibrin degradation products; APTT, activated partial thromboplastin time; FIB, fibrinogen; TT, thrombin time; INR, international normalized ratio.***P < 0.001,**P < 0.01,*P < 0.05.
Concentrations of L-CN and GBB in human blood and urine at different stages of DVT
According to the onset time of DVT, the patients were divided into acute groups (30 days) to analyze L-CN and GBB. The levels of L-CN (P < 0.01) and GBB (P < 0.01) in plasma of the DVT acute group were significantly lower than those of the control group. The level of L-CN continued to increase (Fig. 6b), whereas the level of GBB increased in the subacute group and then decreased gradually (Fig. 6a). The levels of L-CN and GBB in the urine of patients with DVT during the onset of DVT were lower than those in the control group, particularly in the subacute group (P < 0.01; Fig. 6c, d).
γ-Butylbetaine (GBB) and l-carnitine (L-CN) concentration levels in plasma and urine of patients with deep vein thrombosis (DVT) at different times. (a, b) Plasma GBB and L-CN levels in the control, acute, subacute, and chronic groups. (c, d) Urinary GBB and L-CN levels in the control, acute, subacute, and chronic groups. ***P < 0.001,**P < 0.01,*P < 0.05; ###P < 0.001, ##P < 0.01, #P < 0.05; +++P < 0.001, ++P < 0.01, +P < 0.05.
Diagnostic values of L-CN and GBB in human plasma and urine according to DVT stage
In the serum, L-CN differed only slightly for the assessment of DVT in the acute group, and the effect was low (Fig. 7a), whereas GBB differed higher than L-CN in the diagnosis of DVT in the acute and chronic groups (Fig. 7a, b). However, both were inferior to the traditional marker D-dimer (Fig. 7a–c). The area under the curve (AUC) of L-CN combined with GBB in diagnosing acute and chronic DVT was only slightly higher than that of a single diagnosis (Fig. 7a, c). The AUC of combined GBB and D-dimer was higher than that of D-dimer alone in diagnosing DVT. The AUC of combined L-CN and D-dimer was higher than that of D-dimer in the diagnosis of DVT in the subacute group, whereas the AUC of single and combined diagnoses in the chronic group did not show significant differences (Fig. 7a–c). In urine, L-CN and GBB alone, and their combination, could distinguish subacute patients with DVT (Fig. 7e), but there was no diagnostic effect in the acute and chronic groups (Fig. 7d, f).
Diagnostic potency of γ-butylbetaine (GBB) and l-carnitine (L-CN) in the plasma and urine for different periods of deep vein thrombosis (DVT). (a–c) Receiver operating characteristic (ROC) curves of GBB, L-CN, D-dimer, and their combination in plasma of the acute, subacute, and chronic groups. (d–f) ROC curves of GBB, L-CN, and their combination in urine of the acute, subacute, and chronic groups. ***P < 0.001, **P < 0.01, *P < 0.05.
Concentrations of L-CN and GBB according to sex
There was no significant difference in L-CN (P > 0.05) and GBB (P > 0.05) between the control and DVT groups in male plasma (Fig. 8a, b). GBB level in DVT female plasma was significantly lower than that in the control group (P < 0.01) as well as that in DVT male plasma (P < 0.01), whereas there was no significant difference in L-CN (P > 0.05) (Fig. 8a, b). L-CN and GBB in DVT male urine were significantly lower than those in the control group (P < 0.05) (Fig. 8c, d). The Pearson correlation values of L-CN and GBB concentrations with DVT in male and female patients were calculated (Table 3); plasma GBB level was more strongly correlated with DVT in women than that of men (female: R = − 0.398, P < 0.05), and GBB was more closely related to L-CN in women (female: R = 0.559, P < 0.01) than that of men (male: R = 0.458, P < 0.01). The levels of L-CN and GBB in male urine were negatively correlated with DVT (L-CN: R = − 0.223, P < 0.01; GBB: R = − 0.218, P < 0.01). There was no significant difference in the correlation coefficient between L-CN and GBB in urine between women and men (women: R = 0.882, P < 0.01; male: R = 0.893, P < 0.01); however, both were closer than those in plasma.
Median concentration levels of γ-butylbetaine (GBB) and l-carnitine (L-CN) in the plasma and urine of patients of different sexes with deep vein thrombosis (DVT). (a, b) Levels of GBB and L-CN in the plasma of male and female patients. (c, d) GBB and L-CN levels in the urine of male and female patients. ***P < 0.001, **P < 0.01, *P < 0.05.
