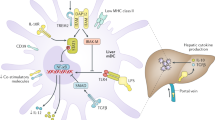
Abstract
The roles of donor liver natural killer (NK) cells in allogeneic liver transplantation remains controversial. Herein, we investigated the effects of liver NK cells on liver allograft tolerance by using liver NK cells and allogeneic spleen T cell co-culture in vitro experiments and the rat orthotopic liver transplantation model. CD8+ T cells exhibited higher apoptotic rate and up-regulated expression of CD95 molecules when co-cultured with allogeneic liver NK cells in vitro, indicating that liver NK cells might induce the apoptosis of CD8+ T cells via CD95 in vitro. The rat liver transplant model showed that recipients with donor liver NK cell immunity, had better survival, less damage, and higher apoptotic rate of intrahepatic infiltrating lymphocytes in liver grafts, as well as higher level of peripheral CD4+CD25+ T cells and lower level of peripheral CD3+CD8+ T cells. These findings indicate that donor liver NK cells can ameliorate allograft rejection via inducing the CD95-mediated apoptosis of alloreactive CD8+ T cells and the generating of CD4+CD25+ regulatory T cells.
Similar content being viewed by others
Introduction
The human liver contains a significant population of lymphocytes, consisting of various types such as memory T (TRM) cells, natural killer T (NKT) cells, natural killer (NK) cells, innate lymphoid cells (ILCs), and γδT cells1. These lymphocytes present in the liver differ significantly from those found in the peripheral blood. In the liver, the CD8+/CD4+ ratio is notably higher compared to peripheral blood2,3. Among the liver-resident lymphocytes, NK cells are the predominant subset, accounting for 30–40% of all lymphocytes, while they make up only 2–18% of lymphocytes in the peripheral blood4. These hepatic NK cells were initially identified as “pit cells” due to their appearance as large granular lymphocytes and possess potent cytolytic function5. It is widely acknowledged that hepatic NK cells exhibit distinctive features compared to peripheral NK cells, including differences in surface marker expression, cytokine profiles, and cytotoxic potential5,6. However, research on the roles of donor-derived hepatic NK cells in liver transplantation remains limited. Determining whether these donor-derived hepatic NK cells contribute to liver tolerance or allograft rejection has proven challenging7.
Recent studies have demonstrated that donor-derived hepatic NK cells can be detected in the recipient’s peripheral circulation approximately two weeks after liver transplantation8. This finding suggests that these NK cells have the ability to migrate from the transplanted liver into the recipient’s peripheral circulation and survive in the new environment. In a previous study conducted on a rat model, it was reported that donor-derived hepatic NK cells did not have any impact on liver transplant tolerance or allograft rejection9. But our previous research shows that while donor-derived hepatic NK cells do not contribute to liver tolerance, they can actually alleviate acute allograft rejection in rat liver transplantation10. The underlying mechanisms by how the donor-derived hepatic NK cells ameliorated liver allograft rejection remain obscure.
T cells are pivotal immune effector cells in the adaptive immune system, and their role is crucial in liver transplantation. During liver transplantation, recipient T cells are responsible for identifying and recognizing the non-self donor alloantigens. This recognition triggers the proliferation and activation of T cells within lymphoid tissue. Once activated, these T cells become key drivers of hepatic rejection through multiple mechanisms. They can directly cause damage through T-cell mediated cytotoxicity, leading to tissue injury. Additionally, activated T cells secrete cytokines that further contribute to the inflammatory response, and they recruit other inflammatory cells that can contribute to tissue destruction11. Therefore, the activated T cells play a central role in orchestrating the process of hepatic rejection in liver transplantation.
It is widely recognized that natural killer (NK) cells serve as modulators of T cell immunity by regulating the activation and differentiation of T cells. NK cells have the ability to interact with T cells through direct cell-to-cell contact and the secretion of soluble factors12,13. NK cells can promote T cell activation by providing co-stimulatory signals or by releasing cytokines that enhance T cell proliferation and effector functions. They can also contribute to the formation of immunological synapses between NK cells and T cells, facilitating communication and T cell activation14. On the other hand, NK cells can exert regulatory functions by suppressing excessive T cell activation and dampening immune responses. They can inhibit T cell proliferation and cytokine production through the release of inhibitory factors or by direct cytotoxicity towards activated T cells15. The intricate interplay between NK cells and T cells highlights the important role of NK cells in modulating the adaptive immune response, but little is known about whether donor-derived hepatic NK cells have effects on the recipient’s alloreactive T lymphocytes. Recently, Cuff et al. has provided important insights into the behavior of donor-derived hepatic NK cells in liver allografts16. our study aims to investigate the specific roles of donor-derived hepatic NK cells in liver allograft tolerance or rejection. We have utilized cell co-culture experiments and a rat liver transplantation model to gain a better understanding of the mechanisms underlying liver allograft tolerance. By studying the interactions between donor-derived hepatic NK cells and other immune cells, we hope to elucidate their impact on the immune response and shed light on the complex processes involved in liver allograft tolerance. The study is reported in accordance with ARRIVE guidelines.
Materials and methods
Ethical approval and animal
All experimental protocols were approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University(Approval number: IACUC-2022081501).
The study was performed according to the international, national and institutional rules considering animal experiments, clinical studies and biodiversity rights.
For the orthotopic liver transplantation (OLT) model in this study, male brown Norway (BN; RT1n) rats and Lewis (LEW; RT1l) rats weighing 180–220 g were purchased from Beijing Vital-River Laboratory Animal Technology Company. The rats were housed in a specific pathogen-free facility located in the Laboratory Animal Center of Sun Yat-Sen University. The animal care and experimental procedures were conducted in accordance with the guidelines approved by the Science and Technology Animal Care and Use Committee of Sun Yat-Sen University, under the protocol number SYXK (Guangdong) 2007-0081.
All surgical procedures were performed with the rats under sodium pentobarbital anesthesia to ensure minimal suffering. Every effort was made to minimize the discomfort and distress experienced by the animals throughout the experiment.
