Abstract
With improved living standards and increased health awareness among Chinese residents, carbohydrate antigen 19-9 (CA 19-9) has become a routine marker in health examinations. Elevated CA 19-9 levels are frequently observed in clinical practice. Although chemiluminescence technology is widely recognized for its high sensitivity and specificity, it cannot eliminate interference, which may lead to deviations and misdiagnoses. This study aims to find a simple and effective method to confirm and exclude the interference of CA 19-9 on the Abbott platform. 342 individuals with unexplained elevated CA 19-9 in serial determinations for more than 6 months were included as cases. Serum samples from individuals with persistently elevated CA 19 -9 levels were evaluated via sialidase treatment, polyethylene glycol (PEG) precipitation, gel filtration chromatography (GFC), gradient dilution assays, heterophile antibody blocking assays and rheumatoid factor titer assays. Out of 342 individuals with elevated CA 19-9, 77 cases were confirmed as interferent by the sialidase treatment. There was a significant consistency between the PEG precipitation and sialidase treatment results (kappa = 0.920). Compared with the sialidase treatment method, the specificity of the PEG precipitation method was 96.2%, and the sensitivity was 100%. When the cutoff value for the CA 19-9 recovery after PEG precipitation was below 37.9%, the area under the curve (AUC) was highest at 0.993 (95% CI 98.5–99.8%). The PEG precipitation method is useful and reliable for patients with unexplained elevated CA 19-9. This method could effectively identify the interference, reduce clinical misdiagnosis, alleviate unnecessary financial burdens on patients.
Similar content being viewed by others
Introduction
Carbohydrate antigen 19-9 (CA 19-9) is a glycoprotein belonging to the sialylated Lewis blood group antigen family and is typically found in trace amounts in the epithelial cells of various tissues, including the salivary glands, prostate, pancreas, breast, stomach, bile ducts, gallbladder, and bronchi, in healthy individuals1. CA 19-9 is a useful tumor marker for diagnosing pancreatic cancer, monitoring treatment efficacy, and tracking recurrence2. However, elevated serum CA 19-9 concentrations are also observed in benign conditions such as benign tumors, inflammation, hyperplasia, and metabolic dysfunction3. In addition to these identifiable causes, some patients experience “unexplained elevated CA 19-9”, posing a diagnostic challenge for clinicians.
Multiple platforms are available for CA 19-9 testing, each varying in methodology, antibody selection, and reference ranges, with no international standardization. The reagents used on different platforms also differ in their ability to prevent nonspecific binding, which can lead to discrepancies in test results4 and complicate clinical diagnosis. The CA 19-9 assay used in this study was performed on the Abbott Alinity i platform, an automated analyzer utilizing chemiluminescent microparticle immunoassay (CMIA) technology. This system employs anti-analyte-coated paramagnetic microparticles and anti-analyte acridinium-labeled conjugates, offering high automation, specificity, sensitivity, and a broad linear range. Despite its excellent performance, the assay remains vulnerable to interference, potentially affecting results and leading to inappropriate treatments or unnecessary invasive procedures5,6.
Traditional interferences in immunoassays include heterophile antibodies, rheumatoid factor, biotin, autoantibodies, and cross-reactivity7. Addressing these interferences to enhance the accuracy of CA 19-9 testing is a key focus for clinical and laboratory professionals. The CA 19-9 assay with Alinity i is based upon a monoclonal antibody, 1116-NS-19-9, which reacts with a carbohydrate antigenic determinant expressed on the circulating antigen. This method can detect tumor-associated antigens present as monosialogangliosides in tissues and carbohydrate-rich glycoproteins in serum.