Blood flow and vascular structure after DVT in SD rats
The blood flow to the lower limbs after DVT model construction was significantly lower than before the operation (Fig. 9a). The blood flow ratio before sampling to that before operation decreased significantly on days 1 and 3, particularly on day 1, and gradually increased on days 7 and 14 (Fig. 9b). When blood samples were drawn from the DVT group at four time points, dark red blocked tissue in the lumen was visible (Fig. 9c). According to HE staining of the cross-section of blood vessel on day 1, compared with the control group, the thrombus occupied the whole lumen, and the lumen and wall contained a large number of infiltrating neutrophils, which lasted until day 3. The thrombus tissue began to organize until day 14 of partial recanalization (Fig. 9d).
Evaluation of deep vein thrombosis (DVT) animal model construction and local tissue structure changes. (a) Postoperative/preoperative perfusion ratio trend chart. (b) Pre-sampling/preoperative perfusion ratio trend. (c) Hematoxylin & eosin staining results of transverse section of the inferior vena cava and thrombus tissue. (d) Images of the inferior vena cava sampled in the control and DVT groups.***P < 0.001, **P < 0.01, *P < 0.05.
GBB and L-CN levels in plasma and urine of SD rats
The overall levels of GBB (P < 0.01) and L-CN (P < 0.001) in the plasma of rats in the model group were lower than those in the control group (Fig. 10a, b). GBB (P < 0.01) and L-CN (P < 0.01) in the urine of DVT rats were still significantly lower than those in the control group (Fig. 10c, d). The level of GBB in plasma decreased significantly from day 1 (P < 0.001) and then increased on day 3, and then gradually decreased until day 7 and was maintained until day 14 (Fig. 10e), whereas the level of L-CN decreased significantly on day 1 (P < 0.001) and then increased slowly until day 14 (Fig. 10f). GBB (P < 0.01) and L-CN decreased significantly on day 1 (P < 0.01), continued to decrease until day 7 (P < 0.001), and then increased by day 14 (Fig. 10g, h) (Supplementary Materials 4).
Analysis of γ-butylbetaine (GBB) and l-carnitine (L-CN) levels in the plasma and urine of rats (a, b) Overall levels of GBB and L-CN in the plasma. (c, d) Overall levels of L-CN and GBB in the urine. (e–h) Plasma and urine levels of GBB and L-CN at different time points of deep vein thrombosis (DVT). ***P < 0.001, **P < 0.01, *P < 0.05.
Discussion
Genomics and proteomics study biological activity at gene and protein levels. Metabolomics, considered the "ultimate omics," explains molecular level cell activity14,15,16. Metabolomics is divided into non-targeted and targeted, with non-targeted metabolomics used to unbiasedly detect small molecule metabolites, whereas targeted metabolomics quantifies targeted metabolites of interest17. Recent DVT research has used metabolomics analysis of human and animal blood or tissue to explore biomarkers, including carnitine, trimethylamine-N-oxide, glucose, phenylalanine, 3-hydroxybutyric acid, lactic acid, tryptophan, and adenosine9,18,19,20,21,22. Additionally, UHPLC/MS is a sensitive and effective technique for separating molecules from biological fluids23 and can provide dynamic coverage for detecting and analyzing fluid samples. Urine is produced by the kidney from the blood to remove toxins, and it contains > 2000 endogenous metabolites23. Therefore, biomarkers in urine could facilitate screening and diagnosis.
Non-targeted detection of urine by UHPLC combined with bioinformatics analysis showed that sucrose, cysteine threonine, 5-hydroxylysine, and some organic compounds in differential metabolites were significantly upregulated, whereas trans-uric acid ester, GBB, and other organic compounds were significantly downregulated in the urine of patients with DVT compared with those in the control group. High-throughput metabolomics analysis of plasma from patients with PE showed that some biomarkers were significantly enriched in the tricarboxylic acid cycle, fatty acid metabolism (acylcarnitine), and purine metabolism9. In vivo experiments using non-targeted NMR found that the serum of the DVT group included ketone bodies, alanine, butyric acid, phenylalanine, amino acid metabolites, glycine, serine, and threonine metabolites24. Here, pathway analysis confirmed that the lysine degradation pathway is associated with DVT. In this pathway, upregulated 5-hydroxylysine and downregulated GBB significantly changed.