The BN and LEW rats were randomly divided into three groups based on the treatments administered to the donor or recipient. Each experimental group was further subdivided into four subgroups. These subgroups corresponded to different observation time points, namely post-transplant day 1, 3, 7, and the recipients’ natural survival time. This division allowed for the evaluation of the study parameters at different stages following transplantation. Rats were euthanized by CO2 asphyxiation after reaching 100 days of follow-up.
Preparation of hepatic NK cells and allogenic spleen T cell subsets
Hepatic NK cells were isolated as previously described17. Briefly, the livers of BN rats were perfused with a warm digestive mixture consisting of 0.05% collagenase IV and 500 U/ml DNase I (Sigma, St. Louis, MO, USA) through the portal vein. The liver tissue was then mashed using a 100 μm cell strainer, resulting in a cell suspension containing both hepatic parenchymal cells and nonparenchymal cells (NPCs). The parenchymal cells were separated by low-speed centrifugation at 300 rpm for 10 min. Subsequently, Ficoll density gradient centrifugation at 1500 rpm for 25 min was performed to eliminate erythrocytes, granulocytes, and cell debris. The mononuclear cells recovered from the interface of the Ficoll-Paque gradient were enriched in hepatic NK cells. To further purify the hepatic mononuclear cells, a Dynabeads FlowComp™ Flexi system (Invitrogen, Waltham, MA, USA) was utilized. Ultimately, CD3−NKR-P1+ hepatic NK cells with a purity of ≥ 90% were obtained and resuspended.
For the isolation of T cells, the spleen was mashed through a 100 μm cell strainer. Similar to the process described above, Ficoll density gradient centrifugation was performed to remove erythrocytes, granulocytes, and cell debris. The resulting mononuclear cells, enriched in T cells, were purified using the Dynabeads FlowComp™ Flexi system. Ultimately, purified CD4+ and CD8+ T lymphocytes were obtained and resuspended. Both CD4+ and CD8+ T cell populations had a purity of ≥ 90%. The viability of the isolated cells was assessed using the trypan blue dye exclusion test, ensuring a trypan blue exclusion rate of ≥ 95%.
Lymphocytes co-culture
The purified BN rat hepatic NK cells and purified Lewis rat spleen CD4+ T/CD8+ T cells were mixed in a 1:1 ratio. As controls, an equal number of CD4+/CD8+ T cells were cultured alone. No cytokines were added to stimulate lymphocyte proliferation during the experiment. The co-cultures and control T cell cultures were maintained for specific time points of 6 h, 12 h, and 24 h.
After the designated co-culture period, the resulting CD4+/CD8+ T lymphocytes and their culture supernatant were collected for further analysis in subsequent experiments. This process was repeated three times to ensure reliable and reproducible results.
Detection of T cell apoptosis
The resultant T cell subsets (CD4+ and CD8+) were collected and fixed with 70% pre-cooled ethanol at 4 ℃ overnight. Subsequently, the fixed T cell subsets were incubated with annexin V-Alexa Fluor 647 and propidium iodide (PI) at 4 ℃ for 15 min. Annexin V binds to phosphatidylserine, which is exposed on the outer membrane of apoptotic cells, while PI stains the DNA of cells with compromised membrane integrity.
The apoptosis of the CD4+/CD8+ T lymphocytes was then detected using the Beckman Coulter Epics Altra flow cytometer (Brea, CA, USA). The flow cytometry data were collected and processed using the FlowJo fluorescent-activated cell sorting (FACS) analysis software (Version 10.8.1, Tree Star, Inc., Ashland, OR, USA). For the analysis of T cell apoptosis, a two-color dot plot of annexin V−Alexa Fluor 647 and PI was generated. Both PI+/annexin V+ and PI-/annexin V+ T cells were considered apoptotic T cells.
Before the apoptotic detection, the CD4+/CD8+ T cells and hepatic NK cells were distinguished by using a fluorescein isothiocyanate (FITC)-conjugated CD4/CD8 probe. This allowed for the separation and identification of the different cell populations during analysis using the FACS Calibur system.
Detection of CD95 expression
After fixation with 70% pre-cooled ethanol at 4 ℃ overnight, the cells were washed and incubated with phycoerythrin (PE)-conjugated CD95 mouse anti-rat monoclonal antibody (Becton Dickinson Company, Franklin Lakes, NJ, USA) for 45 min at 4℃. The CD95 expression on the T cell subsets was detected using the FACS Calibur system (Beckman Coulter, Epics Altra, Brea, CA, USA). The flow cytometry data were collected and processed using the FlowJo FACS analysis software (Tree Star, Inc., Ashland, OR, USA).
To minimize non-specific binding, all samples were incubated with an Fc-blocking antibody before staining. This step helped prevent the binding of antibodies to Fc receptors on the cells, reducing background staining and improving the specificity of the CD95 detection.
Depletion of donor liver leukocytes
To deplete hepatic leukocytes in the donor rats, they were subjected to whole-body irradiation (WBI) using a 60C0 source at 0.6 Gy/min to deplete the hepatic leukocytes as previous described10. The donor rats were placed in a translucent-ventilation plastic box and exposed to a total radiation dose of 10 Gy. The irradiation was administered at a rate of 0.6 Gy/min. This procedure has been previously described in the literature.
Following WBI, the levels of peripheral leukocytes in the donor rats were monitored. If the leukocyte levels were reduced by 99%, indicating successful depletion of hepatic leukocytes, the liver would be considered suitable for transplantation into the appropriate recipients. This monitoring step ensures that the desired level of leukocyte depletion is achieved before proceeding with the liver transplantation procedure.