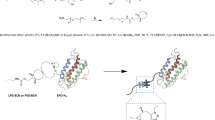
CA 19-9 is a type-1 tetrasaccharide tumor-associated carbohydrate antigen named Sialyl Lewis a (SLea) that includes fucose, N-acetylglucosamine, galactose, and sialic acid8. Altered glycans are often found in cancers that is associated with abnormal expression of the glycosylation biosynthetic pathways, leading to variations in the basic core carbohydrate chains conjugated to glycoproteins and glycolipids, and it particularly affects the expression of sialic acids that cap cell surface glycans9. The SLea tetrasaccharide stems from incomplete synthesis of the normal glycan disialyl Lea which has an additional sialic acid moiety compared to SLea (Fig. 1)9. Borenstein-Katz, et al. presented detailed structural information for the CA 19-9 and its recognition by monoclonal antibody, 1116-NS-19-9, and has shown that binding of full-length antibody to SLea-nanoparticles with multivalent expression of antigen, coated on a solid surface showed binding to SLea, but complete loss of binding to Lea antigen that lacks the terminal sialic acid, which implies that sialic acid recognition plays an important role for the binding of the 1116-NS-19-9 antibody. Sialidase removes terminal sialic acids from gangliosides10and at the cellular level, causes cleavage of a sialic acid residue of glycan moiety linked to cellular glycoproteins11.
Sialidase treatment is effective in identifying CA 19-9 interference; however, its complexity, high cost, and time consumption make it unsuitable for routine clinical screening. In contrast, the polyethylene glycol (PEG) precipitation method is simple, cost-effective, and easy to perform. Early screening via PEG precipitation is a reliable method for ruling out further invasive or costly medical examinations. PEG precipitation has been mentioned in previous reports, such as the interference of macroprolactin, macro-B12, macro-TSH, and macro-troponin12,13,14,15,16. Abnormally elevated CA 19-9 levels may be associated with antibody-mediated analytical interferences such as macro-complexes of CA 19-9 (macro-CA 19-9, generally with immunocomplexes)17. However, it has yet to be systematically validated in routine laboratory practice for CA 19-9 interference.
Our research aims to find a simple, cost-effective analytical method to detect interference in CA 19-9 elevations, ultimately improving clinical outcomes and patient management.
Materials and methods
This project was approved by the Ethics Committees of the First Affiliated Hospital, College of Medicine, Zhejiang University. The study has been conducted in accordance with the principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments, and prior informed consent was obtained from the enrolled patients.
Specimen source and determination
Individuals enrolled in this study underwent healthy physical examinations at The First Affiliated Hospital of Zhejiang University College of Medicine between April 2022 and April 2024 who were serial determinations for more than 6 months. The clinical diagnoses and imaging data of the selected cohort were reviewed, and 342 cases of unexplained CA 19-9 elevation were identified. The other tumor markers and serum biochemical parameters of these cohorts were negative, and they were also negative for other chronic diseases according to laboratory evidence and computed tomography (CT) or contrast-enhanced magnetic resonance (MR). CA 19-9 measurements were performed via the Abbott Alinity i platform, and serum samples were collected and stored at − 80 °C for further analysis.
Additionally, 130 patients with true CA 19-9 elevation which were treated via Sialidase treatment as positive controls, these individuals also had evidences of elevated CA 19-9 based on clinical diagnosis, imaging and pathological data. All positive control samples were subjected to each interference exclusion methods to validate the assay performance.
Sialidase treatment
The sialidase dilution solution (pH 4.5 ± 0.05, 20–25 °C) was prepared using distilled water, acetic acid, NaOH, and BSA. 5 ml of this mixture was added to a tube containing 5 units of sialidase (Sigma, USA) and mixed thoroughly, creating a stock solution with a concentration of 1 U/mL. For the preparation of “Treatment Solution 1” (500 mU/mL), the stock solution was diluted 1:1 with the sialidase dilution solution. The sialidase dilution solution alone was used as “Treatment Solution 2”.