L-CN, known as 3-hydroxy-4-N-trimethyl butyric acid ester, is an amino acid-like compound that plays a broad role in energy metabolism within the body26. l-carnitine in humans is both endogenously synthesized and obtained exogenously through dietary intake.Endogenous l-carnitine biosynthesis involves the amino acids lysine and methionine. Lysine residues within proteins are methylated using S-adenosylmethionine (SAM) as the methyl donor. Upon proteolytic degradation, these residues undergo a series of enzymatic reactions—including hydroxylation, aldol cleavage, and aldehyde oxidation—leading to the formation of 4-trimethylaminobutyric acid (γ-butyrobetaine), which is subsequently converted into l-carnitine through the catalytic action of γ-butyrobetaine dioxygenase (BBOX1)25,26. Thus, γ-butyrobetaine is an intermediate metabolite generated during the endogenous biosynthesis of l-carnitine and serves as its immediate precursor27,28. On the other hand, l-carnitine, primarily sourced from red meat, is obtained exogenously through dietary intake28.Dietary l-carnitine is metabolized in humans in a gut microbiota-dependent manner to form γ-butyrobetaine (GBB), which is subsequently converted into trimethylamine (TMA), a precursor of trimethylamine N-oxide (TMAO)29. This metabolic pathway has been associated with an increased risk of coronary heart disease29.Although blood TMAO and gut microbe-derived metabolites, such as TML and GBB, are not associated with thrombosis in venous thromboembolism (VTE)30, GBB has been shown to prevent myocardial infarction by inhibiting l-carnitine (L-CN) synthesis, limiting long-chain fatty acid oxidation, and promoting glucose metabolism31. An increase in GBB has also been shown to attenuate the development of endothelial dysfunction, one of the key factors in high-glucose-induced deep vein thrombosis (DVT)32. Similarly, elevated levels of L-CN mitigate free fatty acid-induced and obesity-related endothelial dysfunction, which is associated with obesity33. Thus, GBB and L-CN are closely related to each other in terms of mutual transformation in cardiovascular disease. Downregulation of GBB was also observed in our non-targeted study of human urine; to further explore and validate the relationship between GBB and L-CN in DVT, we validated GBB and L-CN in the plasma and urine of humans and SD rats using UHPLC/MS.
To investigate the relationship between GBB, L-CN, and DVT, we correlated GBB and L-CN with changes in blood counts and coagulation function. Hakeshzadeh et al. demonstrated that L-CN supplementation reduced the levels of serum CRP (a marker of systemic inflammation) and plasma fibrinogen (an inflammation-associated coagulation factor) in patients who underwent hemodialysis34, increased total antioxidant capacity, and decreased IL-6 and TNF-α, which were effective in decreasing inflammatory status35. However, L-CN inhibits the accumulation of neutrophil protein kinase C receptor36, and supplementation with L-CN reduces the postoperative neutrophil–lymphocyte ratio in the short term37. In this study, L-CN was closely related to GBB in DVT, and L-CN was more correlated with leukocytes (neutrophils, lymphocytes, monocytes); we also found a positive correlation between GBB and APTT and TT. The most important biological function of L-CN is the transport of fatty acids into mitochondria for β-oxidation38, which leads to esterification of L-CN to form acylcarnitine derivatives39,40, whereas L-CN can catalyze the synthesis of l-palmitoylcarnitine by palmitoyl coenzyme A41. Reduction of GBB with L-CN may lead to impaired conversion of L-CN to acylcarnitines42, which have been shown to be anticoagulants inhibiting factor Xa by metabolomics in VTE10. Recent studies have demonstrated that l-palmitoylcarnitine inhibits thrombosis by enhancing fibrinolytic enzymes and tissue-type activators of fibrinolytic plasminogen41; in the present study, APTT and TT positively correlated with GBB; moreover, we demonstrated its correlation with the generated L-CN and coagulation pathway.
L-CN and GBB did not have the diagnostic potency of the conventional DVT marker; however, D-dimer combined with GBB and L-CN, respectively, was able to better differentiate between acute and subacute groups in DVT diagnosis, while L-CN and GBB in urine, as well as the combination of the two, could distinguish between acute and subacute DVT groups.