Orthotopic liver transplantation (OLT) and post-transplant transfusion of purified donor-type liver NK cells (dtlNKs)
OLT was performed with a modified two-cuff technique as previous described18. The liver allograft combinations were randomly divided into 3 groups based on the donor or recipient treatments as shows in Table 1: A, BN-LEW; B, BN(WBI)-LEW; C, BN(WBI)-LEW (dtlNKs transfusion). At least 6 pairs of rats were used for each time point, including post-transplant day 1, 3, 7(6–8 pairs of rats per subgroup), and the recipients’ natural survival time(8–12 pairs of rats per subgroup). For the tolerant recipients, post-transplant day 100 was considered the natural survival time.
After the grafting of the irradiated liver, once full restoration of blood perfusion in the liver graft was confirmed, dtlNK (donor-derived hepatic NK cell) suspensions containing 3 × 106 NK cells were transfused into the recipient through the portal vein. This transfusion aimed to introduce the donor-derived hepatic NK cells into the recipient’s liver and assess their effects on liver allograft tolerance or rejection.
Liver allograft function measurement
Serum markers related to liver function such as alkaline phosphatase (ALP), bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST), were measured using an automatic biochemical analyzer (Beckman Coulter, Epics Altra, Brea, CA, USA).
Phenotypic analysis of recipients’ peripheral T cells
Blood samples obtained from the recipients were incubated with the mouse anti-rat monoclonal antibodies, FITC-CD3, peridinin-chlorophyll-protein complex (PerCP)-CD3, FITC-CD4, PE-CD4, PE-CD8, PE-CD25 (eBioscience and Becton Dickinson Company, USA) for 45 min at 4℃, respectively. The phenotypic analysis of T cells in the blood samples was performed using the FACS Calibur system from Beckman Coulter (Epics Altra, Brea, CA, USA). The acquired data were then processed using the FlowJo FACS analysis software developed by Tree Star, Inc. (Ashland, OR, USA). All samples were incubated with an Fc-blocking antibody before staining.
cytokine analysis of the culture supernatant and the liver graft extracts
The liver extracts were treated with a protease inhibitor cocktail according to the manufacturer’s instructions (Sigma, St. Louis, MO, USA). The concentrations of Interleukin-2 (IL-2), Interleukin-2 (IL-4), and Interleukin-10 (IL-10) in the culture supernatant and IL-2, Interferon gamma (IFN-γ), IL-4, and IL-10 levels in the recipients’ liver graft extracts were measured using enzyme-linked immunosorbent assay (ELISA) immunoassay kits (Invitrogen, Waltham, MA, USA) and a microplate reader (450 nm, Thermo Scientific, Waltham, MA, USA).
histopathological analysis of liver grafts
The liver graft specimens were first fixed with a 10% formalin solution to preserve their structure and components. After fixation, the specimens underwent a series of steps, including dehydration, paraffin embedding, sectioning, and staining with hematoxylin and eosin (H&E). All liver grafts harvested on days 1, 3, and 7 after transplantation were evaluated semi-quantitatively by three pathologists who were blinded to the experiment. The liver allograft acute rejection was graded according to the criteria of the Banff score19.
Terminal deoxynucleotide transferase-mediated dUTP-biotin Nick end labelling method (TUNEL)
The apoptosis of infiltrating lymphocytes was detected using TUNEL according to the instructions of Roche’s TUNEL Kit (In Situ Cell Death Detection Kit, POD, Basel, Switzerland). Briefly, the paraffin-embedded liver tissue from the liver allografts was sectioned into 5-µm thickness, dewaxed, fixed, and underwent antigen repair. The liver allograft sections were stained with the TUNEL reaction mixture. T cell apoptosis was analyzed using TUNEL positive staining of infiltrating lymphocytes in the portal of the liver graft.
Immunohistochemistry
The paraffin-embedded tissue from liver allografts was sectioned into 5-µm thickness. After dewaxing, hydrazination, and antigen repair, the tissue sections were incubated with mouse anti-rat CD95 monoclonal antibody for 2 h at 37 °C. After washing, sections were incubated with the goat anti-mouse secondary antibody labelled with streptavidin/horseradish peroxide (S-A/HRP) at 37 °C for 30 min. 3,3-diaminobenzidine (DAB) was used for visualization. The CD95 expression of T lymphocytes was analyzed using CD95 positive staining of infiltrating lymphocytes in the portal area of the liver graft.
Statistical analysis
All the collected data were subjected to statistical analysis using IBM SPSS Statistics 22.0 software (IBM, Armonk, NY, USA), R version 3.6.2 (www.r-project.org) and R software (version 4.1.3; https://www.r-project.org/). For the survival analysis, which involved data from long-term survival subgroups, the Kaplan-Meier method was used to estimate overall survival of recipient rats in each group and log-rank test were utilized to compare survival differences in different groups. The chi-square test and analysis of variance (ANOVA) were employed for appropriate data analysis. A p-value of less than 0.05 was considered statistically significant, indicating a significant difference or association. The data are presented as the mean ± standard deviation (SD), providing information on both the average value and the variability of the data.
Results
Liver NK cells promote apoptosis of allogeneic spleen CD8+ T cells via CD95 in vitro
To examine the impact of hepatic NK cells on allogeneic spleen T cells, we conducted in vitro co-culture experiments by combining hepatic NK cells with allogeneic spleen CD4+ and CD8+ T cells separately. FACS results revealed notable findings. Specifically, the apoptosis rate of CD4+ T cells in the liver NK cell co-culture group was significantly lower compared to the control group after 24 h (Fig. 1A). Conversely, the apoptosis rate of CD8+ T cells in the liver NK cell co-culture group was significantly higher than that observed in the control group after 24 h (Fig. 1B). These results suggest that hepatic NK cells have a beneficial effect on the survival of allogeneic spleen CD4+ T cells while inducing apoptosis in allogeneic spleen CD8+ T cells.
Determination of apoptosis and CD95 expression in CD4+ and CD8+ T cells co-culture with NK cells. (A and B) Representative flow cytometry plots using Annexin V-FITC/PI staining for apoptosis in in CD4+ (A) and CD8+ (B) T cells. (C and D) Flow analyses of CD95 expression in CD4+ (C) and CD8+ (D) T cells. *: P < 0.5; two-tailed Student’s t-test.