Equal volumes of the serum samples and the control were added to 50 µL of Treatment Solution 1 and Treatment Solution 2, respectively. The mixtures were sealed with paraffin film and incubated at 37 °C for 24 h. After incubation, the mixtures were thoroughly mixed, and CA 19-9 levels were measured. According to the mechanism of sialidase specificity hydrolyzing on sialic acid, sialidase could remove the terminal sialic acids from gangliosides and eliminate the binding site of the 1116-NS-19-9 antibody10.If the CA 19-9 level in the mixture containing Treatment Solution 1 was below the detection limit (The concentration of CA 19-9 < 2 U/mL), while the CA 19-9 level in the mixture containing Treatment Solution 2 remained unchanged, it indicated that sialidase removes terminal sialic acid and the sialic acid residues linked to cellular glycoproteins. This confirmed the presence of true CA 19-9.
Immunoassay interference exclusion methods
Polyethylene glycol (PEG) precipitation
Polyethylene glycol (PEG) 6000 functions similarly to a sponge, capturing water within protein structures. This process modifies their solubility, leading to protein precipitation18 Proteins with higher molecular weights exhibit lower solubility compared to those with lower molecular weights19. Treating samples with 25% PEG 6000 is a standard approach for detecting autoantibody complexes and is broadly useful in determining whether an antibody is the source of suspected interference13.
An equal volume of 25% PEG 6000 solution was added to each serum sample. After thorough mixing, the samples were incubated at room temperature for 30 min and then centrifuged at 1800 × g for 10 min20. The concentration of CA 19-9 in the supernatant was measured. The percentage of CA 19-9 recovery after PEG precipitation was calculated via the following formula:
Heterophile antibody blocking
Heterophile antibodies are low-affinity, multi-specific antibodies formed early in immune responses that target undefined epitopes, including those from animals such as mice, horses, goats, and sheep21. The heterophile blocking reagent (HBR) contains a unique blocking component designed to inactivate heterophile antibodies. When a specific blocking agent binds to a heterophile antibody, it prevents the antibody from causing immune interference. For this procedure, 100 µL of serum was mixed with 15 µL of HBR (Zen-Bioscience, China) and incubated at room temperature for 30 min before CA 19-9 analysis.
Gradient dilution assay
Patient samples were diluted by factors of 2, 4, and 8 with Abbott Alinity i sample dilution solution, and the average recovery rate was assessed. Under normal conditions without interferences, the concentration of the analyte should decrease linearly as the sample is diluted19. The study of dilution linearity for the CA 19-9 assay was conducted according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) EP6-P2, with the manufacturer’s guidelines indicating an average recovery rate of 100 ± 15%23.
Rheumatoid factor assay
The rheumatoid factor (RF) titer was analyzed via immunoturbidimetry using a fully automated protein analyzer (BN II System, Siemens). The reagents and quality controls were sourced from Siemens, and the reference range was 0–20 IU/mL.
Gel filtration chromatography (GFC) assay
GFC analysis was performed to confirm the presence of immune complex interference. The analysis was carried out via the AKTA-pure 25 system (GE Healthcare) with a Superdex 200 column (10 × 300 mm, GE Healthcare) for sample detection and separation. The column was initially equilibrated with PBS. After reaching equilibrium, 500 µL of the sample was injected at a flow rate of 0.5 mL/min. The detection wavelength was set at 280 nm, and the eluted fractions were collected at one-minute intervals.
The components of each eluted fraction, including CA 19-9, immunoglobulins (IgA, IgG, and IgM), and albumin, were analyzed to generate chromatograms. IgA, IgG, and IgM were measured on the HITACHI platform (Hitachi Diagnostics Co., Ltd. Japan), albumin was measured on the ROCHE Cobas c702 platform (Roche Diagnostics Co., Ltd. Switzerland), and the CA 19-9 concentration was measured on the Abbott Alinity i platform.