Finally, we found that women with DVT outnumbered men, had significantly lower concentrations of GBB in their blood samples compared with those in the control group, and were more strongly associated with L-CN, whereas there was no significant difference in L-CN. However, Liepinsh et al. showed that L-CN concentrations were 10% lower in women than in men regardless of the consumption of carnitine-rich foods (e.g., red meat)42,43,44; although slightly different from the present study, the precursor of L-CN, GBB, was expressed at lower levels in women. In contrast, in urine, both L-CN and GBB concentrations were low in men, and the relationship between the two was more correlated in female urine than in plasma. A possible exogenous cause is the higher intake of carnitine-rich foods in men, including meat, dairy products, and certain types of fish45,46. In non-vegetarians, 75% of the carnitine source is obtained from food, while the endogenous 25% relies on self-production and renal reabsorption related to the maintenance of homeostasis47. There is also a possibility that it is related to variability in absorption.
We chose the SD rat IVC stenosis-induced DVT model as it is suitable for the study of clinical thrombotic disorders48, the mechanism of which is partial stagnation of blood through incomplete ligation of the inferior renal veins of both kidneys14, which is more similar to incomplete thrombosis due to hemodynamic alterations in the clinic49. We examined the concentrations of GBB and L-CN in the plasma and urine of rats at four time points—1, 3, 7, and 14 days—and showed that the levels of GBB and L-CN in the plasma and urine of rats in the DVT group decreased significantly on the first day before gradually increasing with time. The levels of GBB and L-CN in human plasma and urine were lower in patients with DVT than those in the control group. The levels of GBB and L-CN in human plasma and urine were similar to those in rats, suggesting that humans and rats have similar GBB and L-CN expression levels.
This study identified γ-butyrobetaine (GBB) and l-carnitine (L-CN) as promising metabolic markers linked to the progression of deep vein thrombosis (DVT). Consistent alterations in their concentrations were observed across both plasma and urine samples in human subjects and animal models, and these patterns varied with different time points of thrombus formation. The integration of untargeted and targeted metabolomics, along with experimental validation, underscores the potential of GBB and L-CN as diagnostic indicators that may complement traditional markers such as D-dimer.
Building on these findings, several research directions merit further attention. Elucidating the regulatory mechanisms through which GBB and L-CN affect coagulation, vascular inflammation, and endothelial function could reveal novel biological targets. Investigating the contribution of lysine degradation and mitochondrial fatty acid oxidation in DVT-related metabolic shifts may also provide new mechanistic insights. Additionally, experimental studies involving modulation of GBB or L-CN levels in cellular or animal systems could help assess their causal roles in thrombogenesis.
From a clinical perspective, large-scale, longitudinal studies are needed to evaluate the robustness of GBB and L-CN as early biomarkers across diverse populations, considering inter-individual variability in microbiota composition, diet, and genetic background. Integrating metabolomic findings with transcriptomic or proteomic data could also support a more comprehensive view of thrombotic pathophysiology. Furthermore, exploring metabolic differences between sexes may clarify the observed gender-specific expression patterns and support personalized diagnostic or therapeutic approaches.
Altogether, the present study not only provides novel biomarker candidates for DVT but also highlights multiple pathways for further investigation, contributing to a deeper understanding and improved clinical management of venous thromboembolic disorders.
Materials and methods
Study population
A total of 171 blood and 141 urine samples were collected at our hospital. Inclusion criteria for the DVT group included: new-onset DVT diagnosed by Doppler ultrasound and D-dimer test. Exclusion criteria for DVT cases and matched controls included: active cancer, antiphospholipid syndrome, chronic inflammatory disease, personal or family history of VTE, persistent factors for pacemaker installation, pregnancy or puerperium, estrogen therapy, hospitalization for acute illness, heparin-induced thrombocytopenia, and transient factors for venous cannulation. Data related to demographics, clinical history, and hematological parameters of the study patients were collected (supplementary materials 5 and 6). All participants signed a written informed consent form (supplementary materials 7–10), and the protocol was reviewed and approved by the Institutional Ethics Committee of the First Affiliated Hospital of Hebei North University (approval no. K2020240).
Blood and urine sample collection
The study participants underwent forearm venipuncture overnight, and blood was drawn into ethylenediaminetetraacetic acid (EDTA) anticoagulation tubes. The samples were immediately transferred to a low-speed centrifuge at 3000 rpm for 10 min to obtain the supernatant. Morning urine was collected simultaneously in a vacuum tube of enzyme inhibitor protein (500 units) and then placed in a low-temperature high-speed centrifuge at 12,000 rpm for 10 min within 1 h. Aliquots of blood and urine were frozen at − 80 °C and thawed up to three times.