To investigate the mechanism by which hepatic NK cells induce apoptosis in CD8+ T cells, we focused on the CD95 protein, which is a well-known death receptor20. We utilized flow cytometry to assess the expression level of CD95 in allogeneic CD8+ T cells in the presence of hepatic NK cells. Notably, the results showed that hepatic NK cells significantly increased the expression of CD95 in allogeneic CD8+ T cells (Fig. 1C). However, no significant effect on CD95 expression was observed in allogeneic CD4+ T cells afer co-cultured with hepatic NK cells (Fig. 1D). Taken together, these data indicate that hepatic NK cells induce apoptosis in allogeneic spleen CD8+ T cells through the CD95-mediated pathway in the in vitro experimental setting.
Liver NK cells alter cytokine secretion in co-culture media
To further investigate the immunomodulatory role of hepatic NK cells on T cells, we measured the levels of IL-2, IL-4, and IL-10 in the culture supernatant using ELISA. IL-2 is a key cellular immune inducer21, while IL-4 and IL-10 are known cellular immunosuppressive factors22,23. Our results demonstrated that the presence of hepatic NK cells in the co-culture significantly decreased the levels of IL-2 in the culture supernatant compared to the control group (Fig. 2A). Conversely, the levels of IL-4 and IL-10 were significantly higher in the co-culture group compared to the control group (Fig. 2B). These findings suggest that the co-culture of hepatic NK cells with T cells induced the expression of immunosuppressive factors (IL-4 and IL-10) while decreasing the expression of the cellular immune inducer (IL-2).
Donor liver NK cells ameliorate allograft rejection in vivo
To further explore the effects of NK cells on liver allograft tolerance or rejection, OLT model was employed. LEW recipients received BN rat liver grafts and were randomly divided into three groups: Group A, Group B, and Group C. In Group B and Group C, the donor liver leukocytes were depleted through WBI. LEW recipients in Group C were additionally infused with dtlNKs. The survival outcomes of the recipient rats in each group are summarized in Table 2. Kaplan-Meier curves for survival of recipient rats in each group are showed in Fig. 3A. The results showed that LEW recipients in Group A had significantly prolonged survival, with recipients surviving over 100 days after allogeneic liver transplantation (LTx). This survival time was significantly longer compared to both Group B and Group C. In Group B, where donor liver leukocytes were depleted, the recipients had a shortened survival time. However, in Group C, where dtlNKs cells were infused into recipients whose transplanted livers underwent leukocyte depletion, the survival time was significantly prolonged compared to Group B. These findings suggest that the depletion of donor liver leukocytes significantly shortened the survival time of the recipients after liver transplantation. On the other hand, the.
Liver function tests and histological analysis in the recipients’ serum. (A)Kaplan-Meier curves for survival of recipient rats in each group. (B-E) The serum levels of ALP (B), bilirubin (C), ALT (D) and AST (E) were measured by ELISA. *: P < 0.05. (F) Representative hematoxylin-eosin images of the liver grafts on day 7 post-transplantation liver (original magnification, ×100). a-c: Representative liver sections of groups A, B and C. (G) The Banff Score of acute liver allograft rejection for liver grafts on days 1, 3 and 7 post-transplantation liver. 9 rats were used in each group. *: P < 0.5; two-tailed Student’s t-test.
infusion of dtlNK cells, which are donor liver NK cells, had a beneficial effect on prolonging.
the recipients’ lifespan after liver transplantation.
Next, we detected the serum levels of ALP, bilirubin, AST, and ALT to test the liver function in recipients. The results showed that in the group where donor liver leukocytes were depleted (Group B), the serum levels of ALP, bilirubin, AST, and ALT were significantly higher compared to the non-treatment group (Group A). However, in Group C, where dtlNK cells were infused into recipients whose transplanted livers underwent leukocyte depletion, the elevated levels of ALP and bilirubin were significantly reduced compared to Group B (Fig. 3B and C). Furthermore, the serum levels of AST and ALT in Group C were comparable to the control group (Group A) (Fig. 3D and E). This suggests that the infusion of dtlNK cells attenuated hepatocellular damage in the liver transplant recipients, as evidenced by the normalization of AST and ALT levels.
On day 7 post-transplantation, the histological analysis of liver grafts revealed that moderate allograft rejection was rarely observed in Group A, the non-treatment group. However, in the group where donor liver leukocytes were depleted (Group B), moderate to severe allograft rejection was frequently detected (Fig. 3F). Interestingly, in Group C, the inflammation caused by acute rejection in the liver grafts was significantly ameliorated. The assessment of allograft rejection was further evaluated using the Banff rejection activity index (RAI), which considers three components: venous endothelial inflammation, bile duct damage, and portal inflammation19. On day 7 post-transplantation, the RAI score in Group A was comparable to that in Group C, while it was highest in Group B (Fig. 3G). These findings, supported by the hematoxylin-eosin images and RAI score, suggest that the infusion of donor liver NK cells (Group C) ameliorated the allograft rejection, as evidenced by reduced inflammation compared to the leukocyte-depleted group (Group B).
Donor liver NK cells change T lymphocyte populations in peripheral blood
CD25 (IL-2 receptor alpha-chain) marks a population of CD4+ T cells that suppress allograft rejection after liver transplantation24. Here, we examined the proportion of CD4+CD25+ and CD8+ in T lymphocytes (CD3+) in peripheral blood to assess the immune response in different groups. In group B, proportion of CD4+CD25+ T cells was significantly decreased compared to control. However, in Group C, where dtlNKs were infused, the proportion of CD4+CD25+ T cells in peripheral blood was restored to levels similar to the control group (Fig. 4A and B). Interestingly, the proportion of CD4+CD25+ T cells reached its peak on day 3 post-transplantation. The peripheral proportion of CD8+ T cell levels did not show significant changes on day 1 and day 3 post-transplantation in all three groups (Fig. 4C and D). However, it was significantly decreased in control and infusion of dtlNKs groups, while it remained high in group B. These findings suggest that the presence of donor hepatic NK cells increased the peripheral proportion of CD4+CD25+ T cells, which are known as suppressors of allograft rejection. Additionally, donor hepatic NK cells decreased the peripheral proportion of CD8 + T cells, which are considered the main mediators of allograft rejection.