Statistical analysis
Gel filtration chromatography data were analyzed via Excel 2010 software (Microsoft Inc., USA). The chi-square test and Mann‒Whitney U test was used as appropriate for comparing categorical and nonnormally distributed continuous data, respectively. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values for PEG, HBR and dilution, aiming to achieve the best AUC, specificity, and sensitivity. Statistical analyses were performed via SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). Graphical analyses and data visualizations were generated via R version 4.3.1 (R Foundation for Statistical Computing).
Results
Characteristics of the individuals enrolled in this study
A total of 342 cases of unexplained elevation of CA 19-9 were enrolled according to clinical diagnosis, imaging, pathological data and sialidase treatment. On the basis of the results of sialidase treatment, we categorized the 342 cases into two groups: the non-interference group (265 cases) and the interference group (77 cases). The non-interference cases were selected as the negative control group. 130 cases of CA 19-9 positive were selected as positive control group based on clinical diagnosis, imaging, pathological data and confirmed by the sialidase treatment method. We performed immune interference analysis (PEG, HBR, Gradient dilution, RF) for interference group, non-interference group and the Positive Controls. GFC analysis was performed for the group with interference cases. The identification process and distribution of interference for both cases and controls are illustrated in Fig. 2. Among the 77 cases in the interference group, PEG precipitation identified interference in all 77 cases, showing good correlation with the sialidase treatment. The HBR treatment showed 39 cases of interference, while the dilution assay identified interference in 21 cases, additionally, 8 cases had RF levels exceeding the upper limit. The results of the positive control group remained unaffected by both the heterophile antibody blocking reagent and PEG precipitation method. RF was detected in 5 samples among the positive control group. 7 samples exhibited a loss of linearity upon Gradient dilution assay. The characteristics of the cases and controls, along with the results of the interference studies, are summarized in Table 1.
Determination of the optimal cutoff value of different methods for the interference determinant
The ability of PEG precipitation, HBR treatment, and gradient dilution assays to predict CA 19-9 interference was analyzed via ROC curves, which were used to compare the CA 19-9 interference group with the non-interference group (Fig. 3A–C). The areas under the curves (AUCs) for the PEG precipitation, HBR, and gradient dilution assays were 0.993 (95% CI 98.5–99.8%), 0.852 (95% CI 79.6–88.6%), and 0.735 (95% CI 65.1–81.6%), respectively.
PEG precipitation, HBR treatment and gradient dilution assay as predictors of CA 19-9 interference. (A–C) Receiver operating characteristic (ROC) analyses of PEG precipitation, HBR treatment and the gradient dilution assay as predictors of CA 19-9 interference. (D–F) Curves of the specificity, sensitivity and Youden index for PEG precipitation, HBR treatment and gradient dilution assay. The position of the black vertical dotted line represents the maximum Youden index that was used to predict CA 19-9 interference.
Using the Youden index (Fig. 3D–F), we established that when the PEG precipitation rate was lower than 37.9%, it exhibited a sensitivity of 100% and specificity of 96.2%. For HBR treatment, a rate lower than 47.4% resulted in a sensitivity of 50.6% and specificity of 97.4%. For the gradient dilution assay, a rate lower than 85.7% was associated with a sensitivity of 66.2% and specificity of 87.5%. However, as the AUC for the RF titer assay did not achieve the desired value, it failed to predict the interference of CA 19-9.
Consistency analysis of immunoassay interference exclusion methods
Based on the results of the sialidase treatment, the consistency analysis was conducted for the interference exclusion methods. The consistency results of each interference method compared with those of the sialidase treatment were as follows: the PEG method had a kappa value of 0.920, the HBR method had a kappa value of 0.560, the DIL method had a kappa value of 0.285, and the RF method had a kappa value of 0.091. The PEG method has the best consistency (Table 2).
GFC analysis of specimens with CA 19-9 interference
Among the 77 interference cases, we identified 71 cases with elevated CA 19-9 levels were attributed to the presence of Macro-CA 19-9. GFC analysis revealed that the majority of these cases were immunocomplexes involving immunoglobulin G (IgG), accounting for 68 of the 71 cases. In addition, 2 cases involved CA 19-9 bound to immunoglobulin A (IgA), and 1 case involved immunoglobulin M (IgM) (Fig. 4). Our results are consistent with previous reports14indicating that IgG is the predominant immunoglobulin involved in the immunocomplexes and plays a dominant role in interference with CA 19-9 detection.