Non-targeted screening of potential metabolic biomarkers in human urine by UPLC–MS
Gender, age, and body mass index (BMI)-matched urine samples from 30 patients with DVT and 30 control group participants were replenished with water to 100 µl into Eppendorf tubes; then, 400 µl of extraction solution (methanol:acetonitrile = 1:1) was added before vortexing and mixing for 30 s, followed by ultrasonication for 10 min in an ice-water bath. Proteins were then precipitated by standing at − 40 °C for 1 h. After centrifugation at 13,800×g for 15 min, the supernatants were transferred to glass vials for online testing. An aliquot of supernatants from all samples was mixed to prepare quality control samples for testing. Chromatographic separation of the target compounds was carried out using an ultra-high performance liquid chromatography (UHPLC) system (Thermo Fisher, Vanquish type, USA) equipped with ACQUITY UPLC BEH Amide (2.1 × 100 mm, 1.7 um; Waters, 186004801, China) liquid chromatography column. Liquid chromatographic phase A was an aqueous phase containing 25 mmol/L ammonium acetate and 25 mmol/L ammonia; phase B was acetonitrile. The sample tray temperature was set to 4 °C, and the injection volume was 2 µl. Primary and secondary mass spectrometry data acquisition was performed on Orbitrap Exploris 120 mass spectrometer under the control of the Xcalibur data acquisition software (version 4.4, Thermo Scientific). The ESI source conditions were set as following: sheath gas flow rate of 50 arbitrary units (Arb), auxiliary gas flow rate of 15 Arb, capillary temperature of 320 °C, full MS resolution of 60,000, MS/MS resolution of 15,000, collision energy of 10/30/60 in normalized collision energy (NCE) mode, and spray voltage of 3.8 kV (positive) or − 3.4 kV (negative).
Construction of a rat inferior vena cava stenosis-induced DVT model
Rats were randomly divided into model (M, n = 20) and control (F, n = 20) groups. The groups were further divided into four subgroups (n = 5 in each subgroup of the model group and n = 5 in each subgroup of the control group): 1 day, 3 days, 7 days, and 14 days. As previously described14 with slight modification, DVT was induced by incomplete ligation of both infrarenal veins leading to partial stagnation of blood. In brief, after 8 h of preoperative water and food fasting, adult male Sprague–Dawley (SD) rats weighing 275 ± 25 g (purchased from Spearfish, China) were sedated in an induction chamber with isoflurane (oxygen flow rate of 3 L/min, 5 mL/kg) and then injected with an intramuscular injection of 1% pentobarbital (Wampanoag, P11011, Germany) at a concentration of 0.5 mL/100 g, and then observed for 3–5 min until the rats entered deep anesthesia. Subsequently, their limbs were fixed on the operating table in a supine position. The lower abdomen was sterilized, and a 2-cm incision was made along the midline of the abdomen. A moistened cotton ball was used to pivot the viscera to the periphery of the abdominal cavity to expose the inferior vena cava (IVC), the abdominal aorta, and the right and left iliolumbar veins. Next, a 30-gauge needle was inserted parallel to the IVC distal to the left renal vein, and the needle and vein were ligated simultaneously with a 7–0 suture and then carefully removed. The bilateral iliolumbar veins were then ligated with 7–0 sutures, along with the dorsal refluxing veins of the inferior vena cava and visualization of the refluxing inferior vena cava side branches. At the end of the operation, the viscera were returned to their place, and the abdominal muscles and skin were sutured together. After modeling, the rats continued to be housed in a Specific Pathogen-Free (SPF) animal house with unrestricted movement upon awakening. The sham-operated group underwent the same procedure except the vein ligation. All animal experiments performed in this study were approved by the relevant ethics committees, and all experimental procedures complied with local laws and ethical standards. The animal experiments were based on ethical considerations and integrity-based assumptions, and the experimental protocols were approved by the Institutional Animal Ethics Committee of the First Hospital Affiliated to Hebei North College, following the guidelines of the Committee for the Control and Supervision of Animal Experiments.
Evaluation of DVT model construction and sample collection
Ligature stenosis was determined by laser Doppler flowmetry of the femoral veins of both lower limbs of rats before modelling but after deep anesthesia, within 3–5 min post-surgery but before sampling. At four specific time points of 1, 3, 7, and 14 days, the rats were anaesthetized again; the viscera and other tissues were freed by laparotomy, and urine was collected by cystocentesis. Blood was collected through the portal vein in EDTA anticoagulant tubes and then centrifuged for 10 min at 3,000 rpm. The resulting plasma aliquots were frozen in a refrigerator at − 80 °C until further measurements. Next, the tissue at the ligation site was cautiously freed to expose the IVC; after removal of the IVC and thrombus tissue, a part of it was rapidly frozen in liquid nitrogen and another part was fixed in 4% formaldehyde.