Donor liver NK cells are associated with CD95-mediated apoptosis of intrahepatic infiltrating lymphocytes
TUNEL assay was used to study whether impact of donor hepatic NK cells on the apoptosis of intrahepatic infiltrating lymphocytes in liver grafts. The results showed that on day 1 post-transplantation, no observable TUNEL signal was detected in the cells (Table 3). However, as the transplantation time progressed, the number of TUNEL-positive cells gradually increased. On days 3 and 7 post-transplantation, the apoptosis rate of intrahepatic infiltrating lymphocytes in Group B (donor liver leukocytes depletion group) was significantly lower compared to Group A (control) and Group C (infusion of dtlNKs) (Table 3; Fig. 5A). Interestingly, the infusion of dtlNKs increased the apoptosis rate after the reconstruction of the biliary tract and portal vein. These findings suggest that the presence of donor hepatic NK cells may be associated with the apoptosis of intrahepatic infiltrating lymphocytes in the transplant liver.
Determination of apoptosis and CD95 expression in the liver grafts. (A) TUNLE staining of liver grafts sections. The cells staining brown were judged as being TUNEL (+). (B) Immunohistochemistry images of CD95 protein expression in liver grafts sections. (original magnification, ×200). 9 rats were used in each group.
To explore the mechanisms of how donor liver NK cells mediated apoptosis of intrahepatic infiltrating lymphocytes, CD95 protein expression was examined using immunohistochemistry. On day 1 after liver transplantation, no observable CD95 positive staining was detected. However, the CD95 protein expression pattern was similar to the TUNEL staining results, indicating a correlation between CD95 expression and apoptosis (Table 4; Fig. 5B). The expression of CD95 protein in group B (donor liver leukocytes depletion group) was significantly lower compared to groups A (control) and C (infusion of dtlNKs). These findings suggest that the presence of donor hepatic NK cells is associated with upregulation of CD95 expression in intrahepatic infiltrating lymphocytes, indicating a potential role for donor hepatic NK cells in CD95-mediated apoptosis of these cells during liver transplantation.
Donor liver NK cells alter cytokine expression in recipients’ liver grafts extracts
Considering that NK cells co-culture with CD4+ or CD8+ T cells altered the cytokines levels in culture media, ELISA was applied to detect levels of cytokines (IL-2, IFN-γ, IL-4, and IL-10) in the recipients’ liver graft extracts. The results revealed that levels of IL-2 and IFN-γ in the liver grafts of group A (control) and group C (infusion of dtlNKs) were significantly lower compared to group B (donor liver leukocytes depletion group). Conversely, levels of IL-4 and IL-10 were significantly higher in group A and group C compared to group B (Fig. 6). These findings indicate that the absence of donor liver NK cells is associated with abnormal levels of IL-2, IFN-γ, IL-4, and IL-10 in the liver grafts, suggesting a role for donor liver NK cells in modulating the cytokine milieu during liver transplantation.
Discussion
Liver allograft rejection is indeed a common occurrence after liver transplantation. It primarily results from an acute immune response mounted by the recipient’s immune system against the transplanted liver. The process involves the recognition of alloantigens present in the donor liver by recipient T cells. This recognition triggers the proliferation and activation of T cells, which, in turn, drive the rejection process through various mechanisms11. Direct T-cell mediated damage is one of the pathways by which hepatic rejection occurs. Activated T cells directly attack the donor liver cells, leading to tissue damage and destruction. Additionally, T cells secrete cytokines that contribute to the immune response and recruitment of tissue-destructive inflammatory cells, such as macrophages and neutrophils. These inflammatory cells further amplify the immune response and contribute to tissue damage11,25. Hepatic NK cells are a significant component of the liver leukocyte population and have distinct characteristics compared to NK cells found in peripheral blood. They are known to exhibit a more tolerogenic phenotype and have been implicated in immune regulation and tolerance induction in the liver. Hepatic NK cells have been shown to have immunoregulatory functions, including the ability to suppress T-cell responses and promote the expansion of regulatory T cells26. However, the roles of donor-derived hepatic NK cells in liver allograft tolerance and rejection are still unclear.
In this study, we found that the infusion of dtlNK cells, administered via the portal vein, led to a significant prolongation of survival in recipients who had undergone liver transplantation without lymphocytes. This finding suggests that donor hepatic NK cells possess immunoregulatory properties that can impact the outcome of liver transplantation. Previous studies have reported the occurrence of donor chimerism, where donor-derived cells are present in the recipient’s body, particularly in the NK cell subset, following liver transplantation8,27,28. This observation further supports the notion that hepatic NK cells play a role in the immunoregulation of the transplant process. These chimeric NK cells, derived from the donor and present in the recipient following liver transplantation, exhibit unique properties that distinguish them from conventional NK cells. They are characterized by a high activation state, indicating their readiness to engage in immune responses. Additionally, chimeric NK cells are known to possess an abundance of cytolytic enzymes, which are instrumental in their ability to directly eliminate target cells. Moreover, these chimeric NK cells display a distinct pattern of interleukin expression8. Due to their missing-self recognition pattern and high activation state in the graft, donor hepatic NK cell might have the potential to play a significant role in responding to alloantigens from the recipient8,27,28. Therefore, we aimed to investigate the role of donor hepatic NK cells as immunoregulators in the early stages of liver transplantation.