Discussion
Immunoassay interference is a common issue that can significantly affect the accuracy of results. For example, cross-reactivity may occur when other substances bind to detection antibodies, resulting in false-positive or false-negative results. Additionally, high concentrations of interfering substances may occupy antibody binding sites, impacting the measurement of the target substance thereby affecting the assay’s specificity and sensitivity. In our research, we implemented different methods, such as PEG precipitation, HBR treatment, and gradient dilution assays, to mitigate CA 19-9 interference.
Rheumatoid factor (RF), an autoantibody that targets denatured IgG, presents a particular risk in immunoassays. Both IgM and IgG types of RF in human serum can directly bind to antibodies and enzyme-labeled secondary antibodies in the chemiluminescence system, potentially leading to false-positive results23,24. In this study, among the 77 interference cases, 8 samples were positive for RF, which may explain the false elevation of CA 19-9 in this cohort. Although manufacturers claim that immunoassays are designed with specific safeguards against RF interference, these measures do not eliminate interference. Since in interference group, only 8 samples tested positive for RF we could not generate a precise area under the curve (AUC) value for the receiver operating characteristic (ROC) curve, these results are not presented in the manuscript.
Diluting samples could destroy the binding of low-affinity nonspecific substances to the analyte, thereby achieving anti-interference effects. Theoretically, without interference, the analyte concentration should decrease linearly upon dilution. However, if interference exists, the response may not be linear25. Among the 77 interference cases, only 21 samples showed a linear loss, indicating potential interference, whereas 56 maintained a linear relationship. Owing to the unsatisfactory linear results, the dilution method was not effective in identifying interference from suspected cross-reactivity. A previous study revealed that linear outcomes may not confirm the absence of interference26in our study of 265 non-interference cases, 12 samples also lacked linearity after dilution, reinforcing this point. Both linear and nonlinear results were observed in interference group and non-interference group.
Heterophile antibodies are low-affinity nonspecific antibodies that can be either natural or autoimmune and are capable of reacting with various antigens and often appear early in immune responses, including human anti-animal antibodies (HAMAs)21. These antibodies are present in patients receiving monoclonal antibody treatments or those with a history of animal exposure27. Additionally, vaccination may lead to the production of heterophile antibodies28. A heterophile blocking reagent (HBR) can partially inactivate these antibodies to block nonspecific binding. In our study, among the 77 interference cases, 39 samples presented a decrease in CA 19-9 concentration after treatment with HBR, with recovery rates below 47.4%, indicating interference. Notably, HBR does not always reliably identify heterophile antibodies21. In our consistency analysis, the kappa value of HBR was 0.560, with a sensitivity of 50.6% and specificity of 97.4%. Our results showed that CA19-9 concentrations in the interference group were only partially reduced following HBR treatment. The limited efficacy not only indicates that heterophilic antibodies are not the main source of interference in our study, but also reveals the inherent limitations of this method. Moreover, the cost of HBR is significantly greater than that of the PEG method. The superior performance of PEG precipitation further supports this conclusion, which effectively removes the immune complexes29. PEG treatment achieved a greater reduction in CA 19-9 levels and demonstrated higher sensitivity and specificity for identifying assay interference, highlighting its diagnostic advantage over HBR.