Hematoxylin and eosin staining
The specimens were fixed using gradient ethanol solution, sequentially immersed for dehydration, cleared in xylene, and finally embedded in paraffin. The tissue was cut into 5-µm thick sections, attached to glass microscope slides, placed in an oven for 30 min at 60 °C, dewaxed by xylene immersion, and rehydrated by alcohol immersion. After hematoxylin staining, the sections were rinsed slowly under tap water until they turned blue. They were then discolored using an ethanolic hydrochloric acid solution and rinsed again slowly under tap water until they regained their blue color. Eosin staining was performed for 3 min, and samples were briefly rinsed under running water before being subjected to an ethanol dehydration gradient. Transparent xylene immersion was applied to neutral gum for sealing and preservation. Histomorphology was performed using a microscope, and photographs and were captured and recorded.
Pre-treatment of human and rat serum and urine samples
Concentrations of L-CN and GBB in plasma and urine were determined in humans and rats using UHPLC/MS as previously described with minor modifications. Plasma and urine samples stored at − 80 °C were first thawed at 4 °C before sample preparation. Urine was vortexed and then diluted five times with phosphate-buffered saline before proceeding to the next step. The urine and plasma samples were vortexed for 10 s. For protein precipitation and metabolite extraction, 25 µl of plasma and diluted urine were added to 1.5 ml Eppendorf tubes, along with 225 µl of methanol and 10 µl of internal standard (IS) of both substances. The mixture was shaken for 5 min for thorough mixing and centrifuged at 14,000 rpm for 10 min at 4 °C, and the supernatants were directly transferred into specific glass vials until assayed. However, the plasma supernatant was transferred to a new Eppendorf tube, 10 ul of it was removed, and 90 ul of the previously configured precipitation reagent was added again. Finally, 100 ul of the sample was transferred to a specific glass vial for UHPLC/MS analysis.
Measurement of L-CN and GBB
The compounds were separated using triple quadrupole liquid chromatography mass spectrometer (LC–MS, AB Sciex, QTRAPTM 4500, China), with the mass spectrometer set to positive electrospray ionization mode and predetermined multiple reaction monitoring (MRM) scans. L-CN and GBB concentrations were quantified using D9-l-carnitine and D9-γ-butylbetaine as IS, and both were diluted to 200 ppb by adding 50% methanol in water to the master solution, which was stored at − 20 °C; fresh solutions were used for each sample sequence. Standard curves were prepared using plasma and urine-containing standards of both substances (100–50,000 ng/ml for L-CN and 5–2000 ng/ml for GBB); those not in the linear range were re-diluted according to the specifics, and retention time for each analyte was determined by authentic standards. L-CN and GBB analyses were performed in MRM (Table 4), and each prepared sample was injected into ACQUITY HPLC BEH HILIC (1.7 um, 2.1 × 100 mm) column (Waters, 186003461, China) at a column temperature of 40 °C for dissociation, and chromatography was performed using a gradient mobile phase consisting of water and 20 mM of ammonium formate (phase A) and acetonitrile (phase B) (Table 5). Laboratory personnel performing LC–MS/MS analyses were blind to sample set assignments and clinical data during the analysis. Data were extracted and processed using the MultiQuant MD 3.0.3 software (AB Sciex, China).
Data processing and statistical analysis
Categorical variables were expressed as frequencies and percentages, whereas continuous variables were expressed as either mean ± standard deviation (SD) or median and interquartile range. Differences in metabolites between the DVT and control groups were assessed using the independent samples t-test and Mann–Whitney U test, and the chi-square test was used to compare categorical data between groups. One-way analysis of variance (ANOVA) was used to test continuous variables between multiple groups, and the least significant difference (LSD) was used for post hoc multiple comparisons. The Kruskal–Wallis test was used to compare continuous variables between multiple groups that were not normally distributed. Correlations between continuous variables were analyzed using Pearson correlation analysis. Statistical software IBM SPSS Statistics v25 and GraphPad Prism 9.0 were used to perform all statistical analyses, and P < 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Khan, F., Tritschler, T., Kahn, S. R. & Rodger, M. A. Venous thromboembolism. Lancet 398, 64–77 (2021).
Heit, J. A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 12, 464–474 (2015).
Duffett, L. Deep venous thrombosis. Ann. Intern. Med. 175, ITC129–ITC144 (2022).