In our liver transplantation rat model, we made an interesting observation regarding the role of donor liver leukocytes, specifically hepatic NK cells, in liver function and allograft tolerance. Depletion of donor liver leukocytes had a detrimental effect on liver function, as indicated by higher levels of ALP, bilirubin, AST, and ALT. However, when dtlNKs were infused into the recipients, there was a notable improvement in liver function after transplantation. Histopathological examination further supported these findings, showing that dtlNKs infusion attenuated the infiltration of mononuclear lymphocytes and preserved the integrity of hepatic lobules in the liver grafts depleted of donor leukocytes. This suggests that.
the donor liver NK cell population plays a crucial role in suppressing the recipient’s immune.
response, particularly in the early stages following liver transplantation. It is important to note that over time, as the recipients undergo allograft remodeling and the donor liver NK cells undergo apoptosis, the immunological impact of donor liver NK cells on recipients may gradually diminish or even disappear. Nevertheless, our results highlight the significant contribution of donor liver NK cells in modulating the immune response and promoting favorable outcomes in liver transplantation.
NK cells are known to play a crucial role in regulating T cell immunity by activating T cells through the production of cytokines that promote T cell differentiation12,13. However, there is limited research on the effects of donor-derived hepatic NK cells on the recipient’s alloreactive T lymphocytes. In our study, we observed that donor hepatic NK cells induced apoptosis in alloreactive CD8+ T cells while supporting the survival of CD4+ T cells. In addition to the cytotoxic effector function, activated CD8+ T cells could produce multiple proinflammatory cytokines including IFN-γ. IFN-γ is known to have immunostimulatory effects and can increase the expression of alloantigens in the graft liver29.
CD95 is an important transduction molecule of the exogenous apoptosis signal pathway. The apoptosis of intrahepatic infiltrating lymphocytes in the liver grafts of transplant recipients is influenced by both the levels of cytokines, such as IL-2, and the expression of CD9520,30,31. In vitro and In vivo experiments showed that the NK cells induced the CD95 protein level in CD8+ T cells, suggesting that donor liver NK cells promoted CD95-mediated apoptosis of alloreactive CD8+ T cells. Activated T cells are CD95-sensitive, and the interaction between CD95 ligand and CD95 molecule rapidly induces apoptosis of CD8+ T cells32.
Lymphokines, including IL-2, IL-4, and IL-10, are crucial immunomodulatory molecules involved in regulating immune responses. Physiological levels of these cytokines play a significant role in the development and differentiation of regulatory T cells (Tregs)33,34,35. We found that IL-2 level in culture supernatant and liver graft was significantly decreased in the presence of liver NK cells in vitro and in vivo. When the T cells are activated by alloantigen, they both produce and respond to IL-2, which could stimulate the proliferation of active T cells36.
CD4+CD25+ Tregs are a specialized subset of T cells that play a critical role in inducing and maintaining immune tolerance, including in the context of liver allograft transplantation24,37. We found that who received liver transplants with donor liver NK cells had a significantly higher percentage of peripheral CD4+CD25+ T cells compared to those without donor liver NK cells. Additionally, we discovered that CD4+CD25+ T cells were significantly up-regulated in the recipient’s liver graft transfused with dtlNKs. When CD4+CD25+ T cells were depleted in the recipient, it led to liver rejection accompanied by the induction of CD8+ T cells. This suggests that CD4+CD25+ T cells in the recipient are essential for liver tolerance38,39,40. IL-4 enhances the expression of FOXP3 or membrane IL-2Ralpha (CD25) on Tregs41. Therefore, a high level of IL-4 in the serum is beneficial for the differentiation of CD4+CD25+ T lymphocytes into Tregs. Another important factor, IL-10, acts as an inhibitor of cellular immunity during liver transplantation and is associated with the upregulation of CD4+CD25+ Tregs42,43.
It has been noted that liver grafts have low expression of MHC II molecules but high expression of MHC I molecules, which indicates that CD3+CD8+ T cells may become activated following liver transplantation11. In our study, we observed a significant reduction in the proportion of peripheral CD3+CD8+ T cells in recipients who received liver transplants with donor hepatic NK cells, specifically on the seventh day after transplantation. Interestingly, in liver allografts of rhesus monkeys that experienced rejection, there was a significant increase in CD3+ and CD8+ T cells. These findings suggest that CD3+ and CD8+ T cells could potentially serve as indicators for acute rejection after transplantation44.
In summary, our study demonstrated that the presence of donor hepatic NK cells had a beneficial effect on liver allograft injury. This effect was mediated by the modulation of lymphocyte infiltration and inflammatory cytokines. We observed that the presence of donor hepatic NK cells was associated with the apoptosis of recipient’s alloreactive CD8+ T cells and the generation of recipient’s CD4+CD25+ Tregs. These mechanisms facilitated the redistribution of T cell subsets in the recipient, ultimately leading to the amelioration of liver allograft rejection. In short, this study was to discover the role of donor liver NK cells in the immune regulation of allogeneic rat liver transplantation and its possible mechanism, providing new ideas for exploring the mechanism of donor-recipient interaction in human liver transplantation. Besides, immune-suppressive drugs employed to prevent graft rejection in humans whether can inhibit NK cell function need further research.
However, there are several limitations in this study. The data of our study was basic on the spontaneous tolerance model of allogeneic rat liver transplantation in a specific strain combination, whether there is consistency in other rat strain combinations of allogeneic liver transplantation needs further research. In addition, the interaction between donor liver NK cells and other donor-derived immune cells was isolated after donor WBI, and it was difficult to completely restore the immune regulation process of donor liver NK cells in allograft liver transplantation. Donor liver is considered a complex organism, and the interaction between donor NK cells and other liver immune cells is also difficult to isolate. To fully understand the role and mechanism of donor NK cells in allograft liver transplantation, more in-depth studies are needed.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- LTx:
-
allogeneic liver transplantation
- KIRs:
-
killer immunoglobulin-like receptors
- PI:
-
Propidium Iodide
- WBI:
-
whole body gamma-irradiation
- MST:
-
mean survival time
- dtlNKs:
-
purified donor type liver NK cells
- ALP:
-
alkaline phosphatase
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- RAI:
-
rejection activity index
- Tregs:
-
regulatory T cells
- FAS:
-
Fas receptor-related molecule
- OLT:
-
orthotopic liver transplantation
References
Wang, Y. & Zhang, C. The roles of liver-Resident lymphocytes in liver diseases. Front. Immunol. 10, 1582. https://doi.org/10.3389/fimmu.2019.01582 (2019). PMID:31379818.