Gel filtration chromatography (GFC) is a chromatographic technique based on the separation of molecules by size. In the GFC analysis, 71 samples showed immune complex interference. Most of them are immunocomplex IgG. IgG antibodies are produced by B cells and bind to specific antigens to form immune complexes, which could explain the 68 cases of immunocomplex IgG. In China, many healthy individuals use traditional Chinese medicines such as Dendrobium officinale and Cordyceps sinensis30which may produce metabolites that can interfere with measurements. These metabolites may be unstable or poorly resolved in GFC31. This may explain why 6 cases did not exhibit the presence of immune complexes. However, PEG precipitation can lead to the aggregation of large molecules through nonspecific interactions, resulting in the elimination of interference with CA19-9, which may explain why PEG precipitation identified 77 cases of interference, whereas GFC detected 71 cases.
The falsely elevated CA19-9 levels observed in some samples are due to the presence of macro-CA 19-9 formed by the binding of CA 19-9 to endogenous immunoglobulins (mostly IgG). These macro-complexes differ from free CA 19-9 typically associated with disease. However, they also could be recognized by assay antibodies, resulting in artificially elevated CA 19-9 values32. The formation of this complexes may protect CA19-9 epitope from enzymatic degradation, prolong their circulation, or alter their behavior in immunoassays, contributing to misleading results33.
PEG precipitation is a simple and effective approach for detecting such interference. PEG could selectively precipitate high-molecular-weight proteins and complexes, including macro-CA 19-9, while leaving free CA 19-9 in solution. Therefore, a significant reduction in CA 19-9 concentration following PEG treatment serves as an indirect marker for the presence of macro-complexes, improving the analytical specificity of the assay34.
Among the 265 non-interference patients, their biochemical and tumor marker levels were normal, and imaging and endoscopic exams were negative. Traditional Chinese medicine and black tea, which contain components such as Astragalus membranaceus and tea polyphenols that may stimulate CA 19-9 secretion, have been reported35,36. Although reports have suggested that COVID-19 vaccination might increase tumor marker levels, particularly when accompanied by traditional Chinese medicine treatment37,38. While these factors are likely to contribute to an increase in CA 19-9, but we have not found evidence in our research to date.
To our knowledge, we are the first to systematically propose the use of polyethylene glycol (PEG) to screen for CA 19-9 interference. PEG is a nonionic, linear polysaccharide that alters the solubility of proteins by capturing water, leading to precipitation18. PEG 6000 (25%) is commonly used to identify the presence of autoantibody complexes and some nonspecific binding antibodies13. Our study revealed that 22% of cases exhibited antibody-mediated interference, highlighting the significant prevalence of macro-complexes among the selected patients.
PEG precipitation is commonly used in clinical laboratories because of its simplicity, speed, and reproducibility. In this study, among the 342 cases with unexplained elevated CA 19-9, 77 cases were identified as interference, with a significant decrease in the CA 19-9 concentration in the supernatant after PEG precipitation. The current data suggest that PEG precipitation is highly consistent with sialidase treatment for detecting CA 19-9. However, our study still has some limitations. First, duo to PEG precipitation is a nonspecific method, potentially false negatives are also observed in prolactin precipitation experiments18. Among the 265 true positives, 10 cases presented false negative results. Such cases require careful validation and communication between laboratories and clinical doctor. Second, the reference interval for CA19-9 recovery after PEG precipitation was not established in this study, future studies are needed to define the recovery reference interval for clinical application. Third, because the correct interference of interference is indistinct, the internal standard substance of spiking experiments is difficult to determine, we did not perform spiking experiments in this study. In conclusion, ROC curve analysis revealed that PEG precipitation had a sensitivity of 100% and specificity of 96.2%, demonstrating significant consistency with sialidase treatment (Cohen’s kappa coefficient = 0.920). Owing to its cost-effectiveness, simplicity of operation and strong correlation with sialidase treatment via PEG precipitation, we recommend PEG precipitation for individuals with persistently elevated CA 19-9 levels for at least six months without clinical symptoms.
Data availability
Data is provided within the manuscript or supplementary information files.
References
People’s Republic of China Health Industry Standard, Common Used Serum Tumor Marker Tests:clinical Practice and Quality Management, WS/T 459-2018.