Navarrete, S. et al. Pathophysiology of deep vein thrombosis. Clin. Exp. Med. 23, 645–654 (2023).
Mazzolai, L. et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur. Heart J. 39, 4208–4218 (2018).
Anghel, L., Sascău, R., Radu, R. & Stătescu, C. From classical laboratory parameters to novel biomarkers for the diagnosis of venous thrombosis. Int. J. Mol. Sci. 21, 1920 (2020).
Albreht, A. et al. Structure elucidation and mitigation of endogenous interferences in LC–MS-based metabolic profiling of urine. Anal. Chem. 94, 1760–1768 (2022).
Alseekh, S. et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 18, 747–756 (2021).
Bujak, R. et al. Metabolomics reveals metabolite changes in acute pulmonary embolism. J. Proteome Res. 13, 805–816 (2014).
Deguchi, H. et al. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood 126, 1595–1600 (2015).
Jiang, X. et al. Metabolites associated with the risk of incident venous thromboembolism: A metabolomic analysis. J. Am. Heart Assoc. 7, e010317 (2018).
Fraser, K. et al. Plasma biomarkers and identification of resilient metabolic disruptions in patients with venous thromboembolism using a metabolic systems approach. Arterioscler. Thromb. Vasc. Biol. 40, 2527–2538 (2020).
Khamis, M. M., Adamko, D. J. & El-Aneed, A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev. 36, 115–134 (2017).
Liu, H. et al. Inferior vena cava stenosis-induced deep vein thrombosis is influenced by multiple factors in rats. Biomed. Pharmacother. 128, 110270 (2020).
Tynkkynen, T. et al. Proof of concept for quantitative urine NMR metabolomics pipeline for large-scale epidemiology and genetics. Int. J. Epidemiol. 48, 978–993 (2019).
Johnson, C. H., Ivanisevic, J. & Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459 (2016).
Rinschen, M. M., Ivanisevic, J., Giera, M. & Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 20, 353–367 (2019).
Zhou, J. et al. Comparison of widely targeted metabolomics and untargeted metabolomics of wild Ophiocordyceps sinensis. Molecules 27, 3645 (2022).
Franczyk, B., Gluba-Brzózka, A., Ławiński, J., Rysz-Górzyńska, M. & Rysz, J. Metabolomic profile in venous thromboembolism (VTE). Metabolites 11, 495 (2021).
Zeleznik, O. A. et al. Metabolomic analysis of 92 pulmonary embolism patients from a nested case-control study identifies metabolites associated with adverse clinical outcomes. J. Thromb. Haemost. 16, 500–507 (2018).
Voils, S. A., Shahin, M. H., Garrett, T. J. & Frye, R. F. Metabolomic association between venous thromboembolism in critically ill trauma patients and kynurenine pathway of tryptophan metabolism. Thromb. Res. 165, 6–13 (2018).
Luo, P. et al. Large-scale genetic correlation scanning and causal association between deep vein thrombosis and human blood metabolites. Sci. Rep. 12, 7888 (2022).
López-Ruiz, R., Romero-González, R. & Garrido Frenich, A. G. Ultrahigh-pressure liquid chromatography-mass spectrometry: An overview of the last decade. Trends Anal. Chem. 118, 170–181 (2019).
Cao, J. et al. Comparison of the serum metabolic signatures based on (1)H NMR between patients and a rat model of deep vein thrombosis. Sci. Rep. 8, 7837 (2018).
Adeva-Andany, M. M., Calvo-Castro, I., Fernández-Fernández, C., Donapetry-García, C. & Pedre-Piñeiro, A. M. Significance of l-carnitine for human health. IUBMB Life 69, 578–594 (2017).
Strijbis, K., Vaz, F. M. & Distel, B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life 62, 357–362 (2010).
Englard, S. Hydroxylation of gamma-butyrobetaine to carnitine in human and monkey tissues. FEBS Lett. 102, 297–300 (1979).
Fujita, M. et al. Hepatic uptake of gamma-butyrobetaine, a precursor of carnitine biosynthesis, in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G681–G686 (2009).
Buffa, J. A. et al. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota L-carnitine catabolism. Nat. Microbiol. 7, 73–86 (2022).
Canyelles, M. et al. TMAO and gut microbial-derived metabolites TML and gammaBB are not associated with thrombotic risk in patients with venous thromboembolism. J. Clin. Med. 11, 1425 (2022).