Wiggins, B. G. et al. The human liver microenvironment shapes the homing and function of CD4(+) T-cell populations. Gut 71, 1399–1411. https://doi.org/10.1136/gutjnl-2020-323771 (2022). PMID:34548339.
Lurje, I., Hammerich, L. & Tacke, F. Dendritic cell and T cell crosstalk in liver fibrogenesis and hepatocarcinogenesis: implications for prevention and therapy of liver Cancer. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21197378 (2020). PMID:33036244.
Li, Y. et al. Natural killer cells: friend or foe in metabolic diseases?? Front. Immunol. 12, 614429. https://doi.org/10.3389/fimmu.2021.614429 (2021). PMID:33717101.
Gu, X. et al. New insights into iNKT cells and their roles in liver diseases. Front. Immunol. 13, 1035950. https://doi.org/10.3389/fimmu.2022.1035950 (2022). PMID:36389715.
Peng, H., Wisse, E. & Tian, Z. Liver natural killer cells: subsets and roles in liver immunity. Cell. Mol. Immunol. 13, 328–336. https://doi.org/10.1038/cmi.2015.96 (2016). PMID:26639736.
Jiang, Y., Que, W., Zhu, P. & Li, X. K. The role of diverse liver cells in liver transplantation tolerance. Front. Immunol. 11, 1203. https://doi.org/10.3389/fimmu.2020.01203 (2020). PMID:32595648.
Moroso, V. et al. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl. 16, 895–908. https://doi.org/10.1002/lt.22080 (2010). PMID:20583081.
van Leest, Y. et al. No evidence for involvement of donor NK cells in liver transplant tolerance. Transpl. Immunol. 24, 138–139. https://doi.org/10.1016/j.trim.2010.11.001 (2011). PMID:21070857.
Yu, J. D. et al. Donor liver natural killer cells alleviate liver allograft acute rejection in rats. Hepatobiliary Pancreat. Dis. Int. 10, 386–392. https://doi.org/10.1016/s1499-3872(11)60065-9 (2011). PMID:21813387.
Ronca, V., Wootton, G., Milani, C. & Cain, O. The immunological basis of liver allograft rejection. Front. Immunol. 11, 2155. https://doi.org/10.3389/fimmu.2020.02155 (2020). PMID:32983177.
Schuster, I. S., Coudert, J. D., Andoniou, C. E. & Degli-Esposti, M. A. Natural regulators: NK cells as modulators of T cell immunity. Front. Immunol. 7, 235. https://doi.org/10.3389/fimmu.2016.00235 (2016). PMID:27379097.
Pallmer, K. & Oxenius, A. Recognition and regulation of T cells by NK cells. Front. Immunol. 7, 251. https://doi.org/10.3389/fimmu.2016.00251 (2016). PMID:27446081.
Cook, K. D., Waggoner, S. N. & Whitmire, J. K. NK cells and their ability to modulate T cells during virus infections. Crit. Rev. Immunol. 34, 359–388. https://doi.org/10.1615/critrevimmunol (2014). PMID:25404045.
Malhotra, A. & Shanker, A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy 3, 1143–1166. https://doi.org/10.2217/imt.11.102 (2011). PMID:21995569.
Cuff, A. O. et al. Eomeshi NK cells in human liver are Long-Lived and do not recirculate but can be replenished from the circulation. J. Immunol. 197, 4283–4291. https://doi.org/10.4049/jimmunol.1601424 (2016). PMID:27798170.
Lv, L. H. et al. Functional distinction of rat liver natural killer cells from spleen natural killer cells under normal and acidic conditions in vitro. Hepatobiliary Pancreat. Dis. Int. 11, 285–293. https://doi.org/10.1016/s1499-3872(12)60162-3 (2012). PMID:22672823.
Yang, L. et al. A rat model of orthotopic liver transplantation using a novel magnetic anastomosis technique for Suprahepatic Vena Cava reconstruction. J. Vis. Exp. https://doi.org/10.3791/56933 (2018). PMID:29608158.
Demetris, A. J. et al. 2016 comprehensive update of the Banff working group on liver allograft pathology: introduction of Antibody-Mediated rejection. Am. J. Transpl. 16, 2816–2835. https://doi.org/10.1111/ajt.13909 (2016). PMID:27273869.
Levoin, N., Jean, M. & Legembre, P. CD95 structure, aggregation and cell signaling. Front. Cell. Dev. Biol. 8, 314. https://doi.org/10.3389/fcell.2020.00314 (2020). PMID:32432115.
Ghelani, A. et al. Defining the threshold IL-2 signal required for induction of selective Treg cell responses using engineered IL-2 Muteins. Front. Immunol. 11, 1106. https://doi.org/10.3389/fimmu.2020.01106 (2020). PMID:32582190.
Saraiva, M., Vieira, P. & O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 217 https://doi.org/10.1084/jem.20190418 (2020). PMID:31611251.
Yang, W. C. et al. Interleukin-4 supports the suppressive immune responses elicited by regulatory T cells. Front. Immunol. 8, 1508. https://doi.org/10.3389/fimmu.2017.01508 (2017). PMID:29184551.
Lei, H., Reinke, P., Volk, H. D., Lv, Y. & Wu, R. Mechanisms of immune tolerance in liver Transplantation-Crosstalk between alloreactive T cells and liver cells with therapeutic prospects. Front. Immunol. 10, 2667. https://doi.org/10.3389/fimmu.2019.02667 (2019). PMID:31803188.