Pancreatic Surgery Group, Surgery Branch of Chinese Medical Association, Digital Medical Branch of Chinese Medical Association. Chinese expert consensus on digital intelligent precise diagnosis and treatment of pancreatic surgical diseases (2022 edition). Zhonghua Wai Ke Za Zhi. 60 (10), 881–887 (2022).
Pavai, S. & Yap, S. F. The clinical significance of elevated levels of serum CA 19-9. Med. J. Malaysia. 58 (5), 667–672 (2003).
Serdarevic, N. The comparison between different immunoassays for serum carbohydrate antigen (CA 19-9) concentration measurement. Acta Inf. Med. 26 (4), 235–239 (2018).
Clerico, A. et al. A black Swan in clinical laboratory practice: the analytical error due to interferences in immunoassay methods. Clin. Chem. Lab. Med. 56 (3), 397–402 (2018).
Constantinescu, G. et al. Mass spectrometry reveals misdiagnosis of primary aldosteronism with scheduling for adrenalectomy due to immunoassay interference. Clin. Chim. Acta. 507, 98–103 (2020).
Zaninotto, M. & Plebani, M. Understanding and managing interferences in clinical laboratory assays: the role of laboratory professionals. Clin. Chem. Lab. Med. 58 (3), 350–356 (2020).
Kannagi, R. Carbohydrate antigen Sialyl Lewis a—its pathophysiological significance and induction mechanism in cancer progression. Chang. Gung Med. J. 30(3), 189–209 (2007).
Borenstein-Katz, A. et al. Biomolecular recognition of the glycan neoantigen CA19-9 by distinct antibodies. J. Mol. Biol. 433 (15), 167099 (2021).
Sillence, D. J., Raggers, R. J. & van Meer, G. Assays for transmembrane movement of sphingolipids. Methods Enzymol. 312, 562–579 (2000).
Ryu Wang-Shick. Chapter 15—Influenza viruses. Mol. Virol. Hum. Pathogenic Viruses. 195–211 (2017).
Zhao, W. et al. Persistent increase of carbohydrate antigen 19-9 with an unknown reason: A seven-year follow-up case. J. Clin. Lab. Anal. 36 (12), e24792 (2022).
McCudden, C. R., Sharpless, J. L. & Grenache, D. G. Comparison of multiple methods for identification of hyperprolactinemia in the presence of macroprolactin. Clin. Chim. Acta. 411 (3–4), 155–160 (2010).
Delgado, J. A. et al. Challenges in the diagnosis of hypervitaminemia B12. Interference by immunocomplexes. Clin. Chim. Acta. 541, 117267 (2023).
Croce, L. et al. Unexplained hyperthyrotropinemia: A biochemical and clinical challenge. J. Clin. Med. 12 (8), 2934 (2023).
Lam, L. et al. Discrepancy between cardiac troponin assays due to endogenous antibodies. Clin. Chem. 66 (3), 445–454 (2020).
Monaghan, P. J. et al. False positive carbohydrate antigen 19-9 (CA19-9) results due to a low-molecular weight interference in an apparently healthy male. Clin. Chim. Acta. 406 (1–2), 41–44 (2009).
Fahie-Wilson, M. & Smith, T. P. Determination of prolactin: the macroprolactin problem. Best Pract. Res. Clin. Endocrinol. Metab. 27 (5), 725–742 (2013).
Ward, G. et al. The investigation of interferences in immunoassay. Clin. Biochem. 50 (18), 1306–1311 (2017).
Fahie-Wilson, M. N. Polyethylene glycol precipitation as a screening method for macroprolactinemia. Clin. Chem. 45 (3), 436–437 (1999).
Levinson, S. S. & Miller, J. J. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin. Chim. Acta. 325 (1–2), 1–15 (2002).
National Committee for Clinical Laboratory Standards. Evaluation of the Linearity of Quantitative Analytical Methods; Proposed Guideline-Second Edition. NCCLS Document EP6-P2 (NCCLS, 2001).