Liepinsh, E. et al. Inhibition of L-carnitine biosynthesis and transport by methyl-gamma-butyrobetaine decreases fatty acid oxidation and protects against myocardial infarction. Br. J. Pharmacol. 172, 1319–1332 (2015).
Vilskersts, R. et al. Elevated vascular γ-butyrobetaine levels attenuate the development of high glucose-induced endothelial dysfunction. Clin. Exp. Pharmacol. Physiol. 40, 518–524 (2013).
Shankar, S. S., Mirzamohammadi, B., Walsh, J. P. & Steinberg, H. O. L-carnitine may attenuate free fatty acid-induced endothelial dysfunction. Ann. N. Y. Acad. Sci. 1033, 189–197 (2004).
Hakeshzadeh, F., Tabibi, H., Ahmadinejad, M., Malakoutian, T. & Hedayati, M. Effects of L-carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren. Fail. 32, 1109–1114 (2010).
Fathizadeh, H. et al. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 19, 1879–1894 (2020).
Derin, N., Agac, A., Bayram, Z., Asar, M. & Izgut-Uysal, V. N. Effects of L-carnitine on neutrophil-mediated ischemia-reperfusion injury in rat stomach. Cell Biochem. Funct. 24, 437–442 (2006).
Aldemir, M. et al. L-carnitine supplementation reduces short-term neutrophil-lymphocyte ratio in patients undergoing coronary artery bypass grafting. Int. Surg. 100, 1160–1168 (2015).
Almannai, M., Alfadhel, M. & El-Hattab, A. W. Carnitine inborn errors of metabolism. Molecules 24, 3251 (2019).
Reuter, S. E. & Evans, A. M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 51, 553–572 (2012).
McCann, M. R., George De la Rosa, M. V., Rosania, G. R. & Stringer, K. A. L-Carnitine and acylcarnitines: Mitochondrial biomarkers for precision medicine. Metabolites 11, 51 (2011).
Yang, J. et al. l-Palmitoylcarnitine potentiates plasmin and tPA to inhibit thrombosis. Nat. Prod. Bioprospect. 13, 48 (2023).
Mansour, F. R., Wei, W. & Danielson, N. D. Separation of carnitine and acylcarnitines in biological samples: A review. Biomed. Chromatogr. 27, 1339–1353 (2013).
Cederblad, G. Plasma carnitine and body composition. Clin. Chim. Acta 67, 207–212 (1976).
Liepinsh, E. et al. Mildronate treatment alters gamma-butyrobetaine and l-carnitine concentrations in healthy volunteers. J. Pharm. Pharmacol. 63, 1195–1201 (2021).
Liu, T. et al. The association of serum L-carnitine concentrations with the risk of cancer in Chinese adults with hypertension. Nutrients 14, 4999 (2022).
Koeth, R. A. et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Invest. 129, 373–387 (2019).
Liu, T. et al. Carnitine and depression. Front. Nutr. 9, 853058 (2022).
Obi, A. T. et al. Experimental venous thrombus resolution is driven by IL-6 mediated monocyte actions. Sci. Rep. 13, 3253 (2023).
Diaz, J. A. et al. Critical review of mouse models of venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 32, 556–562 (2023).
Acknowledgements
We thank The First Affiliated of Hebei North University for approving the study. This work was supported by the Science and Technology Winter Olympics Special (20110017D).
Funding
This work was supported by Science and Technology Winter Olympics special (20110017D).
Author information
Authors and Affiliations
Contributions
Wenjie Zeng: Conceptualization, Methodology. Qu Ming: Funding acquisition, Conceptualization, Methodology. Wenjie Zeng and Yangyang Gao: Formal analysis, Investigation, Writing—original draft, Writing—review and editing. Qitao Wang, Junyu Chi, Zhicong Yang, Jingqi Li, Zhen Wang, and Xin Li: Investigation. Funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical standards
The experimental operation was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Hebei North University (Approval No.K2020240). The animal experiments were in accordance with the statement on animal Ethics Review of Hebei North University (Approval No.HBNU2023012006). Furthermore, this study adheres to the recommendations of the ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments. All animal experiments performed in this study were approved by the relevant ethics committees, and all experimental procedures comply with local laws and ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, W., Wang, Q., Li, X. et al. Diagnosis of deep vein thrombosis from γ-butylbetaine and l-carnitine in plasma and urine based on untargeted and targeted metabolomics. Sci Rep 15, 22940 (2025). https://doi.org/10.1038/s41598-025-06054-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06054-1