Scalea, J., Hanecamp, I., Robson, S. C. & Yamada, K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. ;19:23–30. PMID:22360750. (2012). https://doi.org/10.1111/j.1399-3089. 2011.00687.x.
Mikulak, J., Bruni, E., Oriolo, F., Di Vito, C. & Mavilio, D. Hepatic natural killer cells: Organ-Specific sentinels of liver immune homeostasis and physiopathology. Front. Immunol. 10, 946. https://doi.org/10.3389/fimmu.2019.00946 (2019). PMID:31114585.
Harmon, C., Sanchez-Fueyo, A., O’Farrelly, C. & Houlihan, D. D. Natural killer cells and liver transplantation: orchestrators of rejection or tolerance?? Am. J. Transpl. 16, 751–757. https://doi.org/10.1111/ajt.13565 (2016). PMID:26690302.
Halma, J., Pierce, S., McLennan, R., Bradley, T. & Fischer, R. Natural killer cells in liver transplantation: can we Harness the power of the immune checkpoint to promote tolerance? Clin. Transl Sci. 15, 1091–1103. https://doi.org/10.1111/cts.13208 (2022). PMID:34866338.
Yap, M., Brouard, S., Pecqueur, C. & Degauque, N. Targeting CD8 T-Cell metabolism in transplantation. Front. Immunol. 6, 547. https://doi.org/10.3389/fimmu.2015.00547 (2015). PMID:26557123.
Yokota, S., Yoshida, O., Ono, Y., Geller, D. A. & Thomson, A. W. Liver transplantation in the mouse: insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transpl. 22, 536–546. https://doi.org/10.1002/lt.24394 (2016). PMID:26709949.
Chen, Y. Y. et al. Human intrahepatic regulatory T cells are functional, require IL-2 from effector cells for survival, and are susceptible to Fas ligand-mediated apoptosis. Hepatology 64, 138–150. https://doi.org/10.1002/hep.28517 (2016). PMID:26928938.
Leonardi, A. J. & Proenca, R. B. Akt-Fas to quell aberrant T cell differentiation and apoptosis in Covid-19. Front. Immunol. 11, 600405. https://doi.org/10.3389/fimmu.2020.600405 (2020). PMID:33408715.
Yang, Y. & Lundqvist, A. Immunomodulatory effects of IL-2 and IL-15; implications for Cancer immunotherapy. Cancers (Basel). 12 https://doi.org/10.3390/cancers12123586 (2020). PMID:33266177.
Iwaszko, M., Bialy, S. & Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells 10 https://doi.org/10.3390/cells10113000 (2021). PMID:34831223.
Wei, H. X., Wang, B. & Li, B. IL-10 and IL-22 in mucosal immunity: driving protection and pathology. Front. Immunol. 11, 1315. https://doi.org/10.3389/fimmu.2020.01315 (2020). PMID:32670290.
Abbas, A. K. The surprising story of IL-2: from experimental models to clinical application. Am. J. Pathol. 190, 1776–1781. https://doi.org/10.1016/j.ajpath.2020.05.007 (2020). PMID:32828360.
Shaban, E. et al. Targeting regulatory T cells for transplant tolerance: new insights and future perspectives. Kidney Dis. (Basel). 4, 205–213. https://doi.org/10.1159/000490703 (2018). PMID:30574497.
Dai, H., Zheng, Y., Thomson, A. W. & Rogers, N. M. Transplant tolerance induction: insights from the liver. Front. Immunol. 11, 1044. https://doi.org/10.3389/fimmu.2020.01044 (2020). PMID:32582167.
Du, X., Chang, S., Guo, W., Zhang, S. & Chen, Z. K. Progress in liver transplant tolerance and tolerance-Inducing cellular therapies. Front. Immunol. 11, 1326. https://doi.org/10.3389/fimmu.2020.01326 (2020). PMID:32670292.
Jiang, X. et al. The importance of CD25 + CD4 + regulatory T cells in mouse hepatic allograft tolerance. Liver Transpl. 12, 1112–1118. https://doi.org/10.1002/lt.20787 (2006). PMID:16724335.
Khumalo, J., Kirstein, F., Hadebe, S. & Brombacher, F. IL-4Ralpha signaling in CD4 + CD25 + FoxP3 + T regulatory cells restrains airway inflammation via limiting local tissue IL-33. JCI Insight. 5 https://doi.org/10.1172/jci.insight.136206 (2020). PMID:32931477.
Nguyen, J. H. et al. Intranodal sirolimus induces regulatory T cells in human hepatic lymph nodes via Interleukin 10 signaling. Liver Transpl. 27, 1669–1672. https://doi.org/10.1002/lt.26214 (2021). PMID:34133835.
Beyzaei, Z., Shojazadeh, A. & Geramizadeh, B. The role of regulatory T cells in liver transplantation. Transpl. Immunol. 70, 101512. https://doi.org/10.1016/j.trim.2021.101512 (2022). PMID:34871717.
Kim, H. et al. Memory T cells are significantly increased in rejected liver allografts of rhesus monkeys. Liver Transpl. 24, 256–268. https://doi.org/10.1002/lt.24983 (2018). PMID:29150986.
Acknowledgements
We thank Dr. Li-Shuo Shi(Department of Probability and Statistics, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, PR China.) for guidance on the statistics of this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 30671987; No.8180102403), PhD Start-up Fund of Natural Science Foundation of Guangdong Province (No. 2015A030310377) and National key Clinical Discipline.
Author information
Authors and Affiliations
Contributions
JD Y, JY W, TZ L and LH L wrote the main manuscript text, ZP C, ZY L, HQ Z, GL L, YL W prepared Figs. 1, 2 and 3. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, J., Wu, J., Long, T. et al. Donor liver natural killer cells ameliorate liver allograft rejection via inducing apoptosis of alloreactive CD8+ T cells generating CD4+CD25+ regulatory T cells. Sci Rep 15, 23499 (2025). https://doi.org/10.1038/s41598-025-06456-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06456-1