Gehin, J. E. et al. Rheumatoid factor and falsely elevated results in commercial immunoassays: data from an early arthritis cohort. Rheumatol. Int. 41 (9), 1657–1665 (2021).
Berth, M. et al. Rheumatoid factor interference in the determination of carbohydrate antigen 9-9 (CA 19-9). Clin. Chem. Lab. Med. 44 (9), 1137–1139 (2006).
Wauthier, L., Plebani, M. & Favresse, J. Interferences in immunoassays: review and practical algorithm. Clin. Chem. Lab. Med. 60 (6), 808–820 (2022).
Favresse, J. et al. Interferences with thyroid function immunoassays: clinical implications and detection algorithm. Endocr. Rev. 39 (5), 830–850 (2018).
Ismail, A. A. Identifying and reducing potentially wrong immunoassay results even when plausible and not-unreasonable. Adv. Clin. Chem. 66, 241–294 (2014).
Bjerner, J. et al. Human heterophile antibodies display specificity for murine IgG subclasses. Clin. Biochem. 38 (5), 465–472 (2005).
Berth, M. & Delanghe, J. Protein precipitation as a possible important pitfall in the clinical chemistry analysis of proteins in human serum. Clin. Chem. Lab. Med. 42 (9), 1023–1031 (2004).
Wu Wanxing, D. et al. Immunity enhancement activity and mechanism of ganoderma lucidum-Panax quiquefolium L-Cordyceps sinensis compound based on network pharmacology. Sci. Technol. Food Ind. 44 (8), 392–404 (2023).
Ó’Fágáin, C., Cummins, P. M. & O’Connor, B. F. Gel-filtration chromatography. Methods Mol. Biol. 1485, 15–25 (2017).
Ismail, A. A. et al. Interference in immunoassay is an underestimated problem. Ann. Clin. Biochem. 39 (Pt 4), 366–373 (2002).
Hoofnagle, A. N. & Wener, M. H. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J. Immunol. Methods. 347 (1–2), 3–11 (2009).
Fahie-Wilson, M. N. & Soule, S. G. Macroprolactinaemia: contribution to hyperprolactinaemia in a district general hospital and evaluation of a screening test based on precipitation with polyethylene glycol. Ann. Clin. Biochem. 34 (Pt 3), 252–258 (1997).
Tong, X., Xiao, D., Yao, F. & Huang, T. Astragalus membranaceus as a cause of increased CA19-9 and liver and kidney cysts: a case report. J. Clin. Pharm. Ther. 39 (5), 561–563 (2014).
Al-Janabi, A. A. H. S. & Tawfeeq, E. F. Interfering effect of black tea consumption on diagnosis of pancreatic Cancer by CA 19 -9. J. Gastrointest. Cancer. 48 (2), 148–150 (2017).
Baydogan, S. et al. Transient CA19-9 elevation Post-COVID-19 vaccine and infection: A case series. Gastro Hep. Adv. 2 (7), 946–947 (2023).
Zhang, X. et al. SARS-CoV-2: an updated review highlighting its evolution and treatments. Vaccines (Basel). 10 (12), 2145 (2022).
Funding
This study was supported by the Zhejiang Natural Science Foundation of China (no. TGY23H200020).
Author information
Authors and Affiliations
Contributions
GM and SL were involved in conceptualization, literature searching, data curation, formal analysis and writing the original draft of the manuscript. SL, QW and XY performed the PEG precipitation experiment and GFC assay. RF titer assay and heterophile antibodies assay were carried out by YT and WM. Gradient dilution assay was performed by YY. All authors were involved in reviewing and editing the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, S., Wang, Q., Yu, X. et al. Polyethylene glycol precipitation is a useful method for excluding the interference of unexplained elevated CA 19-9. Sci Rep 15, 23346 (2025). https://doi.org/10.1038/s41598-025-07065-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07065